Abstract
This paper reports the synthesis, structural, thermal and luminescence properties of Sr1−xZrSi2O7:xEu3+ (x = 0.01 ≤ x ≤ 0.05 mol) phosphors by solid-state reaction method in air. The optimal Eu3+ ion concentration in Sr1−xZrSi2O7:xEu3+ phosphor is 0.04 mol. The monoclinic crystal structure with P21/c space group was confirmed by the powder X-ray diffractometry (PXRD) technique. Thermal behavior of the present phosphors was investigated which shows better characteristics. Under 396 nm excitation, Eu3+ ions activated Sr1−xZrSi2O7 phosphors exhibited a strong red emission centered at 617 nm due to the f–f transition of 5D0 → 7F2 transition. The critical doping concentration of Eu3+ ion was x = 0.04 mol and the critical distance was determined as 19.1117 Å. The energy transfer among Eu3+ ions in Sr1−xZrSi2O7 phosphors was found to be a dipole–dipole interaction. Consequently, optimal phosphor shows a thermal stability up to 420 K, superior to that in analogous reports. And the quantum efficiency of prepared Sr1−xZrSi2O7:xEu3+ phosphor with 396 nm excitation was calculated to be nearly 72%. The photometric results indicate that the synthesized Orange-Red phosphor can be potentially applicable for solid-state lighting and display devices applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last decades, phosphors have become key importance of technological development. Many devices have lighting units that are made by LED [1]. This LED has a basic principle of phosphors that are adjuvant with the luminescent properties of the rare earth (RE) ions doped in the selected host materials [2]. Thus, the type of RE ions and features of the host materials are the main attention during the development of phosphor materials. Due to its vast range of possible applications in solid-state lighting, full-colour display systems and white light-emitting diodes (WLEDs) recently gained great attraction. The phosphor-converted light-emitting diode (pc-LED) technology is a renowned solid-state lighting method [3].
The host lattice and the sort of doped ion has a crystal structure which is recognized to have a substantial influence on the luminescence properties of phosphors [4]. Phosphors with a silicate host are the most useful luminescent material, with several advantages like inexpensive cost, higher luminous efficiency, and a simple production technique. Furthermore, RE doped silicates are known for their charge stability, high thermal stability, and low sintering temperatures, all of which have sparked interest [5]. Zirconium silicates construct an essential class due to their magnificent optical properties associated with photothermal stability and low thermal conductivity. furthermore, zirconia possess low phonon energy and it has been catchy for use as support for RE ions, since the transition possibilities are increased, creating this material fascinating for different applications. [6]. Some zirconium silicate phosphors are reported as the potential for white LEDs application such as CaZrSi2O7:Eu3+ [7]; BaZrSi3O9:Eu3+ [8]; and Ca2ZrSi4O12:Eu3+ [9] have been proved as a good luminescence material to exhibit excellent optical properties.
In this study, we have chosen strontium zirconium silicate [(SrZrSi2O7)–SZSO] as a host matrix. A SZSO phosphor would be ideal from the manufacturing point of view, because the raw materials are abundant and are relatively inexpensive. The SZSO crystal was first time reported by Huntelaar et al. [10] and then Blasse et al. [11] and other researchers subsequently studied the ultraviolet (UV) photoluminescence (PL) of SZSO. The luminescent emission color of phosphor usually depends on types of doped RE ions, which are highly affected by the host [12, 13]. The arrangement of host can affect the crystal field environment of RE ions, bringing about the energy level transition change of RE ions [14,15,16]. As the activators commonly used of trivalent rare-earth (RE3+) ions due to their advantages of narrow emission band in visible range based on the inherent transition properties, resultant high efficiency and high lumen obtain. It is distinguished that Eu3+ is a excellent dopant and has a superior emission spectrum in reddish region [17,18,19,20]. Thus, it is essential to choose a good host material to synthesize orange–red phosphor used for white LEDs excited by Near Ultra–Violet (NUV).
To the best of our knowledge, before this work Sr1−xZrSi2O7:xEu3+ (S1−xZSO:xEu) phosphor has not been reported, therefore, in this research work, structural, thermal and PL properties of S1−xZSO:xEu phosphors have been prepare by solid state reaction method. The luminescence characteristics of the discussed phosphor according to the change in the concentration and effect of the activator ions has been studied. In addition, we also explored the photometric properties [Commission Internationale Eclairage (CIE), Color rendering index (CRI), Color Correlated Temperature (CCT), Color Purity (CP) and Quantum Efficiency (QE)] of the discussed materials. The prepared Eu3+ doped SZSO phosphors to explore orange–red emitting phosphor that may be applicable for solid-state lighting and display devices.
2 Experimental
2.1 Materials preparation
The Sr1−xZrSi2O7:xEu3+ (x = 0.01 ≤ x ≤ 0.05 mol) were prepared in air by the solid-state reaction technique using high temperature programmable furnace. The highly pure oxides of SrCO3, ZrO2, SiO2, H3BO3 and Eu2O3 with proper stoichiometric proportions was weighted to the normal composition, and agate mortar and pestle were used for grinding the mixture thoroughly; pre-heated at 1000 °C for 1 h and then heated at 1450 °C for 4 h in Al2O3 crucibles. A small quantity of H3BO3 was added as flux. After completion of the process of sintering, the temperature of programmable furnace is cooled down to room temperature. The synthesized phosphors were ground to obtain fine powder. The sample code of synthesized powder products has tabulated in Table 1. The chemical reaction used for stoichiometric calculation is:
2.2 Measurement techniques
The PXRD experiments were performed using a Bruker D8-Advance XRD (operated in 40 kV and 20 mA) diffractometer with CuKα radiation in the 2θ range of 10–80° in steps of 0.02. The PXRD patterns obtained were then compared with the standard JCPDS files no. 82–1206. Rietveld refinement was performed by using Full Prof software. In the calculations made, a pseudo-voigt type shape of the peaks was adopted and the graphical interface Win PLOTR. For the analysis of thermal behavior, TGA/ DSC have been carried out separately using TGA2/DSC3 by METTLER TOLEDO, respectively. A baseline was measured with an empty crucible after that the well mixed reactants are placed at alumina crucible. In each measurement approx ~ 0.05 g and ~ 0.5 g of prepared materials were used for DSC and TGA, respectively. The powders were heated under nitrogen gas (70 mL/min) from 40 to 1500 °C with heating rate of 20 K/min. The FESEM (FE-SEM, XL30, Philips) was used for imaging of surface morphology of the prepared sample and the elemental (qualitative and quantitative) analysis was also investigated by the Energy Dispersive X-Ray Spectroscopy (EDS). The combination, composition, purity, vibrational properties and other impurity of all of the functional and finger print groups of synthesized compounds was observed by FTIR, Alpha-II ECO ATR, BRUKER. The PL spectra of the prepared powders are recorded by using a spectrofluorometer Shimadzu (RF 5301-PC). Decay curve was measured by fluoro max-4cp_1715D-2218-FM equipped with a 150 W ozone free Xenon arc lamp as the excitation light source. PL emission spectra of the materials were converted into the CIE 1931 color coordinate system and color coordinates corresponding to the prominent emission were determined.
