Abstract
The world’s energy supply depends heavily on lithium-ion batteries due to the progressive depletion of non-renewable resources. The issue of raising the energy density of lithium-ion batteries must be addressed. We are all aware that the anode material is one area where lithium-ion batteries still have room for development. A new anode material, tin disulfide, not only has a high theoretical specific capacity (645 mAh g−1), but also allows the formation of different microstructures through variable growth rates. In this study, we created three-dimensional nano-spheres of SnS2 using solid-phase synthesis and then wrapped SnS2 and B4C in OUCNTs (SnS2/B4C@OUCNT) using hydrothermal synthesis. Ascribed to the synergy between the highly chemical active B4C and the conductive carbon network of the OUCNTs, SnS2/B4C@OUCNT (149 Ω) effectively overcomes the drawback of high impedance of pure SnS2 (307 Ω) while exhibiting high capacity and cyclic stability. After 100 cycles at a current density of 100 mA g−1, this material displayed good electrochemical properties as the anode for lithium-ion batteries, obtaining a reversible capacity of 1024.7 mAh g−1 and a coulombic efficiency of 98.01%. The discharge capacity is 854.7 mAh g−1 with a coulombic efficiency of 98.57% after 200 cycles at 1000 mA g−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the stark incongruity between the rising demand for fossil fuels and the ever-harsher greenhouse gas emissions standards, it is crucial for humans to create and employ high-energy–density rechargeable batteries today [1,2,3,4,5]. While traditional and commercial lithium-ion batteries (LIBs) have cheap cost, extended cycle stability, and vast storage using graphite as the electrode materials, their limited property (372 mAh g−1) and subpar rate capacity narrow their potential to advance [6]. Therefore, there has been growing interest in exploring ideal electrode materials with superior electrochemical performance. The unique physicochemical properties of the transition metal disulfides have sparked an increase in research activity in recent years due to a variety of possible uses, including energy storage, catalysts, and electronics. Additionally, due to the Li-ion conversion or alloying storage mechanism reaction, such as SnS2, WS2, MoS2, and VS2 [7], their theoretically specific capacity is larger than that of conventional plug-in electrode materials [8,9,10,11]. Among these diverse active materials, SnS2 has a layered CdI2-type model, similar to graphene, and is held together by weak van der Waals interactions. Sn atom layers are situated between two levels of S atom layers. [12]. SnS2 has a greater specific capacity than other metal disulfides like MoS2 and WS2, which may be attributed to the special processes of the conversion and Li-Sn alloying/dealloying [13]. During course of the reaction, SnS2 demonstrates a high theoretically specific capacity (645 mAh g−1) [14], and has been investigated as a potential contender for LIBs electrode material.
Despite the fact that diverse SnS2 nanostructures have been utilized as active electrodes, the limited electronic conductivity of SnS2 prevents their application in electrochemistry, particularly at high rates [15]. During charge/discharge cycles, the enormous volume change of SnS2 would cause rapid capacity loss [16]. In order to get over these restrictions, it has been claimed that SnS2 has been mixed with a variety of carbon materials, including carbon coatings, carbon nanotubes, and graphene [17,18,19,20,21]. Youn et al. synthesized the matrix composite of nitrogen-doped graphene oxide reduction and nanocrystals tin sulfides as lithium-ion battery anodes which showed good performance with expansion ratio reduced by 28%, retaining a reversible capacity of 562 mAh g−1 at the 200th loop at 0.2 A g−1 rate [22]. Yin et al. established the “double-sandwich-like” framework of SnS2-reduced graphene oxide hybrid composite, which demonstrated superlative electrochemical performance, including a high initial or reversible discharge capacity and outstanding cyclic stability with superb coulombic efficiency of ∼96.9% [23]. Kim et al. prepared the carbon-coated SnS2 nanoparticles with a thickness of carbon coating about 5 nm which reduce volume change during the battery running process, so following 50 rounds, the reversal capacity was 668 mAh g−1 with a superior rate property [24]. As a result, carbon materials have the ability to lessen volume expansion caused by special layer structure in addition to improving electron transport. Among them, multi-walled carbon nanotubes are one of the important carbon-based materials whose mechanical and electronic properties depend on the structure, and researchers have been working to selectively improve its performance in certain aspects by blending its structure to meet specific needs. For example, the oxidative unzipping of multi-walled carbon nanotubes using a mixture of H2SO4 and KMnO4 allows multi-walled carbon nanotubes to unfold along the axial direction, obtaining a larger specific surface area and more active sites while bonding a large number of oxygen functional groups at the edges and surfaces [25]. Therefore, oxidative unzipping multi-walled carbon nanotubes have good dispersion and conductivity and are suitable as carbon carriers for SnS2.
Even so, researchers are still keen to find more novel electrode materials to a greater electrochemical performance boost. Boron carbide (B4C) is a lightweight, reasonably priced refractory ceramic material [26]. As a result of its great resistance to general corrosion, it may provide an excellent active area for cells such lithium-oxygen, lithium-sulfur, and lithium-ion batteries [27,28,29]. Chen et al. created a Si/B4C combination with a graphite covering that has an entire structure to increase the transport properties and durability of the rechargeable batteries. This combination exhibits outstanding cyclability by including a theoretical capacity of 822 mAh g−1 with 94% capacity retention after 100 cycles at 0.3 C charge/discharge speed [30]. Due to its light weight (≈2.5 g cm−3), strong conductance, and superior catalytic action, B4C is a suitable choice for electrochemical devices [26], good conductivity, and superior catalytic effect. To the best of our knowledge, B4C is only very seldom used in rechargeable batteries, despite having thrived and grown. In addition, B4C has been used as a raw material for anodes in sodium ion batteries and has achieved excellent electrochemical performance [31]; thus, the integration of B4C into tin-based anode materials for lithium-ion batteries is a very worthy study subject.
In this study, we described a straightforward hydrothermal technique for the synthesis of 3D SnS2/B4C@OUCNT, in which many nanoflakes-assembly flower-shaped SnS2 nanomaterials and evenly distributed B4C were wrapped in oxidative unzipped multi-walled carbon nanotube films (OUCNTs), where highly conductive B4C is used as a filling agent added to flower-shaped SnS2 and also as a conductive connector between SnS2 and OUCNTs. The severe tension brought on by the lattice expansion caused the longitudinal dimension of CNTs to modestly open under the combined action of sulfuric acid and oxidant molecules [32]. The undamaged core CNTs were kept as the framework of electronic transmission at this time. In order to intercalate lithium cations, the target product develops a three-dimensional space that offers more active surface area than the control sample. As a result, the lithium-ion battery with an optimized SnS2 anode electrode produced a discharge capacity of 1840 mAh g−1 at 100 mA g−1 rate.
