Abstract
SrY2O4 belongs to the family of AB2O4 (A = alkaline earth metal, B = rare earth element) type spinel oxides. SrY2O4 is an inter oxide in SrO and Y2O3 pseudo-binary phase diagram. It has attracted immense interest as a promising host lattice owing to its thermal and chemical stability. The luminescent characteristics of SrY2O4 phosphors make them promising candidates for field emission displays. In the present review article, recent progress in the synthesis and luminescent properties of bare and doped SrY2O4 phosphors are reviewed. In the first part, different synthesis routes and their resultant outcomes are discussed. Then, the downconversion and upconversion luminescent characteristics of undoped and doped SrY2O4 phosphors are reviewed. The effect of site occupancy, morphology, reaction conditions, different excitation wavelengths, dopant concentrations, and energy transfer mechanisms are explained in detail. In the end, challenges and future scope in luminescence and synthesis of SrY2O4 are proposed. This review article gives a complete summary for the drawbacks and current challenging issues of SrY2O4 phosphors, which can give potential sprout for future work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the present era, field emission displays (FEDs) are in high demand due to their high brightness, less power consumption, low manufacturing cost, high contrast ratio, high efficiency, etc. [1,2,3]. To manufacture flat panel displays, phosphors play an important role. For this purpose, rare earth-doped inorganic materials have attracted much attention in the lighting industry. This is owing to the unique luminescent properties arising from the f–f orbital electronic transitions. In FEDs, the phosphors are operated at low excitation voltage (3–7 kV) and high current densities (10–100 μA cm−2). Thus, the phosphors which can be efficiently operated at low voltages and resistant to current saturation with stable chemical and thermal properties are required. Till now, many commercial sulfide-based phosphors are used for commercial purposes such as ZnCdS:Ag:Cl, ZnCdS:Cu:Al, ZnS:Cu:Al, Y2O2S:Eu, Gd2O2S:Tb, and ZnS:Ag, Cl, SrGa2S4:Ce [4, 5]. However, these phosphors are not eco-friendly. They degrade under electron beam bombardment and release hazardous sulphur gas. Under electron bombardment, electrons cause the formation of oxides and sulphates which deposit on the screen of the device, also known as “dead layer”. This decreases the lifetime of the device and restricts the use of sulfide-based phosphors in FEDs. Thus, potential phosphors are required, which are thermally stable and eco-friendly. For this purpose, oxide-based phosphors are considered as promising candidates due to their high physical, chemical, and thermal stability, low phonon energy, and high bandgaps, absence of blinking, gas free emission, and corrosion resistant properties.
Among the various rare earth oxides, binary rare earth oxides with structural composition ARE2O4 (A = Ca, Sr, Ba, Sr, RE = rare earth element) have become the topic of luminescent researchers. These have been studied by various researchers and are used in various lighting and display devices due to their unique optical, magnetic, and thermal properties [6,7,8,9]. Out of these inter oxides of alkaline earth metals and rare earth elements; SrY2O4 (SYO) has attracted research interest. It is an inter oxide in Y2O3–SrO pseudo-binary phase diagram. SYO possesses high physical, chemical, and thermal stability and also environment-friendly. Earlier, SYO due to its high thermal and chemical stability was used in thermal barrier coatings and oxide fuel cells [10, 11]. The luminescent characteristics of SYO:Eu synthesized via combustion route were first reported by Park et al. [12]. Due to its high structural stability, it is considered a good host matrix for the doping of various elements. It is a suitable phosphor material for field emission displays (FEDs) owing to its emission properties and thermal stability. In addition, Ghorpade et al. [13] studied the photocatalytic activities of the Dy-activated SYO phosphors to degrade the Rohdamine-B dye. It was found that the efficiency of dye degradation decreased with doping. Moreover, due to the high bandgap around 4.9, it is not an efficient catalyst in comparison to the other existing semiconductor catalysts. However, 50% degradation of the dye was achieved. In another study, Dubey et al. [14] reported the thermoluminescent spectra of Eu-doped SYO. The phosphors were irradiated by radiations of 10 Gy Sr90 beta source. The samples showed second-order kinetics and corresponding trap depth was in between 0.18 and 0.5 eV. However, no other investigations using different irradiation sources, change of dopant concentrations, and variations in irradiation duration are reported. Thus, to use SYO phosphors for dosimetric applications, further investigations are required. The pictorial representation of various properties and applications of SYO is shown in Fig. 1.
In general, SYO is synthesized via high temperature ceramic route [15,16,17]. However, many researchers have attempted to synthesize it via soft chemical routes [18, 19]. Till now, many rare earth activators have been doped in the SYO host lattice and luminescent studies are conducted. The present review article is centered around the recent progress in the synthesis and luminescent properties of SYO phosphors.
The crystal structure of SYO is inverse spinel orthorhombic with Pnam group symmetry [20, 21]. The octahedral structure is composed of [Y2O4]2− framework with Sr2+ ions. In the SYO host lattice, there are two different sites for Y3+ ions, i.e., Y1 and Y2 with Cs group symmetry (shown in Fig. 2). In crystal lattice of SYO, atoms of O are placed at vertices and face-centerd sites of lattice, whereas Y1 is placed at almost octahedral void (body-centerd) and Y2 at the tetrahedral void. Both Y atoms are surrounded by six O-atoms [22]. Y2 atoms distort the environment due to the difference in ionic radius of O2− and Y3+. The Sr atoms are placed in octahedral voids (at edges), giving Cs symmetry [23, 24]. PL technique also predicted the presence of these sites in the SYO lattice. The site occupancy of different dopants at different sites and their emission behavior under different excitations led to different remarks on the various sites in SYO host lattice. These are all discussed in the PL section.
2 Synthesis methods
Undoped and doped SYO phosphors are synthesized via adopting different synthesis routes. Till date, SYO materials are synthesized via solid-state, combustion, sol–gel, aldo–keto, and oil-emulsion methods. The detailed summary of precursors, additives, reaction conditions, and morphology are enlisted in Table 1. The details of synthesis routes are described and discussed in this section:
2.1 Combustion method
Combustion method is a constructive, convenient, eco-friendly, and efficient method for the synthesis of SYO compounds. This method requires the use of fuel such as urea, glycine, and sucrose to synthesize samples. The gases liberated during the burning of fuel generate high temperatures around 1500 to 1600 °C, which helps in the formation of phosphors at very low temperatures around 500 to 600 °C. SYO formed by the combustion route is synthesized using aqueous solution of metal nitrates as reducing agents and fuel as oxidizing agents, the amounts of which are calculated according to propellant chemistry. The aqueous solution is then placed in a preheated furnace at a lower temperature where voluminous ashes after completion of the combustion process are obtained. These ashes are further subjected to higher temperatures to obtain the pure phase, crystalline materials, and also to remove organic residues left behind.
Park et al. in 1999 synthesized Eu-doped SYO from metal nitrates in aqueous solution with urea as fuel. For Eu2+-doped samples, the SYO was further subjected to successive heating ranging from 700 to 1000 °C in an H2 stream [12]. Singh et al. in 2015 synthesized SrY2O4:Eu3+ from metal nitrates and europium nitrate mixed with HMT as fuel. The solution was introduced in a preheated furnace at 600 °C and then further calcined at temperatures ranging from 800 to 1100 °C for 1 h [25]. Tamrakar and Upadhyay in 2017 synthesized SYO from metal nitrates using urea as a fuel. Then as-synthesized SYO was further annealed at 900 °C for 2 h to obtain pure phase [26]. Taikar in 2018 synthesized SYO from SrCO3 and Y2O3 as precursors and adding NH4NO3 and urea as fuel. The mixture in the form of paste was put in a preheated furnace at 500 °C and was calcined at 1000 °C for 3 h to obtain pure phase phosphors [18]. Lojpur et al. in 2018 synthesized SYO from metal nitrates as precursors, citric acid, and glycine as fuel. The solution was prepared by mixing nitrates in deionized water, then citric was added and stirred for 30 min at 60 °C. Further glycine was added and stirred at 120 °C. Finally, the solution was burned in furnace at 500 °C for 1.5 h. Then, it was heated at 1000 °C for 2 h to obtain the phosphors [21]. The schematic presentation of combustion route is shown in Scheme 1.