3 Results and discussion
3.1 XRD analysis
In this study, the crystallinity, crystallite size, micro-strain, dislocation density, and crystal structure of PXRD result were analyzed. The typical PXRD plot of SZSO and S1−xZSO:xEu samples are shown in Fig. 1, it is well matched with standard JCPDS:82–1206 file [21] and comparatively it is observed that monoclinic phase and the space group is P21/c (14) with Z = 4 present in the synthesized samples which is showing that doping of Eu3+ ions does not create any remarkable change in the host crystal structure. Because of valuable ionic radius (r2) of Eu3+ ions [r2 = 1.21 Ǻ coordination number (CN) = 8, r2 = 1.09 Ǻ (CN) = 6] should replace Sr2+ [r1 = 1.332 Ǻ (CN) = 9, r1 = 1.274 Ǻ (CN) = 8, r1 = 1.224 Ǻ (CN) = 7, r1 = 1.128 Ǻ (CN) = 6] rather than Zr4+ [ r = 0.98 Ǻ (CN) = 8, r = 0.86 Ǻ (CN) = 6, r = 0.73 Ǻ (CN) = 4] and Si4+ [r = 0.40 Ǻ (CN) = 6, r = 0.26 Ǻ (CN) = 4] [22, 23]. In the SZSO host lattice, a similar ionic radius and the same valance state predict the Eu3+ ions will occupy the Sr2+ sites according to Eq. (2) [24, 25]. No any extra diffraction peaks were found in the diffraction pattern of the samples and it is indicating that no extra impurities were present in the single phase synthesized materials [23].
where, r1(CN) = the radius of the host cations, r2 (CN) = radius of doped ion, and Dr = radius percentage difference. If the radius difference between host cations and doped ion exceeded than 30%, then new compound will produce. In our case; using Eq. (3), it is found that Dr = 5.0235% [8 (CN)] and 3.3687% [6 (CN)], it should be Dr < 15%. It indicates that the doping of Eu3+ ions have no influence on the crystal structure of the SZSO system [25, 26]. When divalent Sr2+ ions are substituted by trivalent Eu3+ ions, various defects can be induced due to the charge compensation mechanism. In order to keep charge balance is that two Eu3+ ions can put back three Sr2+ ions to stabilize the charge of these phosphors, therefore generate two positive defects (\(Eu_{Sr}^{ \cdot }\)), each having one positive charge and one \(V^{\prime\prime}_{Sr}\) negative defect (Eq. (4)). The vacancy defect (\(V^{\prime\prime}_{Sr}\)) acts as a donor and the defects (\(2 Eu_{Sr}^{ \cdot }\)) as an acceptor of the electrons. This process can be expressed by the Kroger – Vink notations.
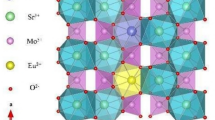
Figure 2 shows the 3D crystal structure of SZSO and S1−xZSOxEu phosphors can be described using VESTA software. The three-dimension crystal framework of SZSO is constructed by alternating layers of corner-sharing [ZrO6] octahedron and of [Si2O7] groups formed from slightly distorted [SiO4] tetrahedron sharing one common oxygen (O) atom and being arranged in nearly eclipsed conformation, and strontium (Sr) atom is coordinated by 8 O atom to form a distorted [SrO8] dodecahedron [10, 27, 28]. The lattice parameters of the optimum SZSO:0.04Eu phosphor was calculated using Rietveld refinement software and compared with the SrZrSi2O7 Crystallographic Information File (CIF): 2,009,819. The refined values of SZSO:0.04 E has monoclinic structure are well matched with the standard lattice parameters and as shown in Table 2.
The crystal structure of the compound SZSO:0.04Eu was determined using Rietveld refinement of PXRD data. The Eu3+ was assumed to substitute the site for Sr2+ ion [7]. Figure 3 displayed the observed, calculated and difference PXRD patterns of prepared SZSO:0.04Eu phosphors. According to the CIF (ID: 2009819) standard host matrix (pure SZSO) was found to be a = 7.76170 Å, b = 8.07130 Å, c = 10.05590 Å, V = 584.510867 Å3 while α = 90.000°, β = 111.9000°, γ = 90.000° and Z = 4. Table 1 represents the Rietveld refinement analysis of the prepared SZSO:0.04Eu phosphor, and the result of the fit obtained was reflected in terms of χ2, RP, Rwp, WRP, Rexp etc. has slightly increased from that the standard host lattice [29]. There is slightly augmentation of lattice parameters due to Eu3+ incorporation into the SZSO lattice. Reason behind was the ionic radii of Eu3+ is lower than Sr2+ and its easily replaceable, causing decrease in unit cell parameters. Alternatively, the decrease in crystal density is ascribed to decreasing cell volume [30]. The detailed comparison among the crystallographic data of SZSO:0.04Eu phosphor with CIF of SZSO refinement parameters are summarized in Table 2. Position of SZSO atoms along with their respective occupancies is listed in Table 3.
Crystallite (grain) sizes were estimated through PXRD pattern using Debye Scherer formula (Eq. (5)) and Micro-crystal strain was calculated by the Williamson–Hall (W–H) plot (Eq. (6) and (7)). Figure 4 displayed the Micro-strain plot of SZSO with S1−xZSO:xEu phosphors of different concentration. The broadening of the peak in the prepared sample grows not only due to crystallite size but also ascribed to be extant strain [31]. The dislocation density was also calculated using (Eq. (8)).