Experimental section
Chemical materials
The following chemicals were used in the synthesis of SnS2 and SnS2/B4C@OUCNTs: stannous chloride dihydrate (SnCl2·2H2O, 98%), thiourea (H2NCSNH2, AR), absolute ethanol (CH3CH2OH, 99.7%), multi-walled carbon nanotubes (C139835, inner diameter: 5–10 nm, outer diameter: 20–30 nm, length: 10–30 µm), boron carbide (B4C, 99%).
SnS2 preparation
In an agate mortar, 2.25 g SnCl2·2H2O and 1.9 g thiourea were placed, where they were uniformly ground into off-white powdery mixture for 30 min. Then, the mixture was moved into 50-mL corundum crucible without cover reacting at 180 °C in the air blast drying oven for 2 h. After cooling, the composition was soaked in distilled water standing for 10 h. The solids were then separated and extracted using centrifugation, which was carried out repeatedly with absolute ethanol and deionized water. By drying at 80 °C in air, the yellow product SnS2 was finally gained.
Preparation of SnS2/B4C@OUCNTs
The oxidative unzipped MWCNTs were synthesized as previously reported [33]. For 3 h, 0.125 g OUCNTs were ultrasonically dispersed in 60 mL ethanol. For 24 h, 0.0625 g SnS2 and 0.0125 g B4C were added into OUCNTs dispersion stirring. The aforementioned dispersion was then transferred to a 100-mL Teflon reactor, heated to 180 °C, and allowed to react for 12 h. The black precipitate that was eventually formed was then cooled to room temperature and repeatedly cleaned with deionized water and absolute ethanol. The target product, SnS2/B4C@OUCNTs, was ultimately successfully abstained by drying it for 12 h at 60 °C in a low-pressure vacuum oven. The SnS2@OUCNTs, for comparison, was created via a hydrothermal technique under identical conditions without the inclusion of B4C.
Assembly of battery
The half-cell lithium-ion battery (LIBs) configuration was assembled so as to examine the electrochemical performance of SnS2/B4C@OUCNTs composite material. SnS2, SnS2@OUCNTs, and SnS2/B4C@OUCNTs are respectively mixed with conductivity (SUPER P) and polyvinylidene fluoride (PVDF) in a mass ratio of 7:1.5:1.5 to obtain three kinds of homogenous slurry with N-methylpyrrolidone (NMP) solvent. After drying in vacuum for 12 h, the dried slurry was cut into discs and adheres to the copper foil with a slurry of approximately 100 µm. The disc and lithium foil were used as the working electrode and the counter electrode, respectively. The Celgard 2200 was acted as the separator, and 1 M LiPF6 mixed with EC and DMC (1:1, v/v) (DodoChem, Suzhou, China) was used as the electrolyte. Assembly of the coin cell (CR2032) was carried out in a glove box with argon gas. Finally, discharge and charge measurements are performed by using LAND for testing.
Results and discussions
Physico-descriptive information
The SnS2/B4C@OUCNTs is prepared as shown in Fig. 1. The stannous chloride dihydrate compound was first ground with thiourea for 30 min into a homogeneous off-white powder, then transferred to a corundum crucible, and reacted by heating at 180 °C for 2 h to produce 3D SnS2 microspheres. A dispersed aqueous solution of OUCNTs was combined with SnS2 and B4C, and the mixture was constantly agitated for 24 h using a magnetic stirrer. A stainless steel hydrothermal synthesis reactor was then used to develop the homogenous solution into 3D materials, with OUCNTs wrapping SnS2 nanoballs and B4C, under high pressure and temperature conditions [34].
Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) were employed for characterization in order to more thoroughly examine the microscopic appearance and structure of the mixture. The SEM picture of pure SnS2 is shown in Fig. 2a, which depicts microspheres made of a number of tightly assembled two-dimensional SnS2 sheets. The gaps between the layers of the sheets facilitate the storage of electrons or lithium ions in the battery during charging and discharging [35]. But SnS2 has obvious shortcomings as an anode material for lithium-ion batteries, such as poor electrical conductivity, low energy storage density, and unstable long-term cycling. Figure 2b shows the SEM image of SnS2@OUCNTs, which shows that SnS2 grows wildly on OUCNTs, thereby improving the poor electrical conductivity of pure SnS2. The extended growth of SnS2 nanosheets on OUCNTs is shown in Fig. 2c, showing the microscopic morphology of SnS2/B4C@OUCNTs, which successfully mitigates the disadvantage of SnS2’s weak electrical conductivity [36]. At the same moment, the partially exposed OUCNTs indicate that SnS2 nanosheets are present on the carbon matrix in moderate amounts, allowing the OUCNTs to still retain the function of storing electrons of ions. In terms of the overall structure, it offers enough room for electrolyte penetration and shortens the Li-ion transport path, strengthening the Li-ion’s adsorption capability and electrochemical characteristics. Therefore, this structure has the potential to further improve the rate performance of the battery.
Figure 2d is a TEM image of the composite SnS2/B4C@OUCNTs, revealing the intertwined OUCNTs that form a conductive carbon network wrapping around SnS2 and B4C. Additionally, Figure S1 illustrates the outer layers of the multi-walled carbon nanotubes being axially oxidative cut and opened, thus creating additional anchoring sites for SnS2 and B4C and improving the integrity of the composite [32, 37]. Figure 2e further shows HRTEM image of SnS2/B4C@OUCNTs, clearly showing the lattice edges of SnS2 and B4C as well as the enlarged carbon layers. Notably, the interlayer spacing of 2.78 nm corresponds to the (101) crystal plane of SnS2, 3.78 nm to the (012) crystal plane of B4C, and 3.37 nm to the (002) crystal plane of the carbon layer. In addition, Fig. 2e shows that SnS2 and B4C are highly encapsulated by the oxidative unzipped multi-walled carbon nanotubes. The (012) face of B4C, the (002) face of the OUCNTs, and the (101) face of SnS2 are all visible in the matching selected area electron diffraction (SAED) pattern, which supports the polycrystalline nature of the trapped B4C and SnS2 with such a different orientation (Fig. 2f).
Figure 2g shows the elemental mapping images of the SnS2/B4C@OUCNTs, which shows that the composites are homogeneous. The homogenous distribution of the components Sn, B, C, and S is seen in Fig. 2g. SnS2 and B4C have apparently been effectively wrapped and disseminated in the network by the OUCNTs, as evidenced by the uniform distribution of Sn and B on the carbon network framework created by the OUCNTs. In addition, the uniform dispersion of B element in the composite (Fig. 2g) indicates that the three materials, SnS2, B4C, and OUCNTs, are well bonded and can synergistically bring out the advantages of each other. Energy-dispersive X-ray microanalysis (EDX) was used to further analyze the content of C, S, B, and Sn elements in SnS2/B4C@OUCNTs, and the results are shown in Fig. 2h. After quantitative analysis, the proportions of C, S, B, and Sn in SnS2/B4C@OUCNTs can be estimated to be ∼46.52%, ∼17.35%, ∼3.40%, and ∼32.73%, respectively, which are revealed the probable experimental ratio of final product (SnS2:B4C:OUCNTs = 10:1:9).