2.2 Sol–gel method
Sol–gel method is an effective method to synthesize nanocrystalline SYO phosphors with uniform size distribution. In this method, metal nitrates of strontium and yttrium are dissolved with a chelating agent such as citric acid or oxalic acid. The solution is heated around 80–150 °C, which leads to the formation of xerogel. The xerogel is further heated to a specific temperature range to get pure phase SYO. The schematic presentation of the sol–gel method is shown in Scheme 2. Wang et al. in 2007 synthesized SYO from Y2O3 and Sr(NO3)2 as precursors and citric acid as a gelating agent via citrate-gel method. The gel was preheated for 2 h at 400 °C and then calcined at 1000 °C for 2 h [20]. Pavitra et al. synthesized Eu3+/Dy3+ co-doped SYO, Eu3+/Sm3+ co-doped SYO, Er3+ and Dy3+/Sm3+ co-doped SYO phosphors using citrate sol–gel method. In their studies, they used citric acid to form a gel at 80 °C. The yellowish gel was further annealed at temperatures ranging from 700 to 1400 °C. However, pure phase SYO was obtained at 1300 °C. For temperatures, lower and above 1300 °C, Y2O3 was obtained as a secondary phase [15, 16, 27]. Zhang and Wang synthesized Eu-doped SYO phosphors via citrate sol–gel method. The xerogel formed was pre-fired at 600 °C for 4 h and further subjected at 1000 °C for 2 h in air [28]. Singh et al. in 2020 synthesized SYO by mixing metal nitrates and citric acid in aqueous solution. They used citric acid to form gel at 120 °C with 1 h stirring. The ratio of metal ions to citric acid was 1:2. Then, dried gel was heated in a furnace at 400 °C for 2 h. The obtained powder was annealed at 1000 °C for 3 h to obtain SYO phosphors [29].
2.3 Solid-state method
Solid-state method is an easy, adaptable, high temperature, and commonly used method to produce bulk SYO samples. This method leads to the formation of polycrystalline particles. The initial precursors are generally carbonates and oxides. In some cases, fluxing agents such as boric acid (H3BO3) in small quantities are used to lower the synthesis temperature. The schematic representation of solid-method is shown in Scheme 3.
Xu et al. in 2001 synthesized SYO by mixing strontium carbonate and yttrium oxide with boric acid as a chelating agent. These samples were pre-fired at 1000 °C for 2 h and then sintered at 1400 °C for 2 h [30]. Atkins et al. synthesized Eu3+-doped SYO using Y2O3, SrCO3 and Eu2O3 as precursors. The raw blend was ground and fired at 1050 °C for 18 h. It was again ground and fired at 1050 °C for 18 h to obtain SrY2O4: Eu3+ phosphors [24]. Fu et al. synthesized SYO by mixing strontium carbonate and yttrium oxide with 0.01 boric acid as flux. After thoroughly mixing, samples were heated at 700 °C for 2 h and then calcined at 1400 °C for 2.5 h in air [23, 31]. Zhang and Wang [28] and Dubey et al. in 2013 [14] synthesized SYO by thoroughly mixing of strontium carbonate and yttrium oxide. Firstly, the samples were subjected to 1000 °C in a furnace and then sintered at 1300 °C in air. The effect of Eu doping was studied in detail. Opravil et al. [32] synthesized SYO by homogenization of strontium carbonate and yttrium oxide via short milling for 10 min in vibration mill. The mixture was then treated in muffle kiln at 1650 °C for 1 h. The sintered pellet sample was next pulverized by grinding and fine milling to get the pure SYO phase. Xianlang et al. [33] synthesized Er3+/Yb3+ co-doped SYO phosphors using SrCO3 and Y2CO3 as initial precursors. NH4Cl was used as a flux agent. The pure phase SYO phosphors were obtained after firing at 1350 °C for 3 h. Wei et al. [34] synthesized Bi3+/Eu3+ co-doped SYO phosphors using SrCO3 and Bi2O3 and Eu2O3 as initial precursors and ethanol as dispersing medium. The ground mixture was further annealed at 1400 °C for 5 h in air atmosphere. The crystallinity was obtained using 2 wt% H3BO3.
2.4 Other methods
Koparkar et al. [35] synthesized SYO by Aldo–keto method. This method consists of mixing of nitrates of strontium and yttrium in oxalic acid solution. Ethanol and benzaldehyde were added into the solution as gelating agents. Then, the pyrolysis of foam was observed at 450 °C. Further, it was burned at 1000 °C to obtain crystalline powder of SYO. In another study, Zhou et al. [36] synthesized SrR2O4:Eu3+ (R = Y,Lu) phosphors via water-in-oil microemulsion route. Two microemulsions (I and II) were prepared and mixed together with same phase. The microemulsion I consisted of Sr(NO3)2, Y(NO3)3/Lu(NO3)3 and Eu(NO3)3 solution while microemulsion II was 1.0 M H2C2O4 solution which was used as a precipitating agent. The organic phase was composed of polyol ethylene octylphenol ether (OP-10), iso-pentanol, and cyclohexane in 2:1:6 volume ratio. The formed oxalate precursors were dried at 120 °C and annealed at different temperatures ranging from 800 to 1100 °C for 2 h. It was observed that temperature below 1000 °C consisted of Y2O3/Lu2O3, which were removed after annealing at temperature higher than 1000 °C.
From Table 1, it can be seen that the SYO phosphors were synthesized at higher temperatures. The morphology of the phosphors also plays a pivotal role in altering the luminescence properties. It can be observed from Table 1 that synthesized SYO phosphors via different routes exhibit different morphologies and particle size depending upon the precursors, reaction conditions, and use of additives. Singh et al. synthesized phosphors via combustion routes and observed narrow size distribution of spherical shape particles [25]. The particle size varied in the range of 10–25 nm. Lojpur et al. synthesized SYO:Eu via combustion route and found that particles were agglomerated having size ~ 50 nm with porous structures [21]. The porous features of the synthesized particles was attributed to the liberation of the gases during synthesis which ruptured the surface of the particles. Pavitra et al. synthesized SYO:Tb via sol–gel method and annealed at different temperatures [27]. It was observed that for pure phase SYO, the tip of every particle was in circular wave like shape, which might help to pack the particle closely. The closed packed particles are considered to prevent the scattering of light, which yields the light output efficiently. Moreover, it was observed that particle size increased gradually with the increase in the temperature. Pavitra et al. [16] synthesized SYO:Dy and found that particles exhibited uniform size spheres with close packing. Zhang and Wang [28] compared morphology of SYOEu synthesized via sol–gel and solid-state method. It was found that particles synthesized via solid-state method were agglomerated, whereas a better dispersion was obtained for samples synthesized via sol–gel method. Singh et al. [29] examined morphological features of SYO doped with Gd synthesized via sol–gel synthesis method. The particles were found to have aggregates of varying shape and size, which consisted of several small crystallites. These particles were highly interconnected having particle size ranging between 1 and 2 µm. Opravil et al. [16] observed the formation of dodecahedral single crystals and twin morphology of SYO:Sm synthesized via solid-state method. Zhou et al. [36] examined the morphology of SYO doped with Eu3+ synthesized by water-in-oil microemulsion and obtained rugby-like shape with diameter of 70–100 nm.
