Abstract
In this work, the SnS2 nanoflakes electrode materials have been synthesized using citric acid as chelating agent for lithium-ions battery by a facile microwave hydrothermal method. Citric acid can form a strong ligand for metal ions, and the crystals growth is controlled by the interaction between ligand and hydrogen bond, which affects the nucleation, growth, and self-assembly in the synthesis process. Therefore, the SnS2 nanoflakes electrodes were obtained with the size and thickness of around 100–200 nm and ~ 30 nm, respectively. The SnS2 nanoflakes electrode delivers the initial discharge capacity of 1485.8 mAh g−1, the reversible capacity of 327.3 mAh g−1 at 100 mA g−1 after 100 cycles, and the Coulombic efficiency (CE) of about 99.8%. The prominent rate capability was exhibited for lithium-ion battery (387.5 mAh g−1 and 339.7 mAh g−1 at 800 and 1000 mA g−1, respectively). The outstanding electrochemical performance and high capacity retention rate mainly are ascribed to the fast charges, lithium-ions diffusion, and stable structure for SnS2 nanoflakes. This work could provide a potential approach to promote the electrochemical performances of sulfides electrode materials for lithium-ions battery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the continuous development of economy and human society, the consumption rate of non-renewable resources is increasing, which makes the search and development of renewable clean energy become a huge challenge for all scientific researchers [1]. Renewable clean energy with obvious discontinuous and intermittent characteristics needs to be used in conjunction with energy storage systems to achieve continuous energy supply [2]. Therefore, it is particularly important to develop energy conversion and energy storage equipment [3,4,5]. As an energy storage and conversion device, lithium-ion batteries can effectively store discontinuous renewable clean energy [6]. Rechargeable lithium-ion batteries have recently attracted worldwide attention owing to their high energy density, long cycle life, and low self-discharge capability, which is a new type of secondary energy source with great potential [7, 8]. However, the electrochemical properties of lithium-ion battery have still been improved to meet more demands in practical applications. The anode materials are very crucial part of battery because they have a critical influence on the performances of lithium-ion battery [9, 10]. Nowadays, the further application of low theoretical capacity and poor rate performance of industrial graphite anode in lithium-ion batteries is limited [11, 12]. Therefore, researchers are constantly developing high-performance and cost-effective anode materials, which are capable of meeting the increasing demand for better cycle performance, higher energy, and power density in the next generation of lithium-ion battery [13, 14]. Recently, tin disulfide (SnS2) as a potential anode material of lithium-ion batteries has high theoretical capacity (645 mAh g−1), low cost, and good cycle life [11, 15,16,17]. SnS2 has a CdI2-type layered structure (a = b = 3.65 Å, space group P3m1), consisting of tin atoms layer sandwiched between two layers of hexagonally close filled sulfur atoms [18,19,20,21]. The large interlayer distance (0.59 nm) promotes the intercalation/deintercalation of (Li-ion) Li+ (0.076 nm) during cycling process [22,23,24]. However, the SnS2 shows poor cycling stability attributing to its larger volume expansion during charging/discharging, which may lead to serious pulverization and collapse of nanostructures [25, 26]. To conquer above problem effectively, researchers have prepared the various nanostructure and morphologies of SnS2 electrode by many methods [27, 28].
2D structure of SnS2 electrode can significantly shorten the diffusion pathway and accelerate the diffusion efficiency of lithium ions and electrons for lithium-ion battery during charging/discharging [29,30,31]. In addition, the nanoflakes structure is able to a certain extent buffering volume change and provides more active site during alloying reaction [29]. Therefore, SnS2 electrode can significantly improve the electrochemical performance of lithium-ion batteries. Nevertheless, the synthesis of high quality and excellent electrochemical performance two-dimensional (2D) SnS2 has remained a difficult challenge. In recent years, many scholars have struggled to synthesize nanoflakes structure of SnS2 electrode. Two-dimensional graphene–SnS2 composite was developed via two-step approach (the first-step thermal annealing and the second-step chemical vapor deposition) [31]. The composite displays a high reversible capacity (ca. 650 mAh g−1 at 50 mA g−1 after 30 cycles) and an excellent rate capability (630 mAh g−1 at 400 mA g−1). Seo et al. [32] prepared SnS2 nanoplates through thermal decomposition of a molecular precursor [Sn(S2CNEt2)4] in organic solvent at high temperature. The discharge capacity of the SnS2 nanoplates was determined to be 583 mAh g−1 after 30 cycles. Gao et al. [11] prepared RGO/SnS2 nanoplates by two-step methods (hydrothermal and sulfuration reaction), which exhibits high discharge capacity of 823 mAh g−1 after 800 cycles. The full battery delivers a high capacity of 642 mAh g−1 at 0.2 A g−1 and has high-output operating voltage of 3.4 V. However, there are few simple methods for preparing the excellent electrochemical performance of SnS2 nanoflakes electrodes.