X-ray photoelectron spectroscopy (XPS) was carried out on the SnS2/B4C@OUCNTs to examine the chemical composition and bonding configuration of the composites. No additional impurities are seen in the entire XPS spectrum (Fig. 3a), which displays signals for Sn, O, C, B, and S. Two distinctive peaks can be seen in the high-resolution Sn 3d XPS spectrum (Fig. 3b), which correspond to Sn 3d5/2 and Sn 3d3/2 in Sn4+, respectively, at 487.5 and 495.9 eV [38,39,40]. The high-resolution XPS spectrum of elemental boron is depicted in Fig. 3c. It is separated into two distinctive peaks at 187.5 and 188.7 eV, which stand for the B-C bonds in B4C and the B-B bonds, respectively [41]. Three unique distinctive peaks with binding energies of 162.2, 163.8, and 165.0 eV, which correspond to S 2p3/2 and S 2p1/2 in S2−, can be seen in the high-resolution S 2p XPS spectrum (Fig. 3d) [42, 43]. The three typical peaks in the high-resolution C 1 s XPS spectrum (Fig. 3e) have binding energies of 284.8, 285.6, and 288.5 eV, respectively, and correspond to C–C bonds, C-O bonds, and O-C = O bonds [36, 44, 45]. It is thought that the oxygenated carbon produced during the hydrothermal treatment makes it easier for the SnS2 nanosheets to be anchored and dispersed through chemical and physical processes.
Pure SnS2, SnS2@OUCNTs (Figure S2), and SnS2/B4C@OUCNTs were subjected X-ray diffraction (XRD) examination to ascertain the structural properties of the composites. The SnS2/B4C@OUCNTs composite’s XRD analysis pattern is depicted in Fig. 4a, where it displays the distinctive XRD diffraction peaks of the composite of hexagonal SnS2 and rhombohedral hexahedral structured B4C. These include the XRD peaks at 2θ values of 28.20°, 32.12°, 41.89°, 49.96°, 52.45°, 60.62°, and 70.33° that are in accordance with the JCPDS data (JCPDS card number: 23–0677) and represent the (100), (101), (102), (110), (111), (201), and (113) crystal planes of hexagonal SnS2. In addition, the typical diffraction peaks at 2θ values of 24.50°, 35.96°, 57.55°, 64.66°, and 76.58° are consistent with the JCPDS data (JCPDS card number: 35–0798) and correspond to the (012), (104), (107), (125), and (217) crystal planes of B4C, respectively. As can be seen from Figure S2, the SnS2@OUCNTs (Figure S2b) with the addition of oxide-cut carbon nanotubes only retains mainly the diffraction peak positions of pure SnS2 (Figure S2a), but its diffraction peak intensity is significantly reduced. In contrast, the SnS2/B4C@OUCNTs composite preserves the majority of the diffraction peaks of pure SnS2 (Fig. S3a) while also adding some of the distinctive diffraction peaks of the dopant B4C and reducing the strength of the diffraction peaks seen in SnS2. However, this does not affect the fact that the characteristic diffraction peaks in Fig. 4a all have a sharp peak pattern, implying that the composite SnS2/B4C@OUCNTs have high crystalline properties. Furthermore, the figure demonstrates that SnS2 and B4C are together successfully composited and encapsulated in oxide-cut carbon nanotubes.
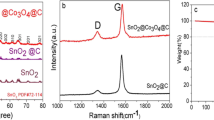
The composite SnS2/B4C@OUCNTs’ molecular structure is further illuminated by the Raman spectroscopy study. The Raman characteristic peaks of C-atom crystals at 1300 cm−1 and 1580 cm−1, respectively, are the D and G peaks. The G peak indicates in-plane stretching vibrations of the C-atom sp2 hybridization, and the D peak represents defects in the C-atom lattice [46]. The inclusion of oxidative unzipped carbon nanotube films effectively raises the degree of graphitization of the material, as shown by the comparison of the Raman spectrum of SnS2@OUCNTs with an ID/IG of 0.96. However, when SnS2/B4C@OUCNTs and SnS2@OUCNTs are compared, the ID/IG increases to 1.02, showing that the addition of boron carbide enhances the degree of flaws in the material’s faulty lattice and adds conductance and adsorption sites for lithium-ion store. Additionally, a pinnacle at 311 cm−1, which is thought to be the A1g pattern of the SnS2 phase, can be seen in the Raman spectrum of SnS2 [23, 47]. All Raman bands of SnS2, B4C, and OUCNTs can be seen in the Raman spectrum of SnS2/B4C@OUCNTs, confirming their inclusion in the composite.
SnS2, SnS2@OUCNTs, and SnS2/B4C@OUCNTs underwent Brunauer–Emmett–Teller (BET) and Barrett-Joyner-Halenda (BJH) studies to examine the specific surface area and pore structure of the materials. Figure 5a illustrates the adsorption/desorption isotherms of nitrogen for SnS2/B4C@OUCNTs, which matches the characteristics of the type IV adsorption/desorption isotherm. The material is a lamellar material with mesopores, as indicated by the visible hysteresis loop of type H3 in the relative pressure range of 0.4 to 1.0 [48]. The specific surface area of SnS2/B4C@OUCNTs, determined by the BET method, is 94.23 m2 g−1, which falls between the other two samples, SnS2 (40.96 m2 g−1) and SnS2@OUCNTs (74.18 m2 g−1), as shown in Figure S3. The BJH pore size distribution of SnS2/B4C@OUCNTs is presented in Fig. 5b, clearly indicating that the material’s pore sizes are concentrated in the mesoporous region. The increased contact area of the electrolyte with the materials enhances the migration channels for ions and shortens the routes, boosting the rate capability. This is facilitated by the moderate specific surface area and mesoporous structure of SnS2/B4C@OUCNTs. Additionally, reducing the formation of the SEI film may increase the initial coulombic efficiency of LIBs.
In addition, thermogravimetric (TGA) analysis was used in this work to determine the compositional content of SnS2/B4C@OUCNTs, which was heated in air from 30 to 800 °C at a ramp rate of 10 °C/min, as shown in Fig. 6. The 1.4% heat loss within ~ 200 °C is due to the evaporation of water and decomposition of oxygen-containing functional groups inside the material. Approximately 8.67% heat loss from 200 to 450 °C is mainly attributed to the oxidation of SnS2 in air to form SnO2, and ~ 45% heat loss occurring from 450 to 800 °C is mainly due to the oxidation of carbon material [33, 49]. After combining the EDX calculations, the contents of SnS2, B4C, and OUCNTs in SnS2/B4C@OUCNTs were obtained to be about 50%, 5%, and 45%, respectively.