3 Photoluminescence (PL) in bare and doped SrY2O4 phosphors
SrY2O4 is a suitable host matrix due to its stable physical, chemical, and thermal structure [18]. It shows luminescent properties under UV and vacuum UV excitation. Zhang and Wang [28] synthesized undoped SrY2O4 phosphors by solid-state method. The excitation spectrum of SYO consisted of a band centered at 324 nm which was due to host absorption band. The band was appeared due to presence of defects which appeared due to preparation of samples at high temperature. It was further found that the antisite defects (Sr at Y site) could be considered to occur in the SrY2O4 structure, and oxygen vacancies were also produced. Therefore, the excitation bands in the region of 290 to 360 nm when monitored at 406 nm, as well as the emission band around 406 nm excited at 324 nm, might have a relationship with defects in the SrY2O4 samples. So, the emission in SYO was considered due to presence of defects only. SYO itself is not a good luminescent material, however doping with different metal ions, it exhibits fascinating luminescent characteristics. In this section, downconversion and upconversion luminescent properties and various energy transfer mechanisms are discussed. The various photometric parameters of SYO phosphors are tabulated in Table 2.
3.1 Downconversion in SYO phosphors
When SYO is doped with Eu, Dy, Sm, Ce, Bi, and Tb, it exhibits downconversion spectra as shown in Fig. 3. The various factors affecting PL characteristics of doped SYO are discussed in this section.
3.1.1 SYO:Eu phosphors
Park et al. [12] prepared SrY2O4:Eu and SrGd2O4:Eu phosphors via combustion method. The excitation spectra of SrY1.98O4:Eu0.02 were obtained at two different emission wavelengths, i.e., 611 nm (having maximum at 270 nm) and 616 nm (having maximum at 255 nm). The excitation bands were attributed to the charge-transfer transition within the [O2−-Eu3+] with diffused reflectance spectra. It was seen that the emission spectrum at 270 nm excitation mainly consisted of peaks corresponding to 5D0–7F0, 5D0–7F1, and 5D0–7F2 transitions of Eu3+. The presence of two resolved 5D0–7F= lines at 580.4 and 581.8 nm in the emission spectrum were believed to be due to the existence of at least two Eu3+ sites in the host lattice. Also, it was interesting to note that different excitation lines showed different emission spectra. The emission lines of two different excitation wavelengths (270 nm and 255 nm) were labeled as A (Eu3+ ion occupying the Y site) and B (Eu3+ ion to occupy the Sr site). It was noticed that the emission intensities of site B were relatively increasing more rapidly than the site A as the concentration of Eu3+ was increased. It was suggested that Eu3+ ions in SYO occupied one Sr and two Y sites. At lower concentration (x = 0.0025), A site emissions dominated, whereas at higher Eu3+ (x = 0.15) concentration as the environment of two Y sites was changed the emission intensity of site B dominated. It was worth noting that apart from charge-transfer bands, each excitation spectra also consisted of an additional excitation band around 295 nm. The emission studies of the same samples showed a broad band emission around 470 nm in addition to Eu3+ emission lines which was too broad for emission from divalent Eu2+ emission. The studies of undoped SrY2O4 (reduced in the H2 stream) showed the blue emission could be due to some crystal defects introduced by the reduction, not to the divalent Eu2+. Further analysis showed that in the excitation spectra obtained by monitoring the B site emissions, the intensity of the defect band was comparable to that of the charge-transfer band. It was concluded that the energy transfer from the defect to Eu3+ ion in the B site might be more effective than that in the A site.
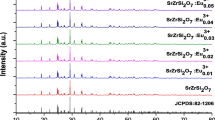
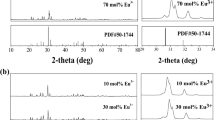
In another study, Zhou et al. [36] prepared SrR2O4:Eu3+ (R = Y, Lu) superfine phosphors with rugby-like shape by microemulsion approach. Upon exciting the samples at different exciting wavelength from 257 to 272 nm, SrY1.96O4:Eu3+0.04 showed strong emission at 595.2 nm and 618 nm, due to f-f transitions of Eu3+. It was worth noting that the purely magnetic transition appeared as five lines which were because of two sites of Eu3+ ions. By considering the different valence states and different ionic sizes of Sr2+ (113 pm) and Eu3+ (95 pm), as the calcination temperature was not high enough for Eu3+ to substitute Sr2+, and the replacement of Eu3+ to Y3+ (89 pm) happened. Hence, two sites of Y3+ ions with the similar dimension of O2− polyhedron in SrY2O4 lattice, led to the charge transfer from the oxygen ions to the substituting Eu3+. A similar splitting for the transition 5D0 to 7F2 was also observed. The excitation studies on SrLu1.96O4:Eu3+0.04 at 613 nm, 615 nm and 618.6 nm, respectively, gave the excitation bands at 257 and 269 nm. Upon excitation with 257 nm and 269 nm, five 5D0 to 7F1 transition peaks also indicated that the Eu3+ ions occupied two non-equivalent Lu3+ ion sites. The highest intensity of SrY2O4:Eu3+ and SrLu2O4:Eu3+ was found at doped Eu3+ concentration of about 9 mol% and 6 mol%, respectively. Higher Eu3+ beyond the optimum concentration of Eu ions led to PL quenching.
Atkins et al. [24] studied the spectroscopic properties of Eu3+-doped SrY2O4 under UV and VUV (vacuum ultraviolet) excitation. It was observed that peaks at 609 and 615 nm were sensitive to the excitation wavelengths. An opposite trend in peak intensity at 609 nm was observed under charge-transfer excitation at 250 nm and host lattice excitation at 150 nm. At 250 nm excitation, the intensity of the 609 nm emission line increased as Eu3+ content was increased, whereas it decreased for 150 nm excitation. These optical results again suggested the presence of two emission centers corresponding to the two Y sites in the host. By using host to activator energy transfer approach, they calculated that energy transfer was slightly more efficient to Y(2) site. It was also found that the 35% of the excitation energy was lost to the surface states. Zhang et al. [28] compared the PL properties of SrY1.98O4:0.02Eu3+ prepared via solid-state and sol–gel method. It was found that the excitation spectra (at 616 nm) consisted of two bands (242 and 262 nm) when Gaussian deconvoluted. The band at 242 nm was attributed to the charge-transfer band (CTB) of Eu3+ ions occupying Sr2+ sites and the peak at 265 nm to the Eu3+ occupying two Y sites. It was interesting to observe that only one band was found for 610 nm, which suggested that this band was due to the Eu3+ ions occupying Sr2+ sites. The excitation spectra of the samples derived through sol–gel and solid-state methods exhibited the same trend, except the bands at 324 and 406 nm were disappeared in case of sol–gel method due to the absence of defects produced at high temperature. The emission spectra at 324 nm excitation showed that the emission intensity increased with increase in the Eu3+ concentration but the intensity of band at 406 nm decreased. This was due to the energy transfer from the defects to the Eu3+ ions at Sr2+ ions. The phosphors were excited at vacuum UV excitation (147 nm). Both the sol–gel and solid-state method derived samples which exhibited the same emission spectra. However, higher PL emission was obtained for samples prepared by sol–gel method when compared to samples prepared by solid-state reaction. It was because the surface quality of the phosphor particles that plays a vital role in luminescence efficiency. The samples prepared by sol–gel method seemed to have uniform dispersion of particles. Hence, it demonstrated that morphology characteristics also influenced the PL properties to a great extent. But unlike in the UV region, the spectra in VUV region did not show much difference except for the luminescence intensity for phosphors prepared by solid-state reaction and sol–gel method.