Dislocation density (δ)
where < Ds > is the mean crystallite size, λ is X-ray wavelength, β is full-width half maxima (FWHM), θ is the angle of diffraction, k is the shape factor (k = 0.9), ε = micro-strain and \(\delta\) = dislocation density. The mean crystallite size of the sintered samples was calculated by the Debye Scherer formula and W–H plot, Micro-crystal strain with dislocation density were calculated and listed in Table 4.
3.2 TGA/DSC
A simultaneous TGA/DSC system is very useful for thermal profiling samples. TGA curve provides in detail mass loss of the sample in the desired temperature range while DSC curve pertain information about the heat flow to detect thermal events such as liquefy and crystallization, furthermore to provide specific and precise transition temperatures. The TGA/DSC curves of prepared SZSO:0.04Eu sample are displayed in Fig. 5. TGA/DSC curve for the prepared sample shows that the weight is decreases for TGA up to ~ 650 °C/DSC (~ 250 °C) than constant up for TGA up to ~ 1150 °C/DSC (~ 750 °C). A total weight loss of 0.5561% was observed in TGA curve. From DSC curve, a melting peak was observed for SZSO: 0.04 Eu phosphor, the melting temperature is observed at 1416.50 °C with a change in enthalpy of − 90 J/g. The weight loss of samples is due to removed carbon impurities and trapped of associated gasses [32, 33]. Hence, carbon dioxide gas must be released during one reaction. Considering the possible reaction between SrCO3, ZrO2, and SiO2 (Eq. 2), we may be seeing the formation of SZSO and carbon dioxide is released in the form of gas.
3.3 Field emission scanning electron microscopy (FESEM)
The micrographs with different magnification of prepared SZSO:0.04Eu phosphor were also recorded through the use of FESEM in Fig. 6a–c. The surface of the SZSO:0.04Eu phosphor has shown irregular distribution of the crystallite sizes. As seen from the images, the discussed phosphors are in the form of microstructures and particles are generally in the shape of sphere [34]. The morphological images displays that the particles are accumulated tightly with each other. From the FESEM image, it can be noticed that the prepared sample are highly distinctive, more or less uniform, and compact grain distribution. Using the SEM picture and the lineal interecept method (Heyn's method, Eq. (9)), the average particle size DSEM is computed.
where, respectively, M, L, and N denote the picture's magnefication, the length of line drawn on the image, and the grain boundaries the line intersects. According to statistic, the mean size is around 0.32353 µm. Image J software was use to identify the mean particle size by the histogram, as shown in Fig. 6d.
3.4 Energy dispersive X-ray spectroscopy (EDS)
The EDS technique was used to identify elemental compositions of SZSO:0.04Eu phosphor is shown in Fig. 7. The different peaks of the spectrum revealed the elemental composition of strontium (Sr), zirconium (Zr), silicon (Si), oxygen (O), and europium (Eu) in the synthesized compound SZSO:0.04Eu phosphor. It is notified that no other peaks are obtained, that result is indicating homogeneity and purity of SZSO:0.04 phosphor. The elemental composition of discussed phosphor is listed in Table 5.
3.5 Fourier transform infrared (FTIR) spectra
Figure 8 shows the FTIR spectra of S1−xZSO:xEu phosphors. In the shown spectrum the absorption bands of zirconate and silicate groups were distinctly visible. In the 1400–500 cm−1 region several bands are typical metal oxygen absorptions Si-Onb, Zr–O2–Zr, Zr–O/ Sr–O, and Si–O–Si stretching frequencies were found [35]. The Si-Onb asymmetrical stretch/bending for the silicate tetrahedral show infrared absorption bands, located at about ~ 1100–700 cm−1. The vibrational stretch at ~ 700–600 cm−1 may be assigned the Zr–O2–Zr symmetric and bending vibrations. The symmetric bonding of Zr–O/Si–O appeared at nearly ~ 600–550 cm−1. The anti-symmetric stretching bands arounds ~ 550–400 cm−1 (in our case 550–500 cm−1) are attributed due to the Si–O–Si vibrations [36, 37]. FTIR assignment of S1−xZSO:xEu phosphors of different concentration were listed in the Table 6.
3.6 Photoluminescence (PL)
3.6.1 PL excitation spectra
Figure 9 shows the PL excitation (PLE) spectra of S1−xZSO:xEu phosphors for varying concentrations monitored at 617 nm emission wavelength at room temperature. PL emission spectra were recorded from 220 to 500 nm range. Recorded PLE spectra has given broadband from 220 to 350 nm and it is centered at around 290 nm assigned to the O2− → Zr4+ charge transfer (CT) and CT from oxygen 2p to an empty 4f7 orbitals of Eu3+ ion (O2− → Eu3+). Other sharp peaks are obtained in the range of 360–500 nm due to the 4f → 4f transition of Eu3+ ions from the ground state of 7F0 to its excitation levels [38, 39]]. The excitation peaks were found at 363 (7F0 → 5D4), 384 (7F0 → 5L7), 396 (7F0 → 5L6), 411 (7F0 → 5D3), 417 (7F0 → 5D2), 450, 466, 488 and 492 (7F0 → 5D1) nm are due to transition. [32, 33]. Among them, the strong excitation peaks are located at 396 nm. In the excitation spectra, it is observed that the intensity of CT band transition is weaker than the intra-f transition (⁓ 396 nm and 466 nm). This phenomenon of transition may be due to the weak covalency of the Eu3+ and O2− in the S1−xZSO:xEu phosphors [34, 35]. The optimum intensity of excitation spectra is obtained for 0.04 mol doping concentration. According to the results, the prepared phosphor may be efficient for excitation in NUV lights. The excitation wavelengths are well-matched with the common commercially available blue Indium Gallium Nitride (InGaN) chip which is very useful for application in a white light generation [40].