Herein, we report an innovative means to construct SnS2 nanoflakes structure using citric acid as chelating agent by microwave hydrothermal method and also investigate the effect of citric acid addition on micromorphology and electrochemical properties for lithium-ion battery. The SnS2 product is represented as SN-0 without the addition of citric acid. When the amount of citric acid added is 4 mmol, 8 mmol, and 15 mmol, the obtained SnS2 electrodes are denoted as SN-1, SN-2, and SN-3, respectively. As a result, the obtained SnS2 samples display remarkably dissimilar electrochemical performances. The SN-3 electrode possesses excellent electrochemical activity, high discharge capacity (327.3 mAh g−1) with the Coulombic efficiency of over 99.8% when the current density is 100 mA g−1 after 100 cycles, and excellent high-rate capacity (361.5 mAh g−1 and 326.4 mAh g−1 at the rates of 800 mA g−1 and 1000 mA g−1, respectively). The significant electrochemical properties of SN-3 electrode are primary ascribed to its lower lithium-ion and electric charge diffusion resistance and stable structure provided by SnS2 nanoflakes.
2 Experimental section
2.1 Synthesis of SnS2 samples
The products of SnS2 nanoflakes were synthesized by cushy microwave hydrothermal method. All the chemicals were analytical grade and used directly without further purification. First, 2 mmol thioacetamide (TAA) was dissolved into 50 mL deionized water with strong stirring for 10 min. Then citric acid monohydrate (the amount of citric acid monohydrate added was 4 mmol, 8 mmol, and 15 mmol, which are, respectively, marked as SN-1, SN-2, and SN-3, respectively) was added to the above solution with magnetic stirring until totally dissolved and the formation transparent solution, followed by addition of 1 mmol SnCl4·5H2O into the above mixture under stirring for 10 min and ultrasonic dispersion for 30 min. Whereafter, the obtained brown suspension was sealed in a special container for 2 h under microwave radiation at 180 °C. SnS2 precipitation was naturally cooled to room temperature, centrifuged and washed with deionized water and absolute ethanol for several times, and then dried in vacuum at 80 °C for 12 h.
As a contrast experiment, the SN-0 sample was prepared under the same conditions, and no citric acid was added.
2.2 Material characterizations
Powder X-ray diffraction (XRD, Rigaku, D/max-2200PC, Cu Kα1 radiation, λ = 1.5406 Å, at 40 kV and 40 mA) was used to characterize the phase composition. The morphologies and microstructures were analyzed by scanning electron microscopy (SEM, Hitachi, S-4800) and transmission electron microscopy (TEM, FEI Tecnai, G2 F20 S-TWIN). The surface compositions were evaluated by X-ray photoelectron spectroscopy (XPS, PHI-5400) spectrum, using C 1s (binding energy 284.6 eV) as a reference.
2.3 Battery assembly and electrochemical measurement
The electrochemical performances of the SnS2 nanoflakes electrodes were evaluated by using CR2032 coin-type batteries. A homogeneous slurry was formed by mixing 10 wt% acetylene carbon black, 10 wt% binder (PAA:CMC = 1:1), and 80 wt% synthetic SnS2 in deionized water of 1–1.5 mL. Whereafter, the copper foil was coated with flowing slurry with a scraper and dried at 70 °C for 24 h. The coin batteries were constructed in an argon-filled glove-box using pure lithium foil as cathode. 1 M LiPF6 was used as electrolyte, and porous polypropylene film was used as separator.
A computer-controlled LAND-CT2001A Battery Testing System was used to measure the galvanostatic charge–discharge of assembled batteries at different current densities in the range of 0.01–3.0 V voltage versus Li+/Li. Electrochemical workstation (CHI660E, Shanghai, Chenhua) at the frequency range of 100 kHz to 0.01 Hz and the voltage range of 0.01–3.0 V was used to characterize cyclic voltammogram (CV) and electrochemical impedance spectroscopy (EIS).