D SnS2/B4C@OUCNTs electrochemical property
The electrochemical property of LIB half-cell made from SnS2/B4C@OUCNTs electrode components was evaluated. In this case, the anode consisted of SnS2/B4C@OUCNTs, while the counter electrode (cathode) was made of lithium chips. First, cyclic voltammetry (CV) was used to assess the half-cell between 0.01 and 3.0 V (versus Li+/Li) at 0.1 mV s−1 scan rate. Figure 7a displays five initial CV curves. Five distinct reduction peaks with centers at 1.65 V, 1.3 V, 1.0 V, 0.71 V, and 0.2 V are shown in the initial cathodic scan curves. The first peak at 1.65 V results from the entrance of lithium into the SnS2 interlayer without phase disruption, which corresponds to the conversion reaction (1), \({SnS}_{2}+{xLi}^{+}+{xe}^{-}\leftrightarrow {Li}_{\mathrm{x}}{SnS}_{2}\) [50]. \({Li}_{x}{SnS}_{2}+{\left(4-x\right)Li}^{+}+{4.4e}^{-}\leftrightarrow {Sn+2Li}_{2}S\) (2) [51] is the conversion reaction mechanism that is responsible for the additional three reduction peaks at 1.3 V, 1.0 V, and 0.71 V. Due to the deterioration of the electrolyte, SnS2 changes into Sn nanoparticles that are incorporated into the Li2S substrate and create a solid electrolyte interface (SEI) [35]. The creation of the LixSn alloy and the introduction of Li+ into the oxidatively unzipped multi-walled carbon nanotubes were credited causing the peak of about 0.2 V. Three anodic peaks at 0.55 V, 1.89 V, and 2.37 V can be seen in the initial anodic scanning. The delithiation process of the LixSn is visible in the oxidation peak at 0.55 V, accompanying by the reversible process of conversion reaction (2). And the reversible process of conversion reaction (1) causes the peak at 1.89 V, corresponding to the synthesis of SnS2 and the partial Sn oxidation [52]. Lithium ions being removed from the SnS2 sheet are likely what caused the oxidative peak around 2.37 V [53]. With the passage of scanning time, the other four laps gradually coincide which suggests that the lithium-ion half-cell with 3D SnS2/B4C@OUCNTs composite as anode electrode possesses stable electrochemical property.
Figure 7b depicts the charge/discharge graphs of SnS2/B4C@OUCNTs during the initial three cycles at a steady current density of 100 mA g−1. The first three discharge processes have specific capacities of 1813.3, 1606.6, and 1513.3 mAh g−1; meanwhile, the first three charge processes have specific capacities of 1510.6, 1426.6, and 1364.6 mAh g−1. So the initial coulomb efficiency (ICE) of the SnS2/B4C@OUCNTs is then calculated as 83.46%. In accordance with the initial CV finding, this lower ICE of the first electrochemical charge/discharge curve is attributed to the development of an SEI thin coating on the surface of active material. In second run, the corresponding coulombic efficiency value rises to 88.79%, and during the third run, it even reaches 90.17%. Additionally, depictions of the charging/discharging curves of SnS2 (Figure S4a) and SnS2@OUCNTs (Figure S4b) at 100 mA g−1 for the first three turns, where the ICEs are 59.39% and 83.20%, respectively, reveal that the components of OUCNTs and B4C may successfully raise the ICE of the active electrode, hence enhancing the materials’ electrochemical performance. Furthermore, Fig. 7b demonstrates that the voltage plateau and the redox peak of the CV curve in Fig. 7a are related. The composite electrode made of SnS2 and B4C@OUCNTs reached a voltage plateau during the first discharge process (lithiation course), which is consistent with the behavior of Li+ when it embeds in the SnS2 lamellas and undergoes transformation into Li2S and Sn [54]. Then, the de-alloying of LixSn, degradation of Li2S, oxidation of metal Sn, and creation of SnS2 all occur at the voltage plateau of the initial charge process (sulfation course), according to research conducted [55].
Figure 7c shows the rate capability of the SnS2/B4C@OUCNTs anode materials. The electrodes’ discharging specific capacities are respectively 1511, 1126, 806, 706, and 514 mAh g−1 at 100, 200, 500, 1000, and 2000 mA g−1 current densities. It is interesting that after cycling at high current densities, the discharge specific capacity also recovers to 79.5% of the original specific capacity when the current density drops to 100 mA g−1, demonstrating strong rate capability. By contrast, it shows the rate performances for pure SnS2 and SnS2@OUCNTs in figure S5, where their discharge capacities respectively recover to 18.39% and 28.09% of the original specific capacities, much lower than the rate capability of SnS2/B4C@OUCNTs. As a result, SnS2/B4C@OUCNTs exhibit high reversal capacity and durable cyclability as an anode material for LIBs.
The charge/discharge graphs of various current densities are displayed in Fig. 7d for SnS2/B4C@OUCNTs. High sweeping rates do not substantially alter the curve’s shape, indicating that the SnS2/B4C@OUCNTs anode has good rate performance. And below are the reasons for this exceptional property: (1) The unique oxidative unzipping of multi-walled carbon nanotube framework structure acts as a scaffold matrix to avoid collapsing and to offer a high active surface area, allowing for the fast passage of lithium ions in an unhindered manner [32, 37]. (2) The oxidating and unzipping of OUCNTs in SnS2/B4C@OUCNTs materials incorporates hydrophilic functional groups, which improve the hydrophilicity of the active material and increase the contact area of the active electrode material and electrolyte to speed up the rate of chemical reaction [33]. (3) Electrochemical impedance spectroscopy (EIS) evidences that doping of conducting material B4C lowers the resistivity of the SnS2/B4C@OUCNTs composite materials [29]. Herein, the B4C of active material also acts as a catalyst, which is responsible for the rapid synthesis and decomposition of SEI films during charging and discharging process, indirectly boosting the reversible capacity and long lifetime upon the working electrode.
With an original discharge specific capacity of 1840 mAh g−1 at 100 mA g−1, SnS2/B4C@OUCNTs (Fig. 8a) exhibit strong energy storage that is much better than that of SnS2 (1188 mAh g−1) (Figure S6a) and SnS2@OUCNTs (1640.8 mAh g−1) (Figure S6b). After a further 100 cycles, the SnS2/B4C@OUCNTs electrode’s discharge specific capacity (1024.7 mAh g−1) and coulombic efficiency (98.01%) remain steady and continue to increase. And the respective discharge specific capacities of SnS2 and SnS2@OUCNTs drop to 85.1 mAh g−1 and 647 mAh g−1 after 100 cycles, with coulombic efficiencies of 98% and 95.66%. And the cycling performance graph of pure B4C is provided in Figure S6c. The initial capacity of B4C in is not high (445.8 mAh g−1) and only 73.4 mAh g−1 reversible capacity remains after 100 cycles, so B4C is not suitable as a LIBs’ anode material alone. However, B4C has catalytic effect and high conductivity, and the synergistic effect with SnS2 and OUCNTs can make SnS2/B4C@OUCNTs achieve better cycling performance. Figure 8b reveals that SnS2/B4C@OUCNTs material has good cycling stability as the electrode material for lithium battery, maintaining a reversible capacity of 854.7 mAh g−1 at 1000 mA g−1 large current density with 98.57% coulombic efficiency after 200 turns of charging/discharging processes. In addition, the specific capacities of the cells have a certain degree of decrease during approximately the first 60 turns of the charging and discharging processes. This is because the electrode material and the electrolyte are in the process of reacting at the solid–liquid phase interface to form a SEI film, resulting in the lithium ions not getting a normal standard. The upward trend in capacity is due to the construction of the SEI film and the expansion of the contact area between the electrode material and the electrolyte. Although the construction of the SEI film consumes a portion of the lithium ions to increase the irreversible capacity, the organic insolubility of the SEI film prevents solvent molecules from damaging the electrode material, greatly improving the cycling performance and service life of the electrode. And the recovery of capacity after about 60 cycles should be attributed to the increased interlayer spacing via activating material, increasing diffusion rate of Li+ and lithium storage space [56]. This will help the material reduce the loss of irreversible capacity.