In another study, Pavitra et al. [27] studied the luminescent properties of SrY2O4 (SYO):Eu3+ phosphors synthesized via sol–gel method. The photoluminescence excitation (PLE) spectra of the SYO:Eu3+ phosphors at different Eu3+ concentrations at the emission wavelength of 616 nm revealed the broad excitation band, also called charge-transfer band (CTB) and the sharp excitation peaks due to f–f transitions of Eu3+ ions. It was found that the CTB position shifted towards the lower energy side (higher wavelength) with increasing annealing temperature, indicating the decreased quantum efficiency of Eu3+ ions. This redshift was explained on the fact that electrons in O2− ions were less stable for the nano-scale particles and the surface to volume ratio was high as compared to the bulk sample; this increased the degree of disorder of the nanostructured system. Therefore, it required less energy to remove an electron from an O2− ion; as a result, the CTB shifted towards the lower energy side. The PL spectra showed that phosphors exhibited emission bands ascribed to transition of the Eu3+ ions. The maximum PL intensity was observed at 9 mol%, and above this concentration quenching followed. Koparkar et al. [35] synthesized SrY2O4:Eu3+ phosphors via aldo–keto gel method. The excitation spectra consisted of a broadband widening from 200 to 310 nm region of UV radiation attributed to the charge-transfer band of O2− to Eu3+ and some sharp lines at near UV–Visible region. The emission spectra showed a number of peaks in the range 500 to 700 nm corresponding to 5D0–7FJ (J=1, 2 and 3) transitions of Eu3+ monitored at 272 nm. The concentration of Eu3+ ion was varied in SrY2(1−x)O4:xEu (x = 0.001, 0.002, 0.005, 0.01, and 0.02). It was found that the emission intensity increased with the increase in Eu3+ concentration. It was ascribed to the fact that dopant did not alter its position in the lattices with increase in concentration. Also, the dipole moments of the Eu3+ were not affected by the increasing concentration.
Xu et al. [30] prepared both polycrystals and single crystals SrY2O4:0.01Eu3+ phosphors. Polycrystals were prepared by combustion method, while single-crystal fibers were grown in the air by the laser-heated pedestal growth (LHPG) method. The emission spectra of SrY2O4:0.01Eu3+ was recorded under two different excitations at 250 and 270 nm. It was found that under excitation at 270 nm, the emission peak intensity at 579.3 nm was comparable to the peaks near 616 nm, whereas under the excitation at 250 nm, the intensity was 20 times stronger than the peak at 581 nm. The authors extended their studies to SrY2O4:0.01Eu3+ single-crystal fibers to clarify the site occupation of Eu3+ doped in SrY2O4. The emission spectra were recorded under different excitation wavelengths at 240, 270, and 320 nm. It was observed that under excitation at 320 nm, peak at 578.1 nm was observed corresponding to 5D0 to 7F0 transition. It was concluded from the SrY2O4:Eu3+ single crystals that emission occurred from the three sites of Eu3+ ions; one at Sr2+ site and two at Y3+ sites, respectively. Wang et al. [20] prepared Eu3+-doped Sr(Gd,Y)2O4 powder samples via citric-gel method. It was found that the excitation spectra of SrY1.9Eu0.1O4 and SrY1.0G d0.9Eu0.1O4 were identical. The excitation bands around 149 and 204 nm were assigned to the host absorption, which was most likely to originate from the related charge-transfer band (CTB) of O2−–Y3+, as these became very weak in SrGd1.9Eu0.1O4. Other two bands were observed in the UV range with peaks at about 230 and 275 nm attributed to the charge-transfer transition from two different Eu sites in SrY2O4:Eu3+. The emission spectra of SrYxGd1.9−xEu0.1O4 was recorded at 147 nm excitation and three samples (SrY1.9Eu0.1O4, SrY1.0Gd0.9Eu0.1O4, and SrGd1.9Eu0.1O4,) had similar emission spectra except intensity. The emission spectra of SrYxGd1.9−xEu0.1O4 were composed of groups of several sharp lines corresponding to the transitions of Eu3+ ions. The calculated CIE coordinates for SrYxGd1.9−xEu0.1O4 at 147 nm were in red region. It was noticed that SrY1.9Eu0.1O4 had the highest emission intensity. This implied that the Eu3+ emission was not efficiently sensitized by Gd3+, which might be due to the narrow host bandgap and no efficient energy transfer occurred in the VUV region.
Fu et al. [31] compared the PL properties of blue and nanocrystalline SrY2O4:Eu phosphors. SrY2O4:Eu3+ nanocrystals were synthesized by PVA + glycine-assisted combustion method, whereas bulk SrY2O4:Eu3+ crystals were prepared by the solid-state method. It was observed that different excitation spectra were obtained at 611 and 616 nm for bulk SrY2O4:Eu3+. The excitation bands were ascribed to the CT from the 2p orbital of O2− to the 4f orbital of Eu3+ observed at 248, 257, and 270 nm. The CT bands in the SrY2O4:Eu3+ nanocrystals shifted to higher energies compared with those in bulk material. It was found that upon UV excitation, emission spectra exhibited characteristic peaks of Eu ions and also emission lines from higher 5D levels were also observed. It was found that in case of bulk SrY2O4:Eu3+, Eu3+ ions occupied three non-equivalent sites: one at the Sr site, two at Y(1) site and Y(2) site, while in nanocrystalline SrY2O4:Eu3+, the Eu3+ ions occupied only two non-equivalent sites, i.e., Y(1) and Y(2) site.
Singh et al. [25] prepared a series of red–orange light-emitting phosphors, MY2O4 (M = Mg, Ca, and Sr) doped with Eu3+ via combustion process using hexamethylenetetramine as an organic fuel and done calcination at 800 and 1100 °C. The excitation spectra for all the phosphors at 612 nm emission wavelength consisted of broad bands due to f-f transitions of Eu3+ ion. It was found that the emission intensity of SrY2O4:Eu3+ nanophosphor was maximum, while MgY2O4:Eu3+ had lowest luminescence intensity. This was considered due to possibility for Eu3+ (1.08 Å) ions to substitute Y3+ (1.04˚A) was more due to similarity in the ionic radii. While in case of Mg2+ (0.86 Å), chances for the substitution were less due to small ionic radius. Also, the substitution of Mg2+/Eu3+ was less likely to happen energetically because of different valence states. It was further observed that on increasing calcination temperature, the luminescence intensity of these materials enhanced.
Lojpur et al. [39] studied the temperature dependence emission spectra of Eu3+-doped SrY2O4 from 20 to 200 °C synthesized via sol–gel method. It was found that increase in temperature resulted in emission quenching. In the range from 20 to 200 °C, emission intensity was reduced by a factor of two. Two distinct emission peaks at 580.8 and 582.1 nm confirmed the two distinctive crystallographic environments for Eu3+ ions in the host lattice. This supported the assumption of two different Y3+ sites in the system. Also, the more intense emission at 611 nm than at 616.5 nm indicated the favored occupancy of Eu3+ at Y1 site. The emission intensity rapidly increased with concentration until the concentration of 3% and then decreased between 3 and 5%.
3.1.2 SYO:Tb phosphors
Pavitra et al. [27] studied the luminescent properties of SYO:Tb3+ phosphors synthesized via sol–gel method. The PLE spectra on monitoring at 543 nm of SYO:Tb3+ consisted of broadband from 220 to 350 nm with band maximum at 293 nm due to f–d transition of Tb3+. It was observed that peaks moved towards the lower energy side with increasing Tb3+ concentration due to the increased crystal field splitting with increasing Tb3+ concentration. The crystal field splitting was increased due to the shortening of the distance between the central metal (Y) ion and its ligands (O). The PL emission spectra at 296 nm for different Tb3+ concentrations consisted of peaks typical of Tb3+ ions. The first group of emission bands associated with the transition 5D3–7FJ (J=5,4, and 3) were found at 419, 437, and 459 nm. Whereas the second group of emission bands were placed at 485, 543, 586, and 623 nm belonging to the 5D4–7FJ(J=6,5,4 and 3) transitions. An intense green color was observed due to the magnetic dipole transition 5D4–7F5 at 543 nm. At higher Tb3+ concentration, the blue emission peaks were suppressed due to the cross-relaxation effect. The PL intensity decreased with increasing Tb3+ concentration at above 1 mol% due to concentration quenching.