3 Results and discussion
3.1 Formation mechanism analysis
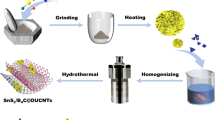
In order to clearly understand the mechanism of excellent electrochemical performance and the effect of morphology and microstructure by the addition of citric acid, the schematic illustration for formation mechanism of the SnS2 nanoflakes is shown in Fig. 1. In generally, the formation of SnS2 electrode materials is fabricated by the coordination of Sn4+ and S2−. Nevertheless, citric acid as a chelating agent for metal ions can form a strong ligand with Bi3+, Al3+, Fe3+, Sn4+, etc. [33]. Citric acid could be hydrolyzed into H+ and [C6O7H5]3− which can frequently chelate with Sn4+ to form [C6O7H5]3−Sn4+. The formation of hydrogen bond mainly attributed to the carboxyl of citric acid and the hydroxyl ions in [C6O7H5]3−Sn4+ [34]. [C6O7H5]3−Sn4+ hydrolyzed in H2O reacts with H+ to form Sn4+. TAA is hydrolyzed to form S2−. Sn4+ reacts with S2− to form SnS2 (chemical equation: Sn4+ + 2S2− = SnS2). The crystals growth of SnS2 nanoflakes is controlled by the interaction between [C6O7H5]3−Sn4+ and hydrogen bond, which will affect the nucleation, growth, and self-assembly of crystals in the synthesis process. In addition, the addition of citric acid can effectively restrain the agglomeration and caking of phase by weakening the interaction between metal ions. Therefore, the SnS2 nanoflakes electrode displays excellent electrochemical performance.
3.2 Structural and morphological characterization
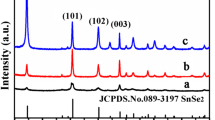
Figure 2 shows XRD patterns of the as-obtained SnS2 nanoflakes samples. Obviously, all the diffraction peaks can be indexed to 2T-type layered structure of SnS2 without any other obvious other impurity peaks (JCPDS Card No. 23-0677), which fully confirms that the four pure phase compositions of SnS2 nanoflakes samples can be fabricated successfully by microwave-assisted hydrothermal method using citric acid as chelating agent. Besides, the diffraction peaks of the four SnS2 nanoflakes samples confirm that the addition of citric acid has no influence on phase composition of SnS2 electrodes.
The average crystallite dimensions of the four SnS2 nanoflakes samples are, respectively, estimated using the (001) diffraction peaks on the basis of the Debye–Scherrer formula (Eq. 1):
Here, k is Scherrer constant, λ is the X-ray wavelength of Cu Kα1 radiation, β is the full width at half-height of the diffraction peak, and θ is the diffraction angle. The average crystallite dimensions of the as-obtained SnS2 nanoflakes are, respectively, calculated as 22.854 nm, 18.422 nm, 19.490 nm, and 30.455 nm.
The morphology and microstructure of the as-obtained SnS2 product are further investigated with scanning electron microscopy (SEM). As shown in Fig. 3a of the SEM image, the microstructure of the SN-0 product is composed of a mass of rounded nanoparticle and rich-shaped nanoparticle, and the size of the SN-0 product nanoparticle is about 50–100 nm. It is unfortunate that the nanoparticles are seriously agglomerated together. When citric acid is added as chelating agent in the synthetic process of SnS2 electrode material, the microstructure of SnS2 samples is significantly changed and the dispersion of nanoflakes is improved. As shown in Fig. 3b, it is obvious that the microstructure of SN-1 sample is stacked up with abundant hexagonal nanoflakes each other, its size is about 40–100 nm. The thickness of SN-1 sample hexagonal nanoflakes is about ~ 15 nm. The amount of citric acid continues to increase to 8 mmol. The flower-like structure of SN-2 sample is assembled from nanoflakes with size of 150–250 nm, and the thickness is about ~ 20 nm (Fig. 3c, d). Figure 3e–f exhibits the SEM image of the SN-3 sample. The microstructure is chaotically composed of nanoflakes with a typical size and thickness of around 100–200 nm and ~ 30 nm, respectively. According to the above analysis, the size of the SN-3 sample nanoflakes is evenly distributed. These vertically stacked nanoflakes are composed of more layers for SnS2 electrodes. Therefore, the uniform SnS2 nanoflakes will provide more active sites and accelerate lithium-ion and electric charge migration during alloying/dealloying between tin and lithium ions.
The effect of citric acid addition on the microstructure and growth behavior of the SnS2 products is subsequently compared by transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM). Figure 4a, b indicates the microstructure of the SN-0 product is inhomogeneous nanoparticles, and the lattice fringes spacing of SN-0 sample nanoparticle is 0.32 nm, which corresponds to (100) crystal plane of the SnS2. As shown in Fig. 4c, the morphology of SN-1 sample significantly grows into nanoflakes compared to the SN-0 sample. HRTEM (Fig. 4d) reveals that the interfacial distances of lattice fringes are 0.32 nm and 0.28 nm, respectively. These distance are consistent with the (100) and (101) crystal planes of hexagonal SnS2. It is evident that the flower-like structure of SN-2 sample is consistent with the analysis result of SEM. HRTEM (Fig. 4f) image of the SN-2 sample distinctly indicates the well-resolved lattice spacing of 0.19 nm and 0.32 nm, which corresponds to (110) and (100) crystal plane of layered SnS2, respectively. The TEM image reveals that the microstructure of SN-3 sample is combined by nanoflakes. The HRTEM image shows two different of lattice fringes (Fig. 4h). The lattice spacing of 0.28 nm corresponds to (101) crystal plane of SnS2. Moreover, the interplanar distance of 0.32 nm matches that of the (100) crystal plane of layered SnS2.