The CV curves of the material were kinetically studied to further understand the SnS2/B4C@OUCNTs’ electrochemical characteristics and capacitive behavior. The CV curves for various sweeping rates, ranging from 0.1 to 1.0 mV s−1, are shown in Fig. 9a. The SnS2/B4C@OUCNTs electrode demonstrates quick responsiveness since the CV curves’ form essentially does not change over time. The connection of peak current (i) and sweeping rate (ν) provides insight into the electrochemical kinetics of the anode, according to Eq. (1) [57, 58]:
where i represents the current of oxidation or reduction peak, ν represents the scan rate, and a and b serve as constants. Through Fig. 9a, Eq. (2) can be obtained by taking the logarithm for different voltage scan rates and corresponding peak current values in both sides of Formula (1):
Consequently, b may be calculated by plotting the slope of the fitting log(i) versus log(ν). In principle, diffusion-controlled cell properties are displayed by the electrode material if b = 0.5, whereas capacity-controlled pseudo-capacity properties are displayed by the electrode material if b = 1.
The slopes of the linear connection of log(i) and log(ν) from the peaks of the cathode and anode, respectively, are 0.657 and 0.666 in Fig. 9b. These findings imply that diffusion-controlled mechanisms and capacitive effects play a role in the electrochemical kinetics of the SnS2/B4C@OUCNTs anode. Additionally, Formula (3) may be used to determine the contribution of the pseudo-capacity [59]:
where V is the specific voltage, k1 and k2 act as the fitting constants, ν is the scanning rate of specific voltage, and k1ν and k2ν1/2 are respectively the pseudo-capacity contribution and the diffusive contribution to the current at a given voltage. Figure 9c demonstrates that at scan speeds of 0.1–1.0 mV s−1, the electrode’s capacity contribution rises from 20 to 51%. The electrochemical action of the SnS2/B4C@OUCNTs electrode is mostly controlled by diffusion at low current densities, whereas capacity control is more prominent at high current densities. Figure 9d shows the CV curve at 1.0 mV s−1 and the closed curve of the pseudo-capacity contribution via fitting analysis, where the pseudo-capacity contribution is calculated as 51%. Because of the unique SnS2/B4C@OUCNTs coating structure and the flower-like structure of the B4C-doped SnS2, the capacitive contribution of Li+ storage mechanism can gradually increase with increasing sweep rate. Thus, the strong capacity contribution, as a result of the short diffusion route and quick transmission speed for ions and electrons, is one of the reasons why SnS2/B4C@OUCNTs electrode can sustain great performance at high sweeping rates.
The electrochemical impedance spectroscopy tests allow a better comparative investigation of the good performance of the SnS2/B4C@OUCNTs anode material. Meanwhile, by using a simulated equivalent circuit to match the electrochemical impedance spectra, it is possible to further examine the kinetic characteristics of the composite electrode. Figure 10d shows the fitted equivalent circuit model, where Rs stands for the resistance of the solution, electrode, and material; CPE and Rct refer to the charge transfer impedance at the contact area between electrode and electrolyte; and Zw is related to the Warburg impedance that is brought on by the diffusion of lithium ions. Figure 10a shows the Nyquist plots for three contrasting electrodes in which each curve consists of a tiny intercept point (Rs) and a semicircle of the high-frequency zone (Rct and CPE) and a sloping line (Zw) of the low frequency zone. In comparison to pure SnS2 and SnS2@OUCNTs, the semicircle of the SnS2/B4C@OUCNTs electrode is obviously smaller. Obviously, the semicircle of the SnS2/B4C@OUCNTs composite is smaller than that of pure SnS2 and SnS2@OUCNTs. According to the fitting analysis, the fitted Rct value of SnS2/B4C@OUCNTs is 149 Ω which is less than the fitted Rct value of pure SnS2 (307 Ω) and SnS2@OUCNTs (150 Ω). This result clearly confirms that SnS2/B4C@OUCNTs has a smaller transfer resistance while charging and discharging. To put it another way, the composite electrode made of SnS2/B4C@OUCNTs material exhibits quicker charge transfer kinetics, indicating that better ionic diffusive channels are crucial for increasing lithium-ion store.
Figure 10b shows three linearly fitted Warburg plots (Z′ vs. ω−1/2), which can obtain accurate Li-ion diffusion coefficients. The Warburg coefficient of the SnS2/B4C@OUCNTs composite electrode material is 170.76 Ω s−1, which is much smaller than the Warburg coefficient of pure SnS2 (780.19 Ω·s−1) and SnS2@OUCNTs (216.94 Ω·s−1). And the diffusion coefficients of lithium ions are calculated by Eq. (4) as follows [60]:
where R denotes the gas constant, T stands for the absolute temperature, A signifies surface area of the electrode sheet, n represents the sum of shifting electrons, F means the Faraday constant, Co is the ionic molarity, and σω expresses the Warburg coefficient. Formula (4) states that the Warburg coefficient, which is a deciding factor, has a square root relationship with the diffusion coefficient of lithium ions. SnS2/B4C@OUCNTs electrode has an estimated lithium-ion diffusion coefficient of 8.02 × 10−22 cm s−1, which is greater than that of pure SnS2 (3.83 × 10−23 cm s−1) and SnS2@OUCNTs (4.96 × 10−22 cm s−1). Due to the high diffusion coefficient, the electrode performs well in terms of rate and charge/discharge even at large current densities. Consequently, the inclusion of OUCNTs and B4C boosts not only the electron conductivity but also the speed at which electrons transfer in during insertion and release of lithium ions at high current densities, greatly enhancing the electrochemical properties of the SnS2/B4C@OUCNTs anode.
