Abstract
In this work, novel Ag/AgCl/PbBiO2Br photocatalysts were synthesized via a hydrothermal and in situ photoreaction method. The microstructure, morphology, composition, electrochemical, and optical properties of the synthesized catalysts were investigated by multiple techniques. The obtained Ag/AgCl, PbBiO2Br, and Ag/AgCl/PbBiO2Br composites were evaluated via degradation of oxytetracycline (OTC) hydrochloride antibiotic under visible-light irradiation. The results show that the Ag/AgCl/PbBiO2Br composites are composed of Ag/AgCl nanoparticles (NPs) and PbBiO2Br nanosheets. The Ag/AgCl/PbBiO2Br (20.4%) composite exhibits the highest visible-light absorption and best photogenerated charge separation efficiency. The photocatalytic degradation experiments show that all Ag/AgCl/PbBiO2Br composites exhibit an enhanced degradation activity under visible-light irradiation, and maintain good stability in the photocatalytic process. The Ag/AgCl/PbBiO2Br (20.4%) composite has the highest degradation activity, which is 1.82 and 2.11 times higher than that of Ag/AgCl and PbBiO2Br, respectively. The enhanced photocatalytic activity of Ag/AgCl/PbBiO2Br can be mainly attributed to the fact that the loading of Ag NPs on the surface of the AgCl promotes the separation efficiency of photoinduced charge and enhance the visible-light absorption. Additionally, active species trapping experiments confirm that superoxide radicals (\({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\)), Cl0 and holes (h+) play an very important role in the degradation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the widespread usage of antibiotics has received the increasing attention because they flow into the water system and cause the water pollution [1]. Oxytetracycline (OTC) hydrochloride is one of the very important antibiotics, extensively used in human and veterinary medicine [2]. Worryingly, the most of OTC is only partially metabolized in humans or animals and hardly biologically degraded, ultimately released into lakes and rivers. These antibiotics have harmful effects on water environment and human health [3]. Hence numbers of research attempts have been made in the past decades to eliminate these refractory antibiotics, such as electrochemical treatments [4], photoelectron-Fenton [5] and advanced oxidation treatment [6]. However, most of the methods use expensive oxidants. Therefore, it is indispensable to develop novel visible-light-driven photocatalysts [7], which are environmentally friendly and efficient approach to remove OTC from waters.
PbBiO2Br is an n-type visible-light-driven semiconductor and has attracted more and more attention in recent years, owing to its physicochemical stability, highly anisotropic layered structure, and outstanding photocatalytic performance [8,9,10,11]. Unfortunately, the photocatalytic activity of bare PbBiO2Br is still unsatisfactory owing to its fast recombination rate of photoexcited electron–hole (e−/h+) pairs [12, 13]. To overcome the above mentioned drawback of PbBiO2Br and improve the degradation efficiency, constructing semiconductor composites is effective in improving the separation efficiency of photoinduced e−/h+ pairs. So far, studies have reported on g-C3N4/PbBiO2Br [14], NbSe2/PbBiO2Br [15], PbBiO2Br/UiO-66-NH2 [16] Cu2O/PbBiO2Br [17], p-Ag2O/n-PbBiO2Br [18], PbBiO2Br/BiOBr composites [19], and etc. These heterojunction composites were found to exhibit superior photocatalytic activity. Despite many PbBiO2Br-based materials have been reported, it is still necessary to be committed to the exploitation of more efficient visible-light-driven PbBiO2Br-based photocatalysts for making the best use of the solar energies.
The surface plasmon resonance (SPR) strategy is widely used in fabricating efficient visible-light-driven photocatalysts [20]. Because of SPR of noble metal nanoparticles (NPs), the absorption range of visible-light region can be expanded, resulting in the enhanced degradation performance of photocatalysts [21]. Recently, Ag/AgCl has been widely considered as a promising photocatalyst due to its being a p-type SPR structure semiconductor [22]. Furthermore, Ag0 NPs dispersed on the surface of AgCl can not only effectively absorb visible light, but also can accelerate the transfer of photo-carriers. By now, a number of Ag/AgCl-based photocatalysts have been successfully synthesized, such as Ag/AgCl/NaTaO3 [23], and BiVO4/MWCNT/Ag@AgCl [24]. These composite photocatalysts exhibited superior photocatalytic performances. To the best of our knowledge, the coupling of PbBiO2Br nanosheets with Ag/AgCl NPs has not been reported yet. Hence, we expect that the new Ag/AgCl/PbBiO2Br composites not only improve the utilization rate of solar energy, but also enhance photocatalytic ability.