The influence of chemical composition on the addition of citric acid is also studied. X-ray photoelectron spectroscopy (XPS) analysis is used to expose the oxidation states of Sn in the SnS2 samples. The XPS survey spectra of the SN-3 products demonstrate that the presence of C, O, S, and Sn elements (Fig. S1a). This result explains that other hybrid phase could not be detected in the as-obtained SN-3 product. The valent state of element Sn is further indicated with a high-resolution XPS spectrum (Fig. S1b), which reveals two strong peaks of SN-3 product located at 486.7 eV and 495.15 eV that are assigned, respectively, to Sn 3d5/2 and Sn 3d3/2 of Sn4+ with a doublet spacing of ~ 8.45 eV [35, 36]. No evidence of Sn2+ is detected in the spectra. The result adequately proves that the compound of Sn2+ is not synthesized when citric acid as chelating agent is added in the synthesis of SnS2 process, which forcefully illustrates Sn4+ of SnS2 is not reduced to Sn2+ in the synthesis process. On the other hand, this result also attests phase composition of SN-3 product has not changed when the certain amount of citric acid is added in the synthesis process, which is consistent with the results of XRD analysis. The high-resolution XPS spectrum of S 2p shows two peaks in Fig. S1c. There are two different strong S 2p3/2 and S 2p1/2 peaks at 161.68 eV and 162.79 eV, respectively, which is characterized by the presence of element S in the chemical state of S2− [37, 38]. On the basis of the integral area of Sn 3d to S 2p, the atomic ratio of Sn to S is approximately 1:2. The coexistence of Sn and S in SN-3 product is confirmed and the phase formation of SnS2 product is not changed with the addition of citric acid, which is consistent with the result of XRD.
3.3 Electrochemical characterization
To evaluate the lithium storage ability, electrochemical measurements of the assembled cells are performed at room temperature. Cyclic voltammetry (CV) cures of the SnS2 anode materials are shown in Fig. 5. Figure 5a clearly shows the CV curves of SN-0 anode during the first cathodic scan. The weak cathodic peak at ~ 1.78 V appearing in the first cathodic scan is found to vanish subsequently, which is attributed to that there is no phase decomposition lithium intercalation in the SnS2 layer (Eq. 1) [39, 40]. The strong peaks at ~ 1.55 V and ~ 1.04 V are caused by the decomposition of SnS2 and the formation of Li2S and Sn (Eqs. 2 and 4) [11, 39], which is generally considered to form the solid electrolyte interphase (SEI) film and the decomposition of the electrolyte, which leads to reversible capacity loss and the low initial Coulombic efficiency (Eq. 3) [28, 40]. The peak at below ~ 0.3 V represents the reversible alloying process between metallic Sn and lithium ions in the first and subsequent cathodic scan (Eq. 5) [11, 22, 30]. The oxidation peak at ~ 0.52 V corresponds to the delithiation reaction of LixSn at the first anode scan to form metallic Sn (Eq. 5) [28, 41]. Another oxidation peak at ~ 1.75 V possibly is related to the oxygenation of metallic Sn to SnS2 (Eq. 4) [30, 42]. In the subsequent cycles, due to the contribution of discharge capacity in the conversion reactions, the peak at ~ 1.04 V in the first cathodic scan moves to ~ 1.34 V at the second cycle. Since a stable SEI film is formed during the first charge/discharge process, the SEI film in the later cycle will also be relatively stable [43]. The CV cures of other three (SN-1, SN-2, and SN-3) anode materials are similar to the CV cures analysis of SN-0 anode material. It is different from that the weak reduction peak at ~ 1.78 V shifts to ~ 1.99 V (Fig. 5b) or ~ 1.83 V (Fig. 5d), which is attributed to that there is no phase decomposition lithium intercalation in the SnS2 layer (Eq. 1). Clearly observation (Fig. 5b) that the peak at ~ 1.45 V and ~ 0.98 V in the first cathodic scan represents conversion reaction, and the formation of Li2S and SEI film (Eqs. 2–4), which is generally believed to the irreversible process [28]. Moreover, the CV cures of the four anode materials at the second and third cycle coincide well with each other, indicating the well electrochemical reaction is accomplished during charge–discharge process. It is noticeable that the prominent peak at ~ 1.33 V of SN-3 anode in the first and later cathodic scan is acuter than other three anode materials, which declares the conversion reaction and the formation of metallic Sn is more complete (Fig. 5d). It effectively improves reversible electrochemical reaction and alloying/dealloying of metallic Sn and lithium result in electrochemical performance is improved. However, the redox peaks of SN-0 anode, SN-1 anode, and SN-2 anode become broad and shift in the following cycles, which reveals that the nanostructure active materials collapse and polarization during lithiation/delithiation process. The CV cures analysis of the four electrode materials corresponding to charge and discharge profiles are shown in Fig. 6.