Moreover, Fig. 10c exhibits the comparison of electrochemical impedance spectra about the SnS2/B4C@OUCNTs composite electrode before and after 100 turns of charging/discharging at 100 mA g−1. The semicircle in high-frequency zone of the impedance curve after cycling can be shown to be substantially smaller than that of the impedance curve without cycling, showing that the resistance of SnS2/B4C@OUCNTs electrode’s charge transmission will decrease after 100 cycles at 100 mA g−1. Repeated studies have consistently shown that the integration of OUCNTs and B4C plays a significant role in cycling, increasing the diffusion and transfer of lithium ions and electrons during cycling, and producing favorable electrochemical characteristics as a result.
In this work, SnS2/B4C@OUCNTs exhibits good cycling performance compared to the reported Sn-based sulfide-based electrodes for LIBs, and the comparisons are shown in Table 1. This result indicates that SnS2/B4C@OUCNTs is a potential and feasible anode material LIBs.
Conclusions
To sum up, three-dimensional oxidized chain-dissolved multi-walled carbon nanotubes encapsulating SnS2 and B4C were successfully synthesized by a straightforward hydrothermal reaction. As an anode for LIBs, the results show that the SnS2/B4C@OUCNTs electrodes exhibit better cycling performance with a specific capacity retention of 1024.7 mAh g−1 cycling 100 times and higher rate performance with a high capacity of 854.7 mAh g−1 at 1000 mA g−1 than the pure phase SnS2 and SnS2@OUCNTs (85.1 mAh g−1 and 647 mAh g−1 after 100 cycles at 100 mA g−1, respectively). This shows that the disadvantages of poor SnS2 conductivity and capacity decay can be effectively improved by combining conductive yet flexible oxidative unzipping carbon nanotubes and highly chemically active boron carbide with nanosheet self-assembled SnS2 and even has potential for usage for LIBs as the high-performance anode material.
The Supporting Information is available free of charge on the experimental section, TEM, XRD, BET, and electrochemical evaluation of the samples.
References
Di Lecce D, Verrelli R, Hassoun J (2017) Lithium-ion batteries for sustainable energy storage: recent advances towards new cell configurations. Green Chem 19:3442–3467. https://doi.org/10.1039/C7GC01328K
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935. https://doi.org/10.1126/science.1212741
Jiao X, Liu Y, Li B, Zhang W, He C, Zhang C, Yu Z, Gao T, Song J (2019) Amorphous phosphorus-carbon nanotube hybrid anode with ultralong cycle life and high-rate capability for lithium-ion batteries. Carbon 148:518–524. https://doi.org/10.1016/j.carbon.2019.03.053
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29. https://doi.org/10.1038/nchem.2085
Lu Y, Yu L, Lou XW (2018) Nanostructured conversion-type anode materials for advanced lithium-ion batteries. Chem 4:972–996. https://doi.org/10.1016/j.chempr.2018.01.003
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
Zhou Y, Tian J, Xu H, Yang J, Qian Y (2017) VS4 nanoparticles rooted by a-C coated MWCNTs as an advanced anode material in lithium ion batteries. Energy Storage Materials 6:149–156. https://doi.org/10.1016/j.ensm.2016.10.010
Yin L, Chai S, Ma J, Huang J, Kong X, Bai P, Liu Y (2017) Effects of binders on electrochemical properties of the SnS2 nanostructured anode of the lithium-ion batteries. J Alloy Compd 698:828–834. https://doi.org/10.1016/j.jallcom.2016.12.215
Bhandavat R, David L, Singh G (2012) Synthesis of surface-functionalized WS2 nanosheets and performance as li-ion battery anodes. J Phys Chem Lett 3:1523–1530. https://doi.org/10.1021/jz300480w
Youn DH, Jo C, Kim JY, Lee J, Lee JS (2015) Ultrafast synthesis of MoS2 or WS2-reduced graphene oxide composites via hybrid microwave annealing for anode materials of lithium ion batteries. J Power Sources 295:228–234. https://doi.org/10.1016/j.jpowsour.2015.07.013
Wang D, Liu Y, Meng X, Wei Y, Zhao Y, Pang Q, Chen G (2017) Two-dimensional VS2 monolayers as potential anode materials for lithium-ion batteries and beyond: first-principles calculations. J Materials Chem A 5:21370–21377. https://doi.org/10.1039/C7TA06944H
Seo J-w, Jang J-t, Park S-w, Kim C, Park B, Cheon J (2008) Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv Mater 20:4269–4273. https://doi.org/10.1002/adma.200703122
Momma T, Shiraishi N, Yoshizawa A, Osaka T, Gedanken A, Zhu J, Sominski L (2001) SnS2 anode for rechargeable lithium battery. J Power Sources 97–98:198–200. https://doi.org/10.1016/S0378-7753(01)00723-6
Zhong H, Yang G, Song H, Liao Q, Cui H, Shen P, Wang C-X (2012) Vertically aligned graphene-like SnS2 ultrathin nanosheet arrays: excellent energy storage, catalysis, photoconduction, and field-emitting performances. The J Physical Chem C 116:9319–9326. https://doi.org/10.1021/jp301024d
Liu Y, Yu X-Y, Fang Y, Zhu X, Bao J, Zhou X, Lou XW (2018) Confining SnS2 ultrathin nanosheets in hollow carbon nanostructures for efficient capacitive sodium storage. Joule 2:725–735. https://doi.org/10.1016/j.joule.2018.01.004
Jiang Y, Feng Y, Xi B, Kai S, Mi K, Feng J, Zhang J, Xiong S (2016) Ultrasmall SnS2 nanoparticles anchored on well-distributed nitrogen-doped graphene sheets for Li-ion and Na-ion batteries. J Materials Chem A 4:10719–10726. https://doi.org/10.1039/C6TA03580A
Du N, Wu X, Zhai C, Zhang H, Yang D (2013) Large-scale synthesis and application of SnS2–graphene nanocomposites as anode materials for lithium-ion batteries with enhanced cyclic performance and reversible capacity. J Alloy Compd 580:457–464. https://doi.org/10.1016/j.jallcom.2013.06.079
Zhang L, Huang Y, Zhang Y, Fan W, Liu T (2015) Three-dimensional nanoporous graphene-carbon nanotube hybrid frameworks for confinement of SnS2 nanosheets: flexible and binder-free papers with highly reversible lithium storage. ACS Appl Mater Interfaces 7:27823–27830. https://doi.org/10.1021/acsami.5b09115