Inspired by previous studies, we have successfully fabricated a series of Ag/AgCl/PbBiO2Br composites by a hydrothermal and in situ photoreaction method. Morphology and microstructure, elements chemical states, optical and electrochemical properties of the Ag/AgCl/PbBiO2Br composites were systematically studied. Their photocatalytic performances were investigated by the degradation of OTC under visible-light irradiation. The possible enhanced photocatalytic mechanism was also proposed.
2 Experimental
2.1 Preparation of the photocatalysts
PbBiO2Br nanosheets were synthesized via a facile hydrothermal method [17]. Detailed experimental process was given in Supporting Information (S1). Ag/AgCl/PbBiO2Br composites were prepared via a photoreduction method. The preparation process was as follows: 1 mmol of PbBiO2Br was dispersed in deionized water, stirred for 20 min to form uniform suspension A. Then, 1 mmol AgNO3 was added into the suspension A and stirred for 20 min. Subsequently, 1 mmol NaCl was transferred into the suspension A under strong stirring for 30 min. The resulting mixture was illuminated under a 500 W xenon lamp for 30 min so that the Ag+ NPs on the surface of AgCl/PbBiO2Br were reduced to Ag0 NPs. Eventually, the precipitate was filtered, rinsed with deionized water and ethanol, and dried at 80 °C for 24 h. The obtained product, in which the mass ratio of Ag to PbBiO2Br was 20.4%, was designated as Ag/AgCl/PbBiO2Br (20.4%). Ag/AgCl/PbBiO2Br (13.6%), Ag/AgCl/PbBiO2Br (40.8%), and bare Ag/AgCl were also obtained with the same conditions by changing the content of PbBiO2Br.
2.2 Characterization and photocatalytic evaluation
The synthesized catalysts were investigated in detail by multiple instruments analyses. The photocatalytic activities of the Ag/AgCl, PbBiO2Br, and Ag/AgCl/PbBiO2Br composites were evaluated via the degradation of the antibiotic OTC under visible-light irradiation. Detailed experimental process was given in Supporting Information (S2).
2.3 Photoelectrochemical measurements
The electrochemical properties of as-prepared samples were investigated on a electrochemical workstation (CS350H, wuhan sikete instrument Co., Ltd, China) with standard calomel electrode (SCE). Preparation of the working electrodes and detailed experimental process were given in Supporting Information (S3).
3 Results and discussion
3.1 XRD analysis
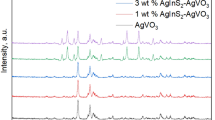
The crystal structures of as-synthesized PbBiO2Br, Ag/AgCl, and Ag/AgCl/PbBiO2Br (20.4%) composite were analyzed using X-ray diffraction (XRD), as presented in Fig. 1a. It can be seen that the XRD pattern of bare PbBiO2Br was consistent with the standard spectrum of tetragonal phase PbBiO2Br (PDF#38-1008). The strong peak located at 30.6° corresponds to the (103) plane of PbBiO2Br, indicating that the obtained catalyst is well-crystallized [25]. For the Ag/AgCl, the peaks at 2θ = 27.7°, 32.1°, 46.1°, 54.7°, and 57.3°correspond to the (111), (200), (220), (311), and (222) planes of cubic AgCl (PDF#31-1238), respectively [26]. In addition, the diffraction peaks at 2θ = 37.9°, 44.1°, 64.3°, and 77.2° match with the (111), (200), (220), and (311) facets of Ag crystal (PDF# 65-2871), respectively [27]. Additionally, as for the Ag/AgCl/PbBiO2Br (20.4%) photocatalyst, all the diffraction peaks correspond to PbBiO2Br and Ag/AgCl, and no additional