Figure 6 compares the charge–discharge voltage profiles for 1st, 2nd, 5th, 50th, and 100th of the four electrode materials in the cutoff voltage range of 0.01–3.0 V vs. Li/Li+ at a current density of 100 mA g−1. The initial discharge capacities of four electrodes are 1313.5 mAh g−1, 1633.7 mAh g−1, 1596.5 mAh g−1, and 1485.8 mAh g−1, respectively. The initial charge capacities of four electrodes are 729 mAh g−1, 974.2 mAh g−1, 829.6 mAh g−1, and 946.4 mAh g−1. The initial Coulombic efficiency (CE) is 55.5%, 59.6%, 52.0%, and 63.7%, respectively. As shown in Fig. 6a, the discharge and charge voltage plateau of SN-0 electrode in the first cycle located at 1.70–1.90 V, 1.00–1.25 V, 0.0–0.50 V, and 0.50–1.00 V are consistent with the result of CV. The overlapping voltage profiles demonstrate well electrochemical reaction during alloying/dealloying process. Similarly, the discharge and charge voltage plateaus of SN-1, SN-2, and SN-3 electrode in the cycles are consistent with the result of CV cures (Fig. 6b–d). According to the above analysis, the excellent charge and discharge capacity and high CE of SN-3 electrode are attributed to the well and intact conversion reaction of SnS2 electrode and alloying reaction with metallic Sn and lithium, which reveals the layer of nanoflakes can accommodate more lithium-ions and electric charge migration.
For comparison the electrochemical performance of the four electrode materials, the tests are performed under parallel test conditions as shown in Fig. 7. As demonstrated in Fig. 7a, the rate performance of the four electrodes is studied by charging/discharging cycles at current density range of 50–1000 mA g−1. The discharge capacities of SN-0, SN-1, SN-2, and SN-3 electrodes are 562.5 mAh g−1, 484.9 mAh g−1, 604.1 mAh g−1, and 598.7 mAh g−1 after 10 cycles at a rate of 100 mA g−1, respectively. Nevertheless, the charging–discharging current density is continuously increased to 200, 500, 800, and 1000 mA g−1. It is evident that the charge and discharge capacity of SN-3 electrodes is superior to other three electrodes. The SN-3 electrode delivers the discharge capacity of 523.7 mAh g−1, 449.7 mAh g−1, 387.5 mAh g−1, and 339.7 mAh g−1 at the current densities of 200, 500, 800, and 1000 mA g−1, respectively. When the current density suddenly recovers to 50 mA g−1, the discharge capacity of the SN-3 electrode recovers to 529.8 mAh g−1, which is nearly equal to the original discharge capacity. Unfortunately, the discharge capacities of the SN-0, SN-1, and SN-2 electrodes are only 354.1 mAh g−1, 44.7 mAh g−1, and 324.3 mAh g−1 at the current density of 800 mA g−1, and 285.7 mAh g−1, 17.5 mAh g−1, and 234.2 mAh g−1 at the current density of 1000 mA g−1, respectively. The discharge capacity retention rates of the four electrodes are, respectively, 47.2%, 3.08%, 34.7%, and 56.7% at the current density from 100 to 1000 mA g−1. The result proves the outstanding nanostructure stability of the SN-3 electrode compared to other three electrodes during lithiation/delithiation process. To demonstrate the excellent cyclic performance and stability of electrode materials, the cycling performances of the four electrodes after 100 cycles are examined at a current density of 100 mA g−1 (Fig. 7b). The discharge capacities of the SN-0, SN-1, SN-2, and SN-3 electrodes are 150.5 mAh g−1, 156.5 mAh g−1, 115.9 mAh g−1, and 327.3 mAh g−1. Meanwhile, the CE of SN-3 electrodes rapid grows to ~ 99.8% after only several cycles at a current density of 100 mA g−1. It is clearly observed that the reversible capacities of the SN-0, SN-1, and SN-2 electrodes decay rapidly, which could be attributed to the pulverization and aggregation of nanostructure that hinder the transport of lithium ions and electrons during charging/discharging process. Compared with other three electrode materials, the SN-3 electrode not only has higher reversible capacity, but also has better cycling stability. Figure 7c shows capacity retention of the four electrodes calculated based on the 5th discharge capacity value at 100 mA g−1. Obviously, the capacity retention of the SN-3 electrode displays absolute advantage and stabilizes at 41.97% after 100 cycles, whereas the capacity retention rates of the SN-0, SN-1, and SN-2 electrodes reveal apparently attenuation and only retain 25.8%, 19.9%, and 14.6% after 100 cycles, which indicates the strong structure stability of SN-3 anode provides more lithium-ions and electric charge migration channel during alloying/dealloying. In addition, the steady nanoflakes of SN-3 anode also afford affluent active sites for the insertion/deinsertion process.