Wang J, Luo C, Mao J, Zhu Y, Fan X, Gao T, Mignerey AC, Wang C (2015) Solid-state fabrication of SnS2/C nanospheres for high-performance sodium ion battery anode. ACS Appl Mater Interfaces 7:11476–11481. https://doi.org/10.1021/acsami.5b02413
Zhou Y, Wang Q, Zhu X, Jiang F (2018) Three-dimensional SnS decorated carbon nano-networks as anode materials for lithium and sodium ion batteries. Nanomaterials 8:135. https://doi.org/10.3390/nano8030135
Zhou Y, Yan D, Xu H, Liu S, Yang J, Qian Y (2015) Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: a high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 7:3520–3525. https://doi.org/10.1039/c4nr07143c
Youn DH, Stauffer SK, Xiao P, Park H, Nam Y, Dolocan A, Henkelman G, Heller A, Mullins CB (2016) Simple synthesis of nanocrystalline tin sulfide/N-doped reduced graphene oxide composites as lithium ion battery anodes. ACS Nano 10:10778–10788. https://doi.org/10.1021/acsnano.6b04214
Yin J, Cao H, Zhou Z, Zhang J, Qu M (2012) SnS2@reduced graphene oxide nanocomposites as anode materials with high capacity for rechargeable lithium ion batteries. J Mater Chem 22:23963–23970. https://doi.org/10.1039/C2JM35137D
Kim HS, Chung YH, Kang SH, Sung Y-E (2009) Electrochemical behavior of carbon-coated SnS2 for use as the anode in lithium-ion batteries. Electrochim Acta 54:3606–3610. https://doi.org/10.1016/j.electacta.2009.01.030
Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM (2009) Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458:872–876. https://doi.org/10.1038/nature07872
Domnich V, Reynaud S, Haber RA, Chhowalla M (2011) Boron carbide: structure, properties, and stability under stress. J Am Ceram Soc 94:3605–3628. https://doi.org/10.1111/j.1551-2916.2011.04865.x
Song S, Xu W, Cao R, Luo L, Engelhard MH, Bowden ME, Liu B, Estevez L, Wang C-M, Zhang J-G (2017) B4C as a stable non-carbon-based oxygen electrode material for lithium-oxygen batteries. Nano Energy 33:195–204. https://doi.org/10.1016/j.nanoen.2017.01.042
Song N, Gao Z, Zhang Y, Li X (2019) B4C nanoskeleton enabled, flexible lithium-sulfur batteries. Nano Energy 58:30–39. https://doi.org/10.1016/j.nanoen.2019.01.018
Wang C, Guo Z, Shen W, Xu Q, Liu H, Wang Y (2014) B-doped carbon coating improves the electrochemical performance of electrode materials for Li-ion batteries. Adv Func Mater 24:5511–5521. https://doi.org/10.1002/adfm.201401006
Chen X, Li X, Ding F, Xu W, Xiao J, Cao Y, Meduri P, Liu J, Graff GL, Zhang J-G (2012) Conductive rigid skeleton supported silicon as high-performance li-ion battery anodes. Nano Lett 12:4124–4130. https://doi.org/10.1021/nl301657y
Sun Y, Yang Y, Shi X-L, Suo G, Lu S, Chen Z-G (2021) Self-standing and high-performance B4C/Sn/acetylene black@reduced graphene oxide films as sodium-ion half/full battery anodes. Applied Materials Today 24:101137. https://doi.org/10.1016/j.apmt.2021.101137
Zhang E, Wang J, Wang B, Yu X, Yang H, Lu B (2019) Unzipped carbon nanotubes for aluminum battery. Energy Storage Materials 23:72–78. https://doi.org/10.1016/j.ensm.2019.05.030
Dimiev AM, Khannanov A, Vakhitov I, Kiiamov A, Shukhina K, Tour JM (2018) Revisiting the mechanism of oxidative unzipping of multiwall carbon nanotubes to graphene nanoribbons. ACS Nano 12:3985–3993. https://doi.org/10.1021/acsnano.8b01617
Deng L, Zhu J, Chen X, Ding M, Liu H (2018) Three-dimensional elastic ultrathin reduced graphene oxide coating SnS2 hierarchical microsphere as lithium ion batteries anode materials. J Alloy Compd 739:1015–1024. https://doi.org/10.1016/j.jallcom.2017.12.210
Zhang Q, Li R, Zhang M, Zhang B, Gou X (2014) SnS2/reduced graphene oxide nanocomposites with superior lithium storage performance. Electrochim Acta 115:425–433. https://doi.org/10.1016/j.electacta.2013.10.193
Zhang Y, Zhu P, Huang L, Xie J, Zhang S, Cao G, Zhao X (2015) Few-layered SnS2 on few-layered reduced graphene oxide as na-ion battery anode with ultralong cycle life and superior rate capability. Adv Func Mater 25:481–489. https://doi.org/10.1002/adfm.201402833
Fan J-J, Fan Y-J, Wang R-X, Xiang S, Tang H-G, Sun S-G (2017) A novel strategy for the synthesis of sulfur-doped carbon nanotubes as a highly efficient Pt catalyst support toward the methanol oxidation reaction. J of Materials Chem A 5:19467–19475. https://doi.org/10.1039/C7TA05102F
Sun W, Rui X, Yang D, Sun Z, Li B, Zhang W, Zong Y, Madhavi S, Dou S, Yan Q (2015) Two-dimensional tin disulfide nanosheets for enhanced sodium storage. ACS Nano 9:11371–11381. https://doi.org/10.1021/acsnano.5b05229
Yu J, Xu CY, Ma FX, Hu SP, Zhang YW, Zhen L (2014) Monodisperse SnS(2) nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl Mater Interfaces 6:22370–22377. https://doi.org/10.1021/am506396z
Zhai C, Du N, Zhang H, Yu J, Yang D (2011) Multiwalled carbon nanotubes anchored with SnS2 nanosheets as high-performance anode materials of lithium-ion batteries. ACS Appl Mater Interfaces 3:4067–4074. https://doi.org/10.1021/am200933m
Zhang R, Chi C, Wu M, Liu K, Zhao T (2020) A long-life Li–S battery enabled by a cathode made of well-distributed B4C nanoparticles decorated activated cotton fibers. J of Power Sources 451:227751. https://doi.org/10.1016/j.jpowsour.2020.227751
Zhang YC, Yao L, Zhang G, Dionysiou DD, Li J, Du X (2014) One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI). Appl Catal B 144:730–738. https://doi.org/10.1016/j.apcatb.2013.08.006
Zheng P, Dai Z, Zhang Y, Dinh KN, Zheng Y, Fan H, Yang J, Dangol R, Li B, Zong Y, Yan Q, Liu X (2017) Scalable synthesis of SnS2/S-doped graphene composites for superior Li/Na-ion batteries. Nanoscale 9:14820–14825. https://doi.org/10.1039/C7NR06044K
Liu H, Chen X, Deng L, Su X, Guo K, Zhu Z (2016) Preparation of ultrathin 2D MoS2/graphene heterostructure assembled foam-like structure with enhanced electrochemical performance for lithium-ion batteries. Electrochim Acta 206:184–191. https://doi.org/10.1016/j.electacta.2016.04.160
Wang J-G, Sun H, Liu H, Jin D, Zhou R, Wei B (2017) Edge-oriented SnS2 nanosheet arrays on carbon paper as advanced binder-free anodes for Li-ion and Na-ion batteries. J Materials Chem A 5:23115–23122. https://doi.org/10.1039/C7TA07553G