In continuation of their study, Pavitra et al. [27] studied the effect of Eu3+ co-doping in SYO:Tb3+ phosphors. The deconvoluted excitation spectra of SrY2(1−(x+y))O4:2xTb3+/2yEu3+ (x and y = 0.5 and 1 mol%) at emission wavelengths of 616 nm (Eu3+) and 543 nm (Tb3+) consisted of peak centerd at 254 nm due to the CTB of Eu3+ ions and peak centered at 275 nm due to the spin-allowed transition between 7F6 ground state and 7DJ(=5,4,3,2,1) multiplets. The other peak centered around 294 nm was due to spin-forbidden transition between the 7F6 ground state and 9DJ(=6,5,4,3) multiplets of the Tb3+ ion. It was found that the excitation intensity of Eu3+ ions increased with increasing Eu3+ concentration at the rate of Tb3+ energy, which confirmed the efficient energy transfer from Tb3+ to Eu3+ ions. The energy transfer from Tb3+ to Eu3+ occurred due to the cross-relaxation process. When excited with 275 or 296 nm, the energy transfer to the 5F3 excitation levels of the Eu3+ ion took place and then it transferred non-radiatively to the 5D0 metastable state. On the other hand, as the emissions bands were overlapped well with the absorption bands which led to energy transfer occurred through the cross-relaxation process. It was found that adjusting the Tb and Eu concentration; white illumination was achieved. The CIE coordinates for 0.5 Tb3+/0.5 Eu3+, 0.5 Tb3+/1 Eu3+, 1 Tb3+/0.5 Eu3+, and 1 Tb3+/1 Eu3+ were calculated to be (0.406, 0.417), (0.407, 0.402), (0.391, 0.406), and (0.401, 0.413), respectively. It was also found that for excitation wavelength at 281 nm, 0.5, and 1 mol% Eu3+ ions co-doped with 0.5 mol% Tb3+:SYO sample, the Eu3+ emission intensity was very high compared to Tb3+ emission intensity due to the high energy transfer efficiency.
Further, Pavitra et al. [27] studied the energy transfer mechanism in Sm3+ co-doped SYO:Tb3+ phosphor. It was observed that excitation spectra of Tb3+- and Tb3+/Sm3+-doped SY phosphors at 543 nm (Tb3+) exhibited similar excitation peaks with a strong broadband due to 4F7–5D1 centered at 296 nm. Whereas the excitation spectra of Tb3+/Sm3+ activated SrY2O4 phosphors at 606 nm (Sm3+) revealed weak HAB of Sm3+, f–d transition of Tb3+ in the high energy region and strong f–f transitions in the lower energy region. It was observed that the emission band edge of Tb3+ overlapped with Sm3+ emission (4G5/2–6H7/2) when excited with 606 nm. It was found that all excitation spectra of SYO at different emission wavelengths showed a common excitation band at 380 nm due to 7F6–5D2 of Tb3+, and 6H5/2–4K13/2 of Sm3+. The emission spectra consisted of blue and green emission bands of Tb3+ ions including weak Sm3+ transitions upon excitation at 296 nm wavelengths. The CIE chromaticity coordinates changed from (0.315, 0.505) to (0.328, 0.451), under different excitation wavelengths. The emission spectra at 380 nm wavelengths covered the total visible spectra due to the presence of both Tb3+ and Sm3+ emission lines, resulting in white emission. It was observed that blue emission lines showed some reasonable intensity in case of SYO:Tb,Eu as compared to SYO:Tb. This indicated the efficient energy transfer from Tb3+ to Sm3+. The white light emission was also observed by adjusting the Sm3+ and Tb3+ concentrations and excitation wavelength.
Taikar [18] studied singly doped SrY2O4 phosphors activated with Tb3+. The excitation spectrum exhibited transitions related to f orbital of Tb3+. The emission spectra of SrY2O4:Tb3+ showed both blue and green emissions due to transitions from 5D3–7Fj and 5D4–7Fj state. The CIE coordinates for SrY2O4:Tb3+ were found to be (0.211, 0.518) with color purity of 62.54%. Zhang et al. [17] explained that the emission intensity of 5D3 decreased with increasing Tb3+ concentration, accompanied by the enhancement of the emission from the 5D4 level due to the cross-relaxation via the resonant energy transfer process: Tb3+ (5D3) + Tb3+ (7F6) → Tb3+ (5D4) + Tb3+ (7F0). In order to support the cross-relaxation phenomena, the critical distance between Tb–Tb ions was calculated. This was because the energy transfer results from the exchange interaction, the critical distance between the sensitizer and activator should be shorter than 4 Å. However, in their study, the critical distance was found to be 21.38 Å, which was much higher than 4 Å, indicating little possibility of cross-relaxation via the exchange interaction mechanism. This led to the conclusion that cross-relaxation between the Tb3+ ions mainly took place via electric dipole–dipole interactions.
3.1.3 SYO:Sm phosphors
Pavitra et al. [27] studied the luminescent characteristics of Sm-doped SYO phosphors synthesized via sol–gel method. The PLE spectra consisted of series of peaks of Sm3+ annealed at 1300 °C when monitored at emission wavelength at 608 nm. The PLE spectra showed a weak host absorption band (HAB) in the shorter and longer wavelength regions. The Sm3+ activated SYO emission under a UV lamp (at 408 nm) exhibited a considerable similarity in the emission color of Sm3+ and Eu3+ in SrY2O4 host lattice. The emission spectra of Sm3+ showed reasonable emission at 566 nm due to 4G5/2–6H5/2 transition, strong, and efficient emission at 606 nm due to 4G5/2–6H7/2 transition, and weak emission at 658 nm due to 4G5/2–6H9/2 transition. Taikar [18] studied singly doped SrY2O4 phosphors activated with Sm3+ synthesized via combustion method. The excitation spectrum gave good overlap in near-UV region and hence on excitation showed orange emission (excited at 409 nm) having high quantum efficiency (75.3%). The CIE coordinates for SrY2O4: Sm3+ were found to be (0.575, 0.425).
3.1.4 SYO:Dy phosphors
Zhang et al. [17] showed that the emission ratio (Y/B) of Dy3+ could be used as a probe to detect the local symmetry of the activator ions as Dy3+ (Y3+) ions have two non-equivalent sites and six oxygen atoms coordinate both sites with Cs symmetry exhibiting an asymmetrical chemical environment. Therefore, the yellow emission dominated the blue emission in the emission spectra. The maximum PL was found for 0.005 and 0.007 concentrations of Tm and Dy ions, respectively, which are of very small concentration values. This was because of short Y–Y bond distance due to the double octahedral of Y2O42− in SrY2O4 compound. It was observed that the concentration quenching of Dy3+ was mainly caused by cross relaxation, i.e., energy transfer among Dy3+ ions via Dy3+ (4F9/2) + Dy3+ (6H15/2) → Dy3+ (6F3/2) + Dy3+ (6F11/2) mechanism.