a Rate performance of the SN-0, SN-1, SN-2, and SN-3 electrodes under various current densities (from 50 to 100, 200, 500, 800, 1000, and 50 mA g−1), b cyclic performance of the SN-0, SN-1, SN-2, and SN-3 electrodes at a current density of 100 mA g−1 for 100 cycles, and Coulombic efficiency (CE) of SN-3 electrodes, c capacity retention of the SN-0, SN-1, SN-2, and SN-3 electrodes as anode for LIBs at 100 mA g−1 in the voltage window of 0.01–3.0 V, Nyquist plots of the SN-0, SN-1, SN-2, and SN-3 electrodes after 3rd (d) and 100th (e) cycle at a current density of 100 mA g−1 in the frequency range of 100 kHz to 0.01 Hz, and f equivalent circuit model for electrode
To further determine the electrochemical property of the four electrodes, the EIS measurement, and equivalent circuit model are implemented after 3rd (Fig. 7d) and 100th (Fig. 7e) cycles at 100 mA g−1 in 100 kHz to 0.01 Hz. The Nyquist plots of the four electrodes consist of a semicircle at high frequency and an oblique straight line at low frequency. The components of the equivalent circuit model are shown in Fig. 7f, where Rs, Rf, CPE, Rct, and Zw represent the contact resistance, SEI film, the double-layer capacitance, charge-transfer, and lithium-ions diffusion for the SnS2 electrode, respectively. The impendences for four electrodes after 3rd and 100th cycles are calculated in Table 1. It is easy to find that the impedance of the charge-transfer and Warburg impedance of SN-3 anode are dramatically less than other three anode materials, indicating the layer nanoflakes can provide ample migration paths of electronic and lithium ions during alloying reaction process, further confirming the addition of citric acid has an evident influence of microstructure resulted in different electrochemical properties.
Meanwhile, the diffusion coefficient of lithium-ions (DLi+) can be estimated by the linear relationship between the impedance (Z′) and ω−1/2 of the four samples (SN-0, SN-1, SN-2, and SN-3 electrodes) after 100 cycles in Fig. 8. The Warburg coefficients (σ) can be calculated from the slope by Eq. 2 (Z′ = Rct + Rs + σω−1/2). The diffusion coefficient of lithium-ions (DLi+) can be obtained from Eq. 3 (DLi+ = R2T2/2A2n4F4C4σ2), where R is the gas constant, T is the absolute temperature, A is the effective surface area of the electrode, n is the number of the electron transfer per mole, F is the Faraday constant, and C is the concentration of lithium-ion in electrode. According to the formula above, it is evident that the Li+ diffusion coefficient (3.873 × 10−10 cm2 s−1) of SN-3 electrode is higher than that of other three electrodes (SN-0: 1.197 × 10−12 cm2 s−1, SN-1: 2.269 × 10−11 cm2 s−1, SN-2: 1.467 × 10−11 cm2 s−1). The result demonstrates the excellent electrochemical performance of SN-3 electrode is related to quickly migration rate of electronic and lithium ions.
To explore the relationship between electrochemical performance and nanostructure during charging/discharging process, the morphological and microstructural changes of the four electrodes after charge/discharge cycling are revealed by TEM (Fig. 9). It is found that the crystalline structures of the four electrodes distinct are changed, which is mainly attributed to the above-mentioned volume expansion causing the collapse and pulverization of structure during insertion/deinsertion process (the mechanism diagram is shown in Fig. S2). However, the part of the SN-3 electrode nanoflakes is destroyed but not form serious aggregation than other three electrodes during charging/discharging process, which can provide more migration paths for electric charge and lithium-ions. The result demonstrates that the SN-3 anode has much better structural stability than other three electrodes, which is consistent with EIS analysis results shown in Fig. 7. The above analysis proves that the nanoflakes of SnS2 electrodes by adding citric acid as chelating agent can effectively improve electrochemical performance for LIBs.