Yu Z, Li X, Yan B, Xiong D, Yang M, Li D (2017) Rational design of flower-like tin sulfide @ reduced graphene oxide for high performance sodium ion batteries. Mater Res Bull 96:516–523. https://doi.org/10.1016/j.materresbull.2017.04.048
Qu B, Ma C, Ji G, Xu C, Xu J, Meng YS, Wang T, Lee JY (2014) Layered SnS2-Reduced graphene oxide composite – a high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv Mater 26:3854–3859. https://doi.org/10.1002/adma.201306314
Yusuf M, Khan MA, Abdullah EC, Elfghi M, Hosomi M, Terada A, Riya S, Ahmad A (2016) Dodecyl sulfate chain anchored mesoporous graphene: synthesis and application to sequester heavy metal ions from aqueous phase. Chem Eng J 304:431–439. https://doi.org/10.1016/j.cej.2016.06.109
Wang X, Zhang Y, Chen H, Sun G, Wang Z, Hou H, Hu Z, Gao Q, Zhang Q (2022) High-capacity and cycling-stable anode for sodium ion batteries constructed from SnS2/MWCNTs nanocomposites. J Alloys Compounds 897:163029. https://doi.org/10.1016/j.jallcom.2021.163029
Wang G, Peng J, Zhang L, Zhang J, Dai B, Zhu M, Xia L, Yu F (2015) Two-dimensional SnS2@PANI nanoplates with high capacity and excellent stability for lithium-ion batteries. J Materials Chem A 3:3659–3666. https://doi.org/10.1039/C4TA06384H
Jiang X, Yang X, Zhu Y, Shen J, Fan K, Li C (2013) In situ assembly of graphene sheets-supported SnS2 nanoplates into 3D macroporous aerogels for high-performance lithium ion batteries. J Power Sources 237:178–186. https://doi.org/10.1016/j.jpowsour.2013.03.048
Du Y, Yin Z, Rui X, Zeng Z, Wu X-J, Liu J, Zhu Y, Zhu J, Huang X, Yan Q, Zhang H (2013) A facile, relative green, and inexpensive synthetic approach toward large-scale production of SnS2 nanoplates for high-performance lithium-ion batteries. Nanoscale 5:1456–1459. https://doi.org/10.1039/C2NR33458E
Zhao Y, Li X, Yan B, Li D, Lawes S, Sun X (2015) Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: a review. J Power Sources 274:869–884. https://doi.org/10.1016/j.jpowsour.2014.10.008
Jana MK, Rajendra HB, Bhattacharyya AJ, Biswas K (2014) Green ionothermal synthesis of hierarchical nanostructures of SnS2 and their Li-ion storage properties. CrystEngComm 16:3994–4000. https://doi.org/10.1039/C3CE42561D
Zhai C, Du N, Yang HZD (2011) Large-scale synthesis of ultrathin hexagonal tin disulfide nanosheets with highly reversible lithium storage. Chem Commun 47:1270–1272. https://doi.org/10.1039/C0CC03023F
Guo J, Li Y, Meng J, Pedersen K, Gurevich L, Stroe D-I (2022) Understanding the mechanism of capacity increase during early cycling of commercial NMC/graphite lithium-ion batteries. J Energy Chem 74:34–44. https://doi.org/10.1016/j.jechem.2022.07.005
He Y, Wang L, Dong C, Li C, Ding X, Qian Y, Xu L (2019) In-situ rooting ZnSe/N-doped hollow carbon architectures as high-rate and long-life anode materials for half/full sodium-ion and potassium-ion batteries. Energy Storage Materials 23:35–45. https://doi.org/10.1016/j.ensm.2019.05.039
Hu M, Jing H, Li T, Wang J, Yang H, Lv R, Chen D (2019) Composite K2Mo4O13/α-MoO3 nanorods: sonochemical preparation and applications for advanced Li+/Na+ pseudocapacitance. J Materials Chem A 7:10954–10961. https://doi.org/10.1039/C8TA11854J
Costentin C (2020) Electrochemical energy storage: questioning the popular v/v1/2 scan rate diagnosis in cyclic voltammetry. The J Physical Chem Lett 11:9846–9849. https://doi.org/10.1021/acs.jpclett.0c02667
Cheng Y, Huang J, Li J, Xu Z, Cao L, Ouyang H, Yan J, Qi H (2016) SnO2/super P nanocomposites as anode materials for Na-ion batteries with enhanced electrochemical performance. J Alloy Compd 658:234–240. https://doi.org/10.1016/j.jallcom.2015.10.212
Chen X, Huang Y, Zhang K, Feng X, Wang M (2017) Synthesis and high-performance of carbonaceous polypyrrole nanotubes coated with SnS2 nanosheets anode materials for lithium ion batteries. Chem Eng J 330:470–479. https://doi.org/10.1016/j.cej.2017.07.180
Xia J, Liu L, Xie J, Yan H, Yuan Y, Chen M, Huang C, Zhang Y, Nie S, Wang X (2018) Layer-by-layered SnS2/graphene hybrid nanosheets via ball-milling as promising anode materials for lithium ion batteries. Electrochim Acta 269:452–461. https://doi.org/10.1016/j.electacta.2018.03.022
Hassan FM, Hu Q, Fu J, Batmaz R, Li J, Yu A, Xiao X, Chen Z (2017) Hot-chemistry structural phase transformation in single-crystal chalcogenides for long-life lithium ion batteries. ACS Appl Mater Interfaces 9:20603–20612. https://doi.org/10.1021/acsami.7b04483
Hu Q, Wang B, Chang S, Yang C, Hu Y, Cao S, Lu J, Zhang L, Ye H (2021) Effects of annealing temperature on electrochemical performance of SnSx embedded in hierarchical porous carbon with N-carbon coating by in-situ structural phase transformation as anodes for lithium ion batteries. J Mater Sci Technol 84:191–199. https://doi.org/10.1016/j.jmst.2020.12.068
Funding
This work was generously supported by the National Natural Science Foundation of China (11874124) and the Science and Technology Planning Project of Guangdong Province, China (2014B03032013, 2015B010114007, and 2016B010129002).
Author information
Authors and Affiliations
Contributions
Wei Su: original manuscript writing, conceptualization, experimentation, validation, investigation, and data analysis. Yandong Xie: desk research, experimentation. Kaidan Wu: experimentation, data acquisition. Deping Xiong: data collection. Li Chen: desk research, experimentation. Zuyong Feng: conception, resources. Kunhua Wen: review and editing of writing. Zhaoying Li: supervision. Miao He: conception, resources, review and editing of writing, supervision, project management, and funds obtainment; all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, W., Xie, Y., Wu, K. et al. SnS2/B4C@OUCNTs as a high-performance anode material for lithium-ion batteries. Ionics 29, 3955–3969 (2023). https://doi.org/10.1007/s11581-023-05117-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05117-5