Pavitra et al. [15] also studied the photoluminescence in Dy3+-doped SrY2O4 phosphors synthesized via citrate sol–gel method. The PL spectra at 578 nm emission, consisted of the charge-transfer state (CTS) between 257 and 307 nm and f–f transitions at 325 nm (6H15/2–6P3/2), 351 nm (6H15/2–6P7/2), 365 nm (6H15/2–6P5/2), 387 nm (6H15/2–4I13/2), 426 nm (6H15/2–4G11/2), and at 451 nm (6H15/2–4I15/2). The emission studies of the phosphors at 351 nm excitation revealed three typical peaks at 488 nm (blue) (4F9/2–6H15/2), 578 nm (yellow) (4F9/2–6H13/2) and 678 nm (red) (4F9/2–6H11/2) of Dy3+ ions. It was reported that Dy-activated SYO nanocrystalline phosphors emitted cool white light. The domination of yellow band over the blue band signified that Dy3+ ions occupied the low symmetry sites. The optimum concentration was found at 1 mol%. Pavitra et al. [15] monitored the luminescent characteristics of SrY2O4:1Dy3+/1Eu3+ nanocrystalline phosphors. The excitation spectra monitored at 578 nm (4F9/2–6H13/2) and 615 nm (5D0–7F2) consisted of broad excitation band with band maxima at 271 nm in the shorter wavelength region, and some sharp excitation peaks in the longer wavelength region due to the f–f transitions of Dy3+ and Eu3+ ions. It was observed that the intensity of excitation peaks were high when monitored under 578 nm as compared to 615 nm. The deconvoluted broad excitation band of the SrY2O4:1Dy3+/1Eu3+ co-activated nanocrystalline phosphor showed three bands using Gaussian fitting. The band centered at 232 nm was HAB and this band occurred due to Dy3+ ions. The remaining two bands with band maxima located at 264 and 275 nm were the CTBs of Eu3+ ions due to the presence of two Y sites in the SrY2O4 host lattice. The PL emission spectra of SrY2O4:Dy3+/Eu3+ nanocrystalline phosphor were studied as a function of Eu3+ ion concentration for a fixed 1 mol% of Dy3+ ion by exciting at 381 nm. The PL emission spectra showed various emission peaks originating from 5DJ(J=0,1,2,3) levels of Eu3+ ions. It was observed that blue and yellow emission bands of Dy3+ ions were split into doublets. The blue emission band splitted into two peaks occurring at 466 and 490 nm, and the yellow emission band was split into two intense peaks occurring at 578 and 590 nm. With the increase in the Eu3+ ion concentration (i.e., 1:2, 1:3 ratios), the emission peak intensity corresponding to the hypersensitive electric dipole transition (5D0–7F2) of Eu3+ ions at 614 nm exhibited a systematic enhancement due to the energy transfer from Dy3+ to Eu3+ ions.
3.1.5 SYO:Er phosphors
Pavitra et al. [16] prepared SrY2O4:Er3+ nanocrystalline phosphors using citrate sol–gel process. PL excitation (PLE) spectra of SrY2O4:Er3+ nanocrystalline phosphors at emission wavelength of 548 nm (4S3/2–4I15/2), consisted of intra 4f transitions of Er3+ ions in the longer wavelength region. Upon excitation at 378 nm (4I15/2–4G11/2), the emission spectra consisted of peaks at 525 (2H11/2–4I15/2) and 548 nm (4S3/2–4I15/2). A small feeble peak at 660 nm (4F9/2–4I15/2) was also observed. The optimum concentration of Er3+ ions was 1 mol% and then concentration quenching occurred. The CIE chromaticity coordinates for the prepared phosphors lie in the green region.
In another study, Pavitra et al. [16] prepared the Dy3+/Er3+ ions co-doped SYO phosphors showing yellowish green emissions. The PLE spectra of SrY2O4:1 mol%Dy3+/1 mol% Er3+ nanocrystalline phosphors at 548 (4S3/2–4I15/2) and 578 nm (4F9/2–6H13/2) were monitored. It was found that when the SrY2O4:1Dy3+/1Er3+ were excited at 548 nm, the spectrum consisted of only sharp excitation peaks in the longer wavelength region corresponding to the f–f transitions of Er3+ ions. Similarly, when SrY2O4:1Dy3+/1Er3+ sample was excited at 578 nm, the spectrum involved HAB in the shorter wavelength region. This was attributed to the efficient energy transfer from the host to the Dy3+ ions. Also, some sharp excitation peaks due to f–f electronic transitions for Dy3+ ions were also observed. In both spectra, a common excitation peak was observed at 365 nm due to electronic transitions of Dy3+ ions (6H15/2–6P5/2) and Er3+ ions (4I15/2–4G9/2). Thus, it was observed that co-doped sample can be efficiently excited by near-UV excitation at 365 nm. The PL emission spectra of SrY2O4:Dy3+/Er3+ consisted of two intense emission bands in the blue and yellow regions at 489 nm and 578 nm and one weak emission band in the red region at 678 nm due to Dy3+ ions. Similarly, the Er3+ ions gave two intense emission bands in the green region and one weak band in the red region. By increasing the Er3+ ion concentration, it was found that the emission peak intensity corresponding to the (4S3/2–4I15/2) transition increased due to the improved energy transfer from Dy3+ to Er3+ ions. The energy transfer efficiencies for the co-doped samples by varying the Er3+ concentration and keeping Dy3+ concentration constant were calculated to be 13.34, 22.09, and 34.76% for 1Dy3+/1Er3+, 1Dy3+/1.5Er3+, and Dy3+/2Er3+, respectively. The CIE chromaticity coordinates for the co-doped samples lie in fair white light region.
3.1.6 SYO:Ce phosphors
Blasse and Bril 1967 [42] studied SrY2O4-Ce phosphors prepared by firing intimate mixtures of high-purity oxides at appropriate temperatures in nitrogen. The fluorescence emission in the case of oxidic host lattices had peaks in the near UV due to the exceptionally large stokes shift of the emission. It was reported that such a large Stokes shift was observed due to the difference between the equilibrium distance of the excited state and that of the ground state was high. For SrY2O4–Ce, it was observed that the light output at 200 K was only 20% as that at l00 K. In another study, Manivannan et al. [43] prepared Ce–SrY2O4 by conventional ceramic method in the hydrogen environment. The samples showed blue and green emission band typical of Ce3+ 5d–4f transitions. It was interesting to note that blue emission band was absent in the air synthesized samples. Also, the decay time of blue band as a function of temperature was single exponential with a decay constant of about 37 ns and was independent of temperature between 10 and 300 K. These observations strongly pointed to the trivalent cerium as being responsible for the blue emission band. The green emission was also observed which was attributed to the presence of Ce4+–O2− charge-transfer transition in Sr2CeO4. It was further found that the decay time of the charge-transfer state in Sr2CeO4 was long (65 ms). With an increase in temperature from 5 to 25 K, the emission intensity increased, beyond which it decreased. Similar results of emission spectra of SrY2O4:Ce3+ phosphors were reported by Philippen et al. [40].
Taikar [18] studied the luminescent properties of Ce3+-doped SrY2O4 phosphors synthesized via combustion method. The excitation spectra of SrY2O4:Ce3+ exhibited broad band at 345 nm which was attributed to transitions from ground state of 4f1 configuration to states of 5d1 configuration of typical Ce3+ ion. Under 345 nm excitation, emission spectra consisted of broadband extending from 390 to 590 nm with a peak at around 460 nm and shoulder around 505 nm. The emission peaks were attributed to the transition from the 5d level to the ground state (2F5/2–2F7/2) of the Ce3+ ions. The Gaussian deconvolution of the emission band gave two well-separated Gaussian components with maxima at 460 nm (21,739 cm‒1) and 505 nm (19,801 cm‒1). It was found that Gaussian component curve on an energy scale was in good agreement with the theoretical difference between the 2F5/2 and 2F7/2 levels (about 2000 cm‒1). The color coordinates for Ce3+-doped SrY2O4 phosphor were found to be (0.1526, 0.2069).
3.1.7 SYO:Bi phosphors
Taikar [18] studied the luminescent properties of Bi3+-doped SrY2O4 phosphors. The excitation spectrum of SrY2O4:Bi3+ consisted of small peak around 240 and highly intense excitation peak at 330 nm. The 330 nm peak was attributed to 1S0–3P1 transition of Bi3+ ion. At 330 nm excitation, PL emission spectra consisted of broadband ranging from 350 to 530 nm with peak maxima around 420 nm. The emission band was attributed to 3P1, 3P0–1S0 transition of Bi3+ ion. The color coordinate for Bi3+-doped SrY2O4 phosphor were found to be (0.1582, 0.035).