4 Conclusions
In summary, we have successfully designed and fabricated a novel SnS2 nanoflakes using citric acid as chelating agent by microwave hydrothermal method. When the amount of citric acid added is 15 mmol in the synthesis process, which acquires the SnS2 nanoflakes with the size and the thickness of around 100–200 nm and 20–30 nm, respectively. The initial charge and discharge of SnS2 nanoflakes are 946.4 mAh g−1 and 1485.8 mAh g−1. The initial Coulombic efficiency is about 63.7%. After 100 cycles, when the current density is 100 mA g−1, the discharge capacity maintains at 327.3 mAh g−1 and the Coulombic efficiency is around 99.8%. When the current density increases to 500 mA g−1, 800 mA g−1 and 1000 mA g−1, the SnS2 nanoflakes delivers the discharge capacity of 449.7 mAh g−1, 387.5 mAh g−1, and 339.7 mAh g−1, respectively. The impedance parameters of the charge-transfer and lithium-ions diffusion are less than other three electrodes after 100 cycles. The excellent electrochemical performance and high capacity retention rate are mainly ascribed to the fast charges and lithium-ions diffusion and more active sites of SnS2 nanoflakes.
References
M. Cao, X. Wang, W. Cao, X. Fang, B. Wen, J. Yuan, Small 14(29), 1800987 (2018)
Y. Cai, W. Cao, Y. Zhang, P. He, J. Shu, M. Cao, J. Alloys Compd. 811, 152011 (2019)
H. Wang, C. Shen, J. Liu, W. Zhang, S. Yao, J. Alloys Compd. 792, 122–129 (2019)
P. He, M. Cao, Y. Cai, J. Shu, W. Cao, J. Yuan, Carbon 157, 80–89 (2020)
C. Hou, J. Wang, W. Du, J. Wang, Y. Du, C. Liu, J. Zhang, H. Hou, F. Dang, L. Zhao, Z. Guo, J. Mater. Chem. 7, 13460–13472 (2019)
W. Cao, W. Wang, H. Shi, J. Wang, M. Cao, Y. Liang, Nano Res. 11(3), 1437–1446 (2018)
F. Tu, X. Xu, P. Wang, L. Si, X. Zhou, J. Bao, J. Phys. Chem. C 121(6), 3261–3269 (2017)
B. Zhao, F. Chen, Z. Wang, S. Huang, Y. Jiang, Z. Chen, Nanoscale 9(45), 17922–17932 (2017)
K. Chang, Z. Wang, G. Huang, H. Li, W. Chen, J.Y. Lee, J. Power Sources 201, 259–266 (2012)
X. Chen, Y. Huang, K. Zhang, X. Feng, M. Wang, Chem. Eng. J. 330, 470–479 (2017)
C. Gao, L. Li, A.R. Raji, A. Kovalchuk, Z. Peng, H. Fei, Y. He, N.D. Kim, Q. Zhong, E. Xie, J.M. Tour, ACS Appl. Mater. Interfaces 7(48), 26549–26556 (2015)
D.H. Youn, S.K. Stauffer, P. Xiao, H. Park, Y. Nam, A. Dolocan, G. Henkelman, A. Heller, C.B. Mullins, ACS Nano 10(12), 10778–10788 (2016)
J. Shan, Y. Liu, P. Liu, Y. Huang, Y. Su, D. Wu, X. Feng, J. Mater. Chem. A 3(47), 24148–24154 (2015)
Y. Li, M.A. Trujillo, E. Fu, B. Patterson, L. Fei, Y. Xu, S. Deng, S. Smirnov, H. Luo, J. Mater. Chem. 1(39), 12123–12127 (2013)
W. Fang, N. Zhang, L. Fan, K. Sun, RSC Adv. 6(102), 99852–99832 (2016)
P. Zhou, X. Wang, W. Guan, D. Zhang, L. Fang, Y. Jiang, ACS Appl. Mater. Interfaces 9(8), 6979–6987 (2017)
L. Yin, S. Chai, J. Huang, X. Kong, L. Pan, Electrochim. Acta 238, 168–177 (2017)
M. Zhao, Q. Zhao, J. Qiu, H. Xue, H. Pang, RSC Adv. 6(98), 95449–95468 (2016)
B. Qu, C. Ma, G. Ji, C. Xu, J. Xu, Y.S. Meng, T. Wang, J.Y. Lee, Adv. Mater. 26(23), 3854–3859 (2014)
P. Chen, Y. Su, H. Liu, Y. Wang, ACS Appl. Mater. Interfaces 5(22), 12073–12082 (2013)