3.6.2 PL emission spectra
Figure 10 shows that PL emission spectra graph of prepared S1−xZSO:xEu phosphors with different doping concentrations were monitored at 396 nm excitation wavelength. The emission spectra show five emission peaks at 581, 594, 617, 654 and 702 nm in agreement with 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 respectively. The strong emission peaks located at 594 nm (5D0 → 7F1) and 617 nm (5D0 → 7F2) in which the most intense peak obtained at 617 nm indicates that favorer in red color emission [27]]. The emission spectra of phosphors indicate similar profile is obtained for different doping concentrations which are shown in Fig. 10. The shape of all emission spectra is similarly demonstrating that the host structure does not affect by the increasing doping concentration of Eu3+ ions [39]. In other words, the valance electron of Eu3+ ions are shielded by the outer electron 5 s and 5p orbitals, and hence the f –f transition of the Eu3+ ions are very small affected by ligand ions of the host lattice [40]. It is observed that the emission peaks are almost same for the 396 nm excitation wavelengths. Most of the f–f transition of the RE ions is very slightly affected by the environment of the host matrix. Some transitions are very sensitive to the environment of the host and become more intense than the other one, such type of transition is known as a hypersensitive transition [34].
From the emission spectra of synthesized sample 5D0 → 7F2 transition is dominant over the 5D0 → 7F1 transition, which indicates the transition 5D0 → 7F2 is hypersensitive, Kanchan Mondal et al. [27] is already reported that the emission peak obtained at 594 nm ascribed to the magnetic dipole transition (MDT). The emission peaks at 617 nm and 702 nm are owing due to electric dipole transition (EDT). The peaks at 581 nm and 654 nm are become forbidden from both MDT and EDT [41, 42]. In the present case, the dominant EDT (5D0 → 7F2) indicates that the Eu3+ ions are located at non-inversion symmetry sites in the SZSO host lattice. Because when MDT are dominating then the Eu3+ ions are located at inversion symmetry sites, and while the EDT are dominating then Eu3+ ions are located at non-inversion symmetry sites in the host matrix [27]. Asymmetric Ratio (R/O) is calculated via emission intensity of the (5D0 → 7F2)/(5D0 → 7F1) transition to identify site symmetry, covalent nature, and polarization of environment of Eu3+ ions in the host lattice. The R/O for optimum doping concentration is found to be around 1.6 for 396 nm for an excitation wavelength. R/O is greater than 1 is suggested that Eu3+ ions occupied a non-centro symmetric site which may be favorable to obtain of high color purity. The value of R/O is strongly depending on the occupation site of cation, bond nature, lattice distortion, and nature of the host material [41, 42].
Under the excitation at 396 nm, different transition possible in case of S1−xZSO:xEu phosphors are illustrated in Fig. 11. Initially, the electrons excited from the ground state (7F0) to the higher excited level (5L6 and 5D2) correspond to 396 nm and 466 nm excitation wavelength, respectively. The electron from that level gets to relax to the lowest excited level (5D0) via the non-radiative (NR) transitions. Then, electrons return back to the ground levels (7F0, 7F1, 7F2, 7F3 and 7F4) with radiative transitions. These different radiative transitions give different colors (orange, orange-red and red) with different intensities and overall give to the orange-red luminescence.
3.6.3 Temperature dependent emission spectra
Thermal stability is a major criterion in solid state lighting, particularly in WLEDs applications. Figure 12 shows the temperature dependent emission spectra of SZSO:0.04Eu phosphor was investigated at various temperatures in the range from room temperature to nearly 420 K. It is evident that the shapes and peak positions of the spectra remain unchanged when the temperature was increased [43]. For the comparison point of view, the excitation wavelength was fixed at 396 nm, which can effectively emit many sharp lines in the emission spectra ranging from 581 to 702 nm. With the increasing temperature, the intensity of the transition originated from 5D0 → 7Fj (J = 0, 1, 2, 3, 4) transition as expected. It is observed that with increasing temperature the peak intensity of the prepared material decreases gradually Fig. 12 (inset). The progressive decrease in PL intensity is due to the augmented non-radiative transition. It can also be noted that with increasing temperature the peak wavelength of discussed phosphor remains constant, thus the thermal stability was ensured [30].
When the Eu3+ concentration is increased up to the 0.04 mol simultaneously emission intensity is also increases and optimum intensity were obtained for 0.04 mol. After then further Eu3+ concentration increases emission intensity is decreased due to the concentration quenching (CQ). The CQ graph is shown in Fig. 10 (inset). It is prominent that when the doping concentration of Eu3+ has increased, the distance between the Eu3+ ions become miniature which amplified non-radiative energy transfers between neighboring Eu3+ ions [31]. The energy transfer mechanism originated from, radiation re-absorption, exchange interaction or multipolar interaction which depends on the value of critical distance (Rc) which is calculated using the Blasse formula [43] shown in Eq. (10).
Where Xc is the critical concentration of the dopant ion, Z is the number of cation sites in SZSO, and V is the volume of the unit cell. In the present case, the experimental and analytic values of V = 584.508948 (Å)3, Z = 4 and Xc = 0.04 mol respectively. Calculations are made to determine the value of Rc is 19.1117 Å. It is well known that when the Rc < 5 Å, Exchange interactions are usually predominant. In our case, Rc > 5 is a non-radiative energy transfer that was mostly attributable to electric multipolar interactions in S1−xZSO:xEu phosphors. According to Dexter's theory, luminescence intensity and activator are related, as shown by the Eq. (11) [32].
where x represents the concentration of dopant, \(\theta\) is a multipolar interaction constant there are nearest neighbor ions numbers 6, 8, and 10, which mean dipole–dipole (d–d), dipole–quadrupole (d–q), and quadrupole–quadrupole (q–q) interaction respectively and K and β are constant for each interaction at the same excitation. Figure 13 shows the graph between Log (I/x) versus Log(x). The fitted line's slope was determined to be -0.858; thus, the calculated value is 5.17, which is close to 6. Therefore, d–d interaction could be attributed quenching effect in S1−xZSO:xEu phosphors.