Wei et al. [22] studied the energy transfer mechanism in SrY2O4:Bi3+,Eu3+ (SYO:Bi3+,Eu3+) phosphors. Firstly, the concentration of Bi ions was varied in the host lattice. The excitation spectra consisted peak of 330 nm (1S0–3P1 transition of Bi3+) when monitored at 410 nm. Then, under excitation at 330 nm, the emission spectra consisted of emission peak at 410 nm originating from 3P1–1S0 transition of Bi3+ ions. The maximum PL was found for 0.024 of Bi ions. To this optimized sample (SYO:0.024Bi3+), Eu ions were co-doped and the maximum intensity was found for 0.32 of Eu ions. Under excitation at 330 nm, the emission spectra of SYO:Bi, Eu phosphors consisted of emission peaks in the blue and as well as in red region ranging from 550 to 720 nm. It was noted that the energy transfer efficiency from Bi3+ to Eu3+ was found to be 92.65%. A tunable emission was achieved from blue (0.179, 0.266) to red (0.620, 0.339) in SYO:Bi3+,Eu3+ by changing the concentration of Bi3+ and Eu3+. It was also observed that emission intensity of the phosphors at 155 °C was maintained over 98% emission intensity of that at 35 °C. In continuation of their earlier studies, Wei et al. [34] also studied the temperature-dependent emission behaviors of SYO:0.024Bi3+, 0.09Eu3+ under excitation at 330 nm. It was observed that Bi3+ and Eu3+ showed significantly different temperature-dependent emission behaviors. The luminescent intensity of Bi3+ increased, whereas Eu3+ weakened gradually when temperature was increased from 313 to 563 K. The CIE color coordinates changed from (0.4268, 0.2270) to (0.2281, 0.1157) with enhancing temperature from 313 to 563 K.
3.2 Upconversion in SYO phosphors
3.2.1 SYO:Er phosphor
Mukhopadhyay et al. [44] studied the upconversion luminescent properties of Er-doped SrY2O4 phosphors synthesized via chemical co-precipitation method. Under laser excitation at 980 nm, the emission spectra consisted of peaks at 490 nm, 525 nm, 550 nm, 667 nm, and 798 nm corresponding to the 4F7/2–4I15/2, 2H11/2–4I15/2, 4S3/2–4I15/2, 4F9/2–4I15/2 and 4I9/2–4I15/2 transitions of Er3+ ions, respectively. The maximum PL was obtained for 5 mol% of Er ions. It was found that two photons accompanied the UC process. The CIE coordinates of the SYO:Er phosphors were found to be (0.23, 0.73), lying in the pure green region.
3.2.2 SYO:Er, Yb phosphors
Shen et al. [33] studied the luminescent properties of SrY2O4:Er, Yb under 1550 and 980 nm laser excitations. The UC emission spectra under excitation at 1550 nm consisted of peaks in the regions of 510–542 nm, 543–570 nm, and 634–681 nm, of Er3+ ions, respectively. The different transitions and energy transfer involved in SYO:Er, Yb under 1550 nm excitation are shown in Fig. 4. Similar results for the UC spectra at 980 nm excitation were found but different relative intensities. The emission studies showed that maximum red and green emission intensity under excitation at 980 and 1550 nm was found for SrY2O4:0.12Er, 0.01Yb sample. The red to green emission intensity ratio varied as a function of pumping current, at 980 and 1550 nm excitation. It was observed that the said ratio increased with increasing Yb3+ ion concentration but decreased as the excitation power increased under 980 nm and 1550 nm pumping. The change in red to green emission intensity ratio was also higher at 1550 nm excitation than at 980 nm excitation.
Schematic diagram of SYO:0.12Er,0.01Yb/SYO:0.12Er,0.08Yb energy levels [33] (with permission)
3.2.3 Tri-doping in SYO
In another study, Pavitra et al. [16] studied the luminescent features with tri-doping of Er3+/Dy3+/Sm3+ ions in SrY2O4 host lattice. The PLE spectra of 1Dy3+/1Er3+/1Sm3+ triple-doped SrY2O4 nanocrystalline phosphors at different emission wavelengths of 578 nm (Dy3+), 561 nm (Er3+), 548 nm (Er3+), and 606 nm (Sm3+) exhibited host absorption band in shorter wavelength region along with respective sharp excitation bands due to f–f transitions of Dy3+, Er3+, and Sm3+ ions in the longer wavelength region. It was found that a common excitation peak around 365 nm was observed in all PLE spectra, so 365 nm was used as an excitation wavelength. The PL emission spectra of 1Dy3+/1Er3+/xSm3+ (x = 0.25, 0.5, 1, and 2 mol%) ions triple-doped SrY2O4 nanocrystalline phosphors as a function of Sm3+ ion concentration under excitation at 365 nm exhibited all three RE ions (Dy3+, Er3+, and Sm3+) emission bands, suggesting that the availability of triple co-doped ions in the host lattice. The PL intensity increased with increased concentration of Sm ions. The emission peak intensity corresponding to the (4G5/2–6H7/2) transition of Sm3+ ions was increased, which was attributed to the energy transfer from 4F9/2 energy levels of Dy3+ ion and 4F7/2 level of Er3+ to 4G5/2 level of Sm3+ ions. The energy transfer efficiencies for 1Dy3+/1Er3+/0.25Sm3+, 1Dy3+/1Er3+/0.5Sm3+, 1Dy3+/1Er3+/1Sm3+, and 1Dy3+/1Er3+/2Sm3+ were calculated to be 14.37, 33.68, 63.49, and 69.32%, respectively. PL analysis showed that the triple-doped SrY2O4 phosphors exhibited a fair white light emission and the CIE chromaticity coordinates were lying in warm-white light region.
Further, Pavitra et al. [45] studied the luminescent properties of tri-doped (Er, Tm, Tb) SrY2O4 nanocrystalline phosphors. The UC emission spectra consisted of blue emission band at 487 nm due to Tm3+ ions, green emission bands at 526 nm and 551 nm due to Er3+ ions and red emission band at 664 nm due to the Er3+ and Tm3+ ions, respectively. The various transitions and enrgy transfer mechanisms involved in the UC process of Er3+, Tm3+ and Yb3+tri-doped SYO are shown in Fig. 5. It was observed that when pump power was changed from 100 to 300 mW, the red emission band at 664 nm was dominant in the spectra. With further increase in pump power from 300 to 900 mW, the blue emission intensity increased gradually. This occurred due to possible energy transfer between the Er3+ and Tm3+ ions. When the Er3+/Tm3+/Yb3+ ions were tri-doped, the 4I11/2 level of the Er3+ ions and the 3H5 level of the Tm3+ ions were populated by the energy transfer from the 2F5/2 level of the Yb3+ ions, there was a chance for an energy transfer from the 4I11/2 (Er3+) level to the 3H5 (Tm3+) level by phonon assistance.