J. Johny, S. Sepulveda-Guzman, B. Krishnan, D. Avellaneda, S. Shaji, Appl. Surf. Sci. 435, 1285–1295 (2018)
J. Wang, C. Luo, J. Mao, Y. Zhu, X. Fan, T. Gao, A.C. Mignerey, C. Wang, ACS Appl. Mater. Interfaces 7(21), 11476–11481 (2015)
P. Zheng, Z. Dai, Y. Zhang, K.N. Dinh, Y. Zheng, H. Fan, J. Yang, R. Dangol, B. Li, Y. Zong, Q. Yan, X. Liu, Nanoscale 9(39), 14820–14825 (2017)
R. Thangavel, A.S. Pandian, H.V. Ramasamy, Y.S. Lee, ACS Appl. Mater. Interfaces 9(46), 40187–40196 (2017)
Y. Liu, Y. Yang, X. Wang, Y. Dong, Y. Tang, Z. Yu, Z. Zhao, J. Qiu, ACS Appl. Mater. Interfaces 9(18), 15484–15491 (2017)
Y. Jiang, M. Wei, J. Feng, Y. Ma, S. Xiong, Energy Environ. Sci. 9(4), 1430–1438 (2016)
L. Yin, S. Chai, J. Huang, X. Kong, J. Wang, Y. Liu, J. Alloys Compd. 727, 1006–1013 (2017)
D. Kong, H. He, Q. Song, B. Wang, Q. Yang, L. Zhi, RSC Adv. 4(45), 23372–23376 (2014)
W. Sun, X. Rui, D. Yang, Z. Sun, B. Li, W. Zhang, Y. Zong, S. Madhavi, S. Dou, Q. Yan, ACS Nano 9, 11371–11381 (2015)
G. Wang, J. Peng, L. Zhang, J. Zhang, B. Dai, M. Zhu, L. Xia, F. Yu, J. Mater. Chem. A 3(7), 3659–3666 (2015)
B. Luo, Y. Fang, B. Wang, J. Zhou, H. Song, L. Zhi, Energy Environ. Sci. 5(1), 5226–5230 (2012)
J. Seo, J. Jang, S. Park, C. Kim, B. Park, J. Cheon, Adv. Mater. 20(22), 4269–4273 (2008)
H. Meng, T. Wang, X. Yu, Y. Zhu, Y. Zhang, RSC Adv. 5(129), 107088–107097 (2015)
K. Zhao, L. Zhang, J. Wang, Q. Li, W. He, J.J. Yin, J. Am. Chem. Soc. 135(42), 15750–15753 (2013)
M.S.A. Sher Shah, A.R. Park, A. Rauf, S.H. Hong, Y. Choi, J. Park, J. Kim, W.J. Kim, P.J. Yoo, RSC Adv. 7(6), 3125–3135 (2017)
H. Liu, Y. Su, P. Chen, Y. Wang, J. Mol. Catal. A 378, 285–292 (2013)
L. Ma, L. Xu, X. Zhou, X. Xu, L. Zhang, RSC Adv. 5(128), 105862–105868 (2015)
T. Wang, H. Meng, X. Yu, Y. Liu, H. Chen, Y. Zhu, J. Tang, Y. Tong, Y. Zhang, RSC Adv. 5(20), 15469–15478 (2015)
F. Lu, Q. Chen, Y. Wang, Y. Wu, P. Wei, X. Kuang, RSC Adv. 6(29), 24366–24372 (2016)
Y. Jiang, Y. Feng, B. Xi, S. Kai, K. Mi, J. Feng, J. Zhang, S. Xiong, J. Mater. Chem. A 4(27), 10719–10726 (2016)
B. Luo, Y. Hu, X. Zhu, T. Qiu, L. Zhi, M. Xiao, H. Zhang, M. Zou, A. Cao, L. Wang, J. Mater. Chem. A 6(4), 1462–1472 (2018)
S. Liu, X. Lu, J. Xie, G. Cao, T. Zhu, X. Zhao, ACS Appl. Mater. Interfaces 5(5), 1588–1595 (2013)
L. Yin, R. Cheng, Q. Song, J. Yang, X. Kong, J. Huang, Y. Lin, H. Ouyang, Electrochim. Acta 293, 408–418, (2019)
Acknowledgements
This research work was supported by National Natural Science Foundation of China (Nos. 51772178, 21701107), the Serve Local Special Projects of Shaanxi Provincial Department of Education (No. 17JF004), Xi’an Key Laboratory of Green Manufacture of Ceramic Materials Foundation (No. 2019220214SYS017CG039), and the China Postdoctoral Science Foundation (No. 2016M592737).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, L., Li, H., Cheng, R. et al. Controllable synthesis of SnS2 nanoflakes as high-performance anode for lithium-ion batteries. J Mater Sci: Mater Electron 32, 191–203 (2021). https://doi.org/10.1007/s10854-020-04753-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04753-1