3.7 Decay
The effect of SZSO:Eu0.04 content on the 5D0 → 7F2 transition decay curve is shown in Fig. 14. As Eu.3+ ions at different sites were excited at the same time, the decay curve cannot be fit by a single exponential equation so it is fits well with a two-exponential equation [44]. The corresponding decay curve can be well fitted with a double exponential function, described by the Eq. (12)
Here Y and Y0 are luminescence intensity at time t and 0, A1 and A2 are constant and fast decay, and slow decay is described by t1 and t2 in the 0.04Eu single doped SZSO sample. The lifetime decay for an exponential component using these parameters, the fitting result of the sample is listed in Table 7. Using Eq. (12), we obtain τ1 = 136.16263 ms and τ2 = 2719.47971 ms which is responsible for shallow and deep trap.
The average decay time (τav) can be determined using the Eq. (13) which is given below[34].
On the basis of the above Eq. (13), the average luminescence decay time is determined to be 2665.59187 ms for SZSO:Eu0.04 phosphor.
3.8 Photometric properties
3.8.1 CIE chromaticity coordinate
The 1931 CIE chromatic color coordinate usually refers to the color in lighting specifications, recognizing that humans see three primary colors: red, blue, and green [31]. The chromaticity coordinates of the S1−xZSO:xEu phosphor is calculated from PL spectra. A region of orange-red light, the color coordinate of the prepared sample, appears on the CIE chromaticity coordinates [45]. Figure 15 represents the CIE 1931 chromaticity diagram of S1−xZSO:xEu phosphor. The number from 0.01 to 0.05 mol presents the concentration of Eu3+; and their chromaticity coordinates are (0.4140, 0.2893), (0.4171, 0.2890), (0.4795, 0.2945), (0.4168, 0.2858) and (0.4793, 0.2980) respectively.
A region of orange-red light, the color coordinate of the prepared sample, appears on the CIE chromaticity coordinates. Luminescence colors of S1−xZSO:xEu phosphors represented by the symbols [*]. Based on the Eq. (14), the color purity (CP) of all samples is calculated [45].
These coordinates correspond with the CIE coordinates of the sample point (x, y), standard source (xi, yi), and dominant wavelength (xd, yd). The (xi, yi) is taken (0.33, 0.33) for standard white light [46]. The variation of percentage in CP with increasing Eu3+ ion concentration is displayed in Fig. 16a. The calculated CP values of prepared S1−xZSO:xEu phosphors are also listed in Table 8. It is seen that the CP values lies in between 42.76 and 45.72; it is noted that the low CP values signifies the emission of color near white region. Thus, the above outcome specify that the prepared phosphors emit orange–red color will be a potential candidate for the solid-state lighting and other display applications.
3.8.2 Correlated color temperature (CCT)
According to the literature, CCT is essentially a characteristic indicating how yellow or blue the light output by a light bulb appears [47]. It is measured in the between 2200 and 6500 Kelvin degrees. Cooler color temperatures range from 3500 to 5000 K + , whereas warmer color temperatures are between 2200 and 3000 K, featuring more light in the red, orange and yellow range [48]. The CCT values are calculated by using McCamy's approximation (Eq. (15)) [49].
where n = x − 0.332/y − 0.186 and (x, y) are CIE coordinates, S1−xZSO:xEu phosphor samples are listed in Table 8. The calculated CCT values lies within a range from 2207 to 2360 K in the warm region of visible for different doping concentrations of Eu3+ ion. The variation of CCT with increasing Eu3+ ion concentration is displayed in Fig. 16b. It was observed that the CCT value decreases with rising, dopant concentration. It means prepared phosphors emits light in the warm region for higher dopant concentration, whereas cool in lower concentrations [50].
3.8.3 Color rendering index (CRI)
The CRI is calculated for the significance of the light source (orange-red), it is an important parameter that describes the quality of the spectrum [51]. The values of ranges from scale of 0 to 100. CRIs in the range of 75–100 is considered excellent, while 65–75 is good. The range of 55–65 is fair, and 0–55 is poor. By using Eq. 16, the CRI values are calculated and all values is tabulated in Table 8 [37].
The calculated CRI values of prepped S1−xZSO:xEu phosphors are varies from 72 to 77, showing in excellent and good range. In addition, the variation of CCT with increasing Eu3+ ion concentration is displayed in Fig. 16c and CRI value depends upon the concentration of Eu3+. The CRI shows the maximum for the 0.04 mol Eu3+ concentration which is a good agreement with the result of PL emission spectrum.
3.8.4 Quantum efficiency (Q.E.) analysis
The QE of prepared S1−xZSO:xEu phosphors has been calculated using Eq. (17) through the conventional methods using a standard material.
where, Isam. = PL intensity of emission spectra of the samples, Esam = PL intensity of excitation spectra of samples, Eref = PL intensity of excitation spectra of reference. The internal (IQE) and external quantum efficiency (EQE) [52] was also calculated using Eqs. (18) and (19) for synthesized S1−xZSO:xEu phosphors.
where, γab = total number of photons absorbed by sample, γex = total number of photons emitted by the excitation, and γr = total number of photons reflected and not absorbed by the phosphor.
The QE, IQE, EQE and absorption rate (AR) were calculated for the sintered S1−xZSO:xEu phosphors and listed in Table 9 and it is shown that, the IQE is always larger than the EQE. The calculated QE values of prepped S1−xZSO:xEu phosphors were estimated under 396 nm excitation are varies from 71.3% to 73.1% and optimum for SZSO:0.04Eu phosphor which is 73.1%. Here, SZSO:0.04Eu has found optimise and efficient sample with respect to other samples which has given excellent result. The variation of QE with increasing Eu3+ ion concentration is displayed in Fig. 16d and value of QE depends upon the concentration of Eu3+ ions.
4 Conclusion
In summary, a series of S1−xZSO:xEu phosphors were synthesized by a high- temperature solid-state reaction method. It is found that the Eu3+ ions successfully incorporated on Sr2+ ions lattice site of SZSO host. The luminescence studies of phosphor exhibited strong absorption near UV light under the excitation of 396 nm. The phosphor indicates two strong emission peaks at 595 nm (5D0 → 7F1) and 617 nm (5D0 → 7F2) where electric dipole transition (EDT) 5D0 → 7F2 is hypersensitive which is favored in red color emission. The optimum luminescence properties are obtained at a 0.04 mol doping concentration of Eu3+ ions in the SZSO host. After then concentration quenching obtains proved owing due to the energy transfer between nearest-neighbor ions. The average luminescence decay time is determined 2665.59187 ms for optimized phosphor due to florescent persistence luminescence phenomenon. The photometric studies are well within the defined acceptable range indicate that prepared phosphor emitted orange-red light and useful for the solid-state lighting applications.