Energy level diagram of Er3+, Tm3+ and Yb3+ tri-doped SYO nanocrystalline phosphors [45] (with permission)
4 Outlook and summary
In conclusion, it is observed that SYO is a promising host lattice for doping different rare and non-rare earth elements. The chemical and thermal stability makes it a prestigious host lattice in the family of AB2O4 oxides. SYO is synthesized via different routes such as solid-state, sol–gel, combustion, microemulsion and aldo–keto methods. Out of these, citrate sol–gel method is found to be effective than other methods. The SYO phosphors obtained by this method are monodisperse, less aggregated and having close-packed structures. SYO on doping with different elements give different emission spectra under different excitation wavelengths in UV and vacuum UV excitation. In addition to this, SYO phosphors are more appealing due to their high water-resistant and unique luminescence characteristics. However, there are challenges in the synthesis of SYO. The methods as mentioned above, are either high temperature routes or proceed via complicated routes. It is also observed that the synthesized materials have impurities of SrO and Y2O3 upto some extent. The synthesized phosphors are generally microcrystalline in nature and agglomerated. So, it is necessary to find the effective synthesis routes to synthesize nanocrystalline, monodisperse and phase pure SYO phosphors. The use of surfactants can be done to avoid the agglomeration and synthesis of particles in the nanoregime. Moreover, the morphology can be engineered to alter the luminescent properties. The crystal structure of SYO needs to be refined by some refinement tools to study the effective occupancy of ions at sites. To the best of our knowledge, there are very few studies reported in this context. From PL results, it is observed that there are different sites in the SYO lattice. However, the percentage occupancy of different sites and their relation with luminescent behavior are not studied. Also, the local environment of the activators in SYO crystal field is not studied on theoretical grounds. The photometric parameters such as CIE coordinates, CCT, CRI, decay curves, quantam yield, and activation energies can be studied in detail. The reports which deal with the SYO are generally related to the doping of the rare earth elements. However, doping with non-rare earth elements such as Mn2+/Mn4+, Cr3+, and Cu2+ are not studied. In addition to this, co-doping with non-rare earth elements such as alkali and alkaline earth metals can be done to improve the luminescent characteristics further.
References
N. Hirosaki, R. Xie, K. Inoue, T. Sekiguchi, B. Dierre, Appl. Phys. Lett. 2, 061101 (2007)
G. Li, X. Xu, C. Peng, M. Shang, D. Geng, J. Chen, J. Lin, Fluoresc. Lumin. Mater. 19, 3020 (2011)
C.S. Kamal, T.K.V. Rao, T. Samuel, P.V.S.S.S.N. Reddy, J.B. Jasinski, Y. Ramakrishna, M.C. Rao, K.R. Rao, RSC Adv. 7, 44915 (2017)
A.A. Talin, K.A. Dean, J.E. Jaskie, Solid State Electron. 45, 963 (2001)
P.H. Holloway, T.A. Trottier, B. Abrams, C. Kondoleon, S.L. Jones, J.S. Sebastian, W.J. Thomest, J. Vac. Sci. Technol. B 17, 758 (1999)
A. Manohar, C. Krishnamoorthi, J. Alloys Compd. 722, 818–827 (2017)
J. Singh, J. Manam, Ceram. Int. 44, 10912–10920 (2016)
R.W. Grimes, A.B. Anderson, A.H. Heuerg, J. Am. Soc. 111, 1 (1989)
J. Choisnet, M. Hervieu, B. Raveau, P. Tarte, J. Solid State Chem. 45, 280 (1982)
K. Kurosaki, T. Tanaka, T. Maekawa, S. Yamanaka, J. Alloys Compd. 395, 318 (2005)
K. Kurosaki, T. Tanaka, T. Maekawa, S. Yamanaka, J. Alloys Compd. 398, 304 (2005)
S.-J. Park, C.-H. Park, Yu Byung-Yong, H.-S. Bae, C.-H. Kim, C.-H. Pyun, J. Electrochem. Soc. 146, 3903 (1999)
S.P. Ghorpade, N.R.P. De, R. Soc. Chem. 10, 21049 (2020)
V. Dubey, J. Kaur, S. Agrawal, N.S. Suryanarayana, K.V.R. Murthy, Optik (Stuttg) 124, 5585 (2013)
E. Pavitra, G. Seeta Rama Raju, W. Park, J.S. Yu, New J. Chem. 38, 163 (2014)
E. Pavitra, G.S.R. Raju, J.S. Yu, J. Alloys Compd. 592, 157 (2014)
Y. Zhang, D. Geng, M. Shang, X. Zhang, X. Li, Z. Cheng, H. Lian, J. Lin, Daltan Trans. 42, 4799 (2013)
D.R. Taikar, J. Lumin. 204, 24 (2018)
C.M. Mehare, Y.R. Parauha, N.S. Dhoble, S.J. Dhoble, J. Mol. Struct. 2, 127957 (2020)
D. Wang, Y. Wang, L. Wang, J. Lumin. 126, 135 (2007)
V. Lojpur, S. Stojadinović, M. Mitrić, Sci. Sinter. 50, 347 (2018)
R. Wei, Z. Zheng, Y. Shi, X. Peng, H. Wang, X. Tian, F. Hu, H. Guo, J. Alloys Compd. 767, 403 (2018)
Z. Fu, S. Zhou, S. Zhang, J. Opt. Soc. Am. B 23, 1852 (2006)
R. Atkins, A.L. Diaz, J. Lumin. 128, 1463 (2008)
D. Singh, V. Tanwar, S. Bhagwan, V. Nishal, S. Sheoran, S. Kadyan, A.P. Samantilleke, P.S. Kadyan, Indian J. Mater. Sci. 2015, 1 (2015)
R.K. Tamrakar, K. Upadhyay, J. Electron. Mater. 47, 651 (2018)
E. Pavitra, G.S.R. Raju, Y.H. Ko, J.S. Yu, Phys. Chem. Chem. Phys. 14, 11296 (2012)
J. Zhang, Y. Wang, J. Mater. Res. 25, 2120 (2010)
V. Singh, K. Swapna, S. Kaur, A.S. Rao, J.L. Rao, J. Electron. Mater. 5, 11296 (2020)
W. Xu, W. Jia, I. Revira, K. Monge, H. Liu, J. Electrochem. Soc. 148, H176 (2001)
Z. Fu, S. Zhou, Y. Yu, S. Zhang, J. Phys. Chem. B 109, 23320 (2006)
T. Opravil, P. Ptáček, F. Šoukal, E. Bartoníčková, J. Wasserbauer, J. Therm. Anal. Calorim. 123, 181 (2016)
X. Shen, M. Xing, Y. Tian, Y. Fu, Y. Peng, X. Luo, J. Rare Earths 34, 458 (2016)
R. Wei, J. Guo, K. Li, L. Yang, X. Tian, X. Li, F. Hu, H. Guo, J. Lumin. 216, 116737 (2019)
K.A. Koparkar, N.S. Bajaj, S.K. Omanwar, Int. J. Lumin. Appl. 5, 134 (2015)
L. Zhou, J. Shi, M. Gong, Mater. Lett. 59, 2079 (2005)
S.P. Ghorpade, R.H. Krishna, R.M. Melavanki, V. Dubey, Opt. Int. J. Light Electron Opt. 208, 164533 (2020)
L. Zhou, J. Shi, M. Gong, J. Lumin. 113, 285 (2005)
V. Lojpur, Z. Antic, M.D. Dramicanin, R. Soc. Chem. 35(15), 4428–4435 (2014)
J. Philippen, C. Guguschev, D. Klimm-Leibniz, J. Cryst. Growth 459, 17 (2018)
E.B. Shaik, S. Rajyalakshmi, M.S.S. Dharmaja, B.V. Sangeetha, R.S. Lakshmi, Y.R. Krishna, K.R. Rao, AIP Conf. Proc. 1992, 3 (2018)
G. Blasse, A. Bril, J. Chem. Phys. 47, 5139 (1967)
V. Manivannan, H.A. Comanzo, A.A. Setlur, A.M. Srivastava, J. Lumin. 103, 635 (2003)
L. Mukhopadhyay, V. K. Rai, in 2018 3rd Int. Conf. Microw. Photonics (ICMAP 2018), 9–11 February (2018).
E. Pavitra, G. Seeta-Rama-Raju, J.-H. Oh, J.S. Yu, R. Soc. Chem. 38, 3413 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors claim no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priya, R., Kaur, S., Sharma, U. et al. A review on recent progress in rare earth and transition metals activated SrY2O4 phosphors. J Mater Sci: Mater Electron 31, 13011–13027 (2020). https://doi.org/10.1007/s10854-020-03930-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03930-6