Data availability
The author’s states that analysed and relevant data of synthesized materials, which are including and described in the manuscript will be freely available to researchers and scientists who are working purpose of research and social welfare.
References
H. Guo, Q. Shi, K.V. Ivanovskikh, L. Wang, C. Cui, P. Huang, A high color purity red-emission phosphor based on Sm3+ and Eu3+ co-doped Ba3Bi(PO4)3. Mater. Res. Bull. 126, 110836 (2020). https://doi.org/10.1016/j.materresbull.2020.110836
B. Yu, Y. Li, R. Zhang, H. Li, Y. Wang, A novel thermally stable eulytite-type NaBaBi2(PO4)3:Eu3+ red-emitting phosphor for pc-WLEDs. J. Alloys Compd. 852, 157020 (2021). https://doi.org/10.1016/j.jallcom.2020.157020
Y. Peng, Q. Sun, J. Liu, H. Cheng, Y. Mou, Fabrication of stacked color converter for high-power WLEDs with ultra-high color rendering. J. Alloys Compd. 850, 156811 (2021). https://doi.org/10.1016/j.jallcom.2020.156811
Y. Gao, Y. Li, X. Sun, P. Jiang, R. Cong, T. Yang, Dopant and excitation wavelength dependent color tunability in Dy3+ and Eu3+ doped CaBi2B2O7 phosphors for NUV warm white LEDs. Mater. Res. Bull. 122, 110649 (2020). https://doi.org/10.1016/j.materresbull.2019.110649
M. Qu, X. Zhang, X. Mi, H. Sun, Q. Liu, Z. Bai, Luminescence color tuning of Ce3+ and Tb3+ co-doped Ca2YZr2Al3O12 phosphors with high color rendering index via energy transfer. J. Lumin. 228, 117557 (2020). https://doi.org/10.1016/j.jlumin.2020.117557
T. Takeda, K. Kato, H. Kiyono, N. Hirosaki, Powder synthesis and luminescence properties of green emitting Ba2LiSi7-xAlxN12-xOx:Eu2+ phosphor. J. Alloys Compd. 850, 156358 (2021). https://doi.org/10.1016/j.jallcom.2020.156358
V.R. Bandi, M. Jayasimhadri, J. Jeong, K. Jang, H.S. Lee, S.S. Yi, J.H. Jeong, Host sensitized novel red phosphor CaZrSi2O7:Eu3+ for near UV and blue led-based white LEDs. J. Phys. D Appl. Phys. 43, 395103 (2010). https://doi.org/10.1088/0022-3727/43/39/395103
Z. Zhou, G. Liu, J. Ni, W. Liu, Q. Liu, Simultaneous multi-wavelength ultraviolet excited single-phase white light emitting phosphor Ba1−x(Zr, Ti)Si3O9:xEu. Opt. Mater. 79, 53–62 (2018). https://doi.org/10.1016/j.optmat.2018.03.018
P. Feng, J. Zhang, C. Wu, Z. Liu, Y. Wang, Self-activated afterglow luminescence of un-doped Ca2ZrSi4O12 material and explorations of new afterglow phosphors in a rare earth element-doped Ca2ZrSi4O12 system. Mater. Chem. Phys. 141(1), 495–501 (2013). https://doi.org/10.1016/j.matchemphys.2013.05.049
M.E. Huntelaar, E.H.P. Cordfunke, P.V. Vlaanderen, SrZrSi2O7. Acta Cryst. C 50, 988 (1994). https://doi.org/10.1107/S0108270193008364
G. Blasse, W.J. Schipper, M.E. Huntelaar, D.J.W. Ijdo, Luminescence of SrZrSi2O7. J. Phys. Chem. Solids 54(9), 1001–1003 (1993). https://doi.org/10.1016/0022-3697(93)90004-B
I. Ahemen, F.B. Dejene, R.E. Kroon, H.C. Swart, Effect of europium ion concentration on the structural and photoluminescence properties of novel Li2BaZrO4:Eu3+nanocrystals. Opt. Mater. 74, 58–66 (2017). https://doi.org/10.1016/j.optmat.2017.03.029
D.V. Deyneko, I.V. Nikiforov, B.I. Lazoryak, D.K. Spassky, I.I. Leonidov, S.Y. Stefanovich, D.A. Petrova, S.M. Aksenov, P.C. Burns, Ca8MgSm1–x(PO4)7:xEu3+, promising red phosphors for WLED application. J. Alloys Compd. 776, 897–903 (2019). https://doi.org/10.1016/j.jallcom.2018.10.317
X. Lu, H. Liu, X. Yang, Y. Tian, X. Gao, L. Han, Q. Xu, A single-phase white-emitting La10(SiO4)6O3:Eu2+ /Eu3+ phosphor for near-UV LED-based application. Ceram. Int. 43(15), 11686–11691 (2017). https://doi.org/10.1016/j.ceramint.2017.05.358
Y. Yuan, H. Lin, J. Cao, Q. Guo, F. Xu, L. Liao, L. Mei, A novel blue-purple Ce3+ doped whitlockite phosphor: synthesis, crystal structure, and photoluminescence properties. J. Rare Earths 39(6), 621–626 (2021). https://doi.org/10.1016/j.jre.2020.07.013
S. Kaur, M. Jayasimhadri, A.S. Rao, A novel red emitting Eu3+ doped calcium aluminozincate phosphor for applications in w-LEDs. J. Alloys Compd. 697, 367–373 (2017). https://doi.org/10.1016/j.jallcom.2016.12.150
J.D. Xiaopyan, X. Pan, Z. Liu, Y. Jing, B. Wang, L. Luo, J. Wang, P. Du, Highly efficient Eu3+-activated Ca2Gd8Si6O26 red-emitting phosphors: a bifunctional platform towards white light-emitting diode and ratio metric optical thermometer applications. J. Alloys Compd. 859, 157843 (2021). https://doi.org/10.1016/j.jallcom.2020.157843
B. Fan, J. Liu, W. Zhao, L. Han, Luminescence properties of Sm3+ and Dy3+ co-doped BaY2ZnO5 phosphor for white LED. J. Lumin. 219, 116887 (2020). https://doi.org/10.1016/j.jlumin.2019.116887
G.L. Bhagyalekshmi, D.N. Rajendran, Luminescence dynamics of Eu3+ activated and co-activated defect spinel zinc titanate nanophosphor for applications in WLEDs. J. Alloys Compd. 850, 156660 (2021). https://doi.org/10.1016/j.jallcom.2020.156660
H. Duan, R. Cui, J. Li, C. Deng, Synthesis and photoluminescence properties of a novel red emitting Ba3ZnTa2O9: Eu3+ phosphor. J. Mol. Struct. 1224, 129075 (2021). https://doi.org/10.1016/j.molstruc.2020.129075
D. Lundberg, D. Warmińska, A. Fuchs, I. Persson, On the relationship between the structural and volumetric properties of solvated metal ions in O-donor solvents using new structural data in amide solvents. Phys. Chem. Chem. Phys. 20(21), 14525 (2018). https://doi.org/10.1039/c8cp02244e
C. Lorbeer, F. Behrends, J. Cybinska, H. Eckertbd, A.V. Mudring, Charge compensation in RE3+ (RE = Eu, Gd) and M+ (M = Li, Na, K) co-doped alkaline earth nanofluorides obtained by microwave reaction with reactive ionic liquids leading to improved optical properties. J. Mater. Chem. C 2, 9439 (2014). https://doi.org/10.1039/c4tc01214c
M. Peng, Z. Pei, G. Hong, Q. Su, The reduction of Eu3+ to Eu2+ in BaMgSiO4: Eu prepared in air and the luminescence of BaMgSiO4:Eu2+ phosphor. J. Mater. Chem. 13, 1202 (2003). https://doi.org/10.1039/B211624C
Y. Ma, S. Chen, J. Che, J. Wang, R. Kang, J. Zhao, B. Deng, R. Yu, H. Geng, Effect of charge compensators (Li+, Na+, K+) on the luminescence properties of Sr3TeO6:Eu3+ red phosphor. Ceram. Int. 47(6), 8518–8527 (2021). https://doi.org/10.1016/j.ceramint.2020.11.219
X. Li, Z. Zou, Z. Wang, C. Wu, J. Znang, Y. Wang, A novel un-doped long lasting phosphorescence phosphor: SrZrSi2O7. J. Rare Earths 33(1), 37–41 (2015). https://doi.org/10.1016/S1002-0721(14)60380-X
H. Guo, X. Huang, Low-temperature solid-state synthesis and photoluminescence properties of novel high-brightness and thermal-stable Eu3+-activated Na2Lu(MoO4)(PO4) red-emitting phosphors for near-UV-excited white LEDs. J. Alloys Compd. 764, 809–814 (2018). https://doi.org/10.1016/j.jallcom.2018.06.156
R. Nagaraj, A. Raja, S. Ranjith, Synthesis and luminescence properties of novel red-emitting Eu3+ ions doped silicate phosphors for photonic applications. J. Alloys Compd. 827, 154289 (2020). https://doi.org/10.1016/j.jallcom.2020.154289
X. Meng, S. Huang, M. Shang, Red emitting Ba2GdVO6:Eu3+ phosphors for blue light converted warm white LEDs. Inorg. Chem. Commun. 113, 107768 (2020). https://doi.org/10.1016/j.inoche.2020.107768
R.D. Shannon, Revised effective ionic radii and systematic studis of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767 (1976). https://doi.org/10.1107/S0567739476001551
G.V. Kanmani, V. Ponnusamy, G. Rajkumar, M.T. Jose, Development of novel Na2Mg3Zn2Si12O30:Eu3+ red phosphor for white light emitting diodes. Opt. Mater. 96, 109350 (2019). https://doi.org/10.1016/j.optmat.2019.109350
S. Ray, P. Tadge, S. Dutta, T.M. Chen, G.B. Nair, S.J. Dhoble, Synthesis, luminescence and application of BaKYSi2O7:Eu2+: a new blue-emitting phosphor for near-UV white-light LED. Ceram. Int. 44(7), 8334–8343 (2018). https://doi.org/10.1016/j.ceramint.2018.02.022
R. Cao, Y. Ren, T. Chen, W. Wang, Q. Guo, Q. Hu, C. Liao, T. Fan, Emission improvement and tunable emission properties of SrZrSi2O7: R (R = Sm3+and Sm3+ /Bi3+) phosphors. J. Lumin. 225, 117350 (2020). https://doi.org/10.1016/j.jlumin.2020.117350
S.J. Park, J.Y. Kim, J.H. Yim, N.Y. Kim, C.H. Lee, S.J. Yang, H.K. Yang, The effective fingerprint detection application using Gd2Ti2O7:Eu3+ nanophosphors. J. Alloys Compd.. 741, 246–255 (2018). https://doi.org/10.1016/j.jallcom.2018.01.116
Golja DR, Dejene FB (2020) Effect of Eu3+ ion concentration on the structural and photoluminescence properties of Ba1.3Ca0.7SiO4 ceramic-based red phosphors for solid- state lighting applications. J. Alloys Compd. 827: 154216. https://doi.org/10.1016/j.jallcom.2020.154216
C. Manjunath, M.S. Rudresha, R. HariKrishna, B.M. Nagabhushana, B.M. Walsh, K.R. Nagabhushana, B.S. Panigrahi, Spectroscopic studies of strong red emitting Sr2SiO4:Eu3+ nanophosphors with high color purity for application in WLED using Judd-Ofelt theory and TL glow curve analysis. Opt. Mater. 85, 363–372 (2018). https://doi.org/10.1016/j.optmat.2018.08.070
X. Huang, Q. Sun, B. Devakumar, Preparation, crystal structure, and photoluminescence properties of high-brightness red-emitting Ca2LuNbO6:Eu3+ double- perovskite phosphors for high-CRI warm-white LEDs. J. Lumin. 225, 117373 (2020). https://doi.org/10.1016/j.jlumin.2020.117373
S. Som, A.K. Kunti, V. Kumar, V. Kumar, S. Dutta, M. Chowdhury, S.K. Sharma, J.J. Terblans, H.C. Swart, Defect correlated fluorescent quenching and electron phonon coupling in the spectral transition of Eu3+ in CaTiO3 for red emission in display application. J. Appl. Phys. 115(19), 1–14 (2014). https://doi.org/10.1063/1.4876316
S.K. Sharma, S. Som, R. Jain, A.K. Kunti, Spectral and CIE parameters of red emitting Gd3Ga5O12:Eu3+ phosphor. J. Lumin. 159, 317–324 (2015). https://doi.org/10.1016/j.jlumin.2014.11.010
P. Aryal, H.J. Kim, A. Khan, S. Saha, S.J. Kang, S. Kothan, Y. Yamsuk, J. Kaewkhao, Development of Eu3+-doped phosphate glass for red luminescent solid- state optical devices. J. Lumin. 227, 117564 (2020). https://doi.org/10.1016/j.jlumin.2020.117564
Q. Wu, Y. Xie, F. She, Q. Zhao, J. Ding, J. Zhou, CsBaB3O6:Eu3+ red-emitting phosphors for white LED and FED: Crystal structure, electronic structure and luminescent properties. J. Rare Earths 39(9), 1040–1048 (2021). https://doi.org/10.1016/j.jre.2020.07.014
K. Mondal, D.K. Singh, J. Manam, Spectroscopic behavior, thermal stability and temperature sensitivity of Ca2SiO4: Eu3+ red emitting phosphor for solid state lighting application. J. Alloys Compd. 761, 41–51 (2018). https://doi.org/10.1016/j.jallcom.2018.05.161
Z. Zhang, L. Sun, B. Devakumar, G. Annadurai, J. Liang, S. Wang, Q. Sun, X. Huang, Synthesis and photoluminescence properties of a new blue-light- excitable red phosphor Ca2LaTaO6:Eu3+ for white LEDs. J. Lumin. 222, 117173 (2020). https://doi.org/10.1016/j.jlumin.2020.117173
K.K. Rasu, S.M. Babu, Impact of Eu3+ concentration on the fluorescence properties of the LiGd(W0.5Mo0.5O4)2 novel red phosphors. Solid State Sci. 98, 106028 (2019). https://doi.org/10.1016/j.solidstatesciences.2019.106028
T. Sakthivel, G. Annadurai, R. Vijayakumar, X. Huang, Synthesis, luminescence properties and thermal stability of Eu3+-activated Na2Y2B2O7 red phosphors excited by near-UV light for pc-WLEDs. J. Lumin. 205, 129–135 (2019). https://doi.org/10.1016/j.jlumin.2018.09.008
G. Blasse, Energy transfer in oxidic phosphors. Phys. Lett. A 28(6), 444–445 (1968). https://doi.org/10.1016/0375-9601(68)90486-6
D.L. Dexter, A theory of sensitized luminescence in solids. J. Chem. Phys. 21(5), 836–850 (1953). https://doi.org/10.1063/1.1699044
L. Zhang, Y. Xie, X. Geng, B. Deng, H. Geng, R. Yu, Double perovskite Ca2MgTeO6:Eu3+ red-emitting phosphors with high thermal stability for near UV/blue excited white LEDs. J. Lumin. 225, 117365 (2020). https://doi.org/10.1016/j.jlumin.2020.117365
S.K. Baghel, N. Brahme, D.P. Bisen, Y. Patle, T. Richhariya, E. Chandrawanshi, C. Belodhiya, Luminescence properties of a novel cyan-blue light emitting Ce3+doped SrZrSi2O7 phosphor. Opt. Mater. 126, 112141 (2022). https://doi.org/10.1016/j.optmat.2022.112141
C.S. McCamy, Correlated color temperature as an explicit function of chromaticity coordinates. Color. Res. Appl. 17(2), 142–144 (1992). https://doi.org/10.1002/col.5080170211
Y. Zhong, P. Sun, X. Gao, Q. Liu, S. Huang, B. Liu, B. Deng, R. Yu, Synthesis and optical properties of new red-emitting SrBi2Ta2O9:Eu3+ phosphor application for w-LEDs commercially based on InGaN. J. Lumin. 212, 45–51 (2019). https://doi.org/10.1016/j.jlumin.2019.03.057
J. Xue, H.M. Noh, S.H. Park, B.C. Choi, J.H. Kim, J.H. Jeong, P. Du, W. Ran, M. Song, Eu3+-activated Ca3Mo0.2W0.8O6 red-emitting phosphors: a near-ultraviolet and blue light excitable platform for solid-state lighting and thermometer. J. Lumin. 223, 117212 (2020). https://doi.org/10.1016/j.jlumin.2020.117212
R.K. Singh, S. Som, C.H. Lu, Spectroscopic investigation of red Eu3+ doped ceria nanophosphors and promising color rendition for warm white LEDs. J. Alloys Compd. 816, 152653 (2020). https://doi.org/10.1016/j.jallcom.2019.152653
Acknowledgements
The corresponding author; Dr. Ishwar Prasad Sahu, would like to acknowledge the Science & Engineering Research Board (SERB), New Delhi, Government of India for funding FTIR Spectrophotometer (CRG/2018/004139). This instrument is used for the IR spectroscopic analysis of the prepared sample from the study.
Funding
This work was supported by UGC-BSR Start-up Fellowship: No. F.30–420/2018 (BSR), dated 23/10/2018, the Corresponding Author Dr. Ishwar Prasad Sahu, has received research support from University Grant commission (UGC), New Delhi, Government of India.
Author information
Authors and Affiliations
Contributions
Ishwar Prasad Sahu and Manorama Sahu: Investigate, designed the whole research, Conceptualization, synthesized all the samples, and collected experimental data, Data plotting, writing of the manuscript, review, editing and formatting, and editing of manuscript corresponding to the journals.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This is an experimental study on materials science and no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahu, M., Sahu, I.P. Studies on the structural, thermal and luminescence properties of Sr1−xZrSi2O7:xEu3+ phosphors for solid state lighting. Appl. Phys. A 130, 322 (2024). https://doi.org/10.1007/s00339-024-07458-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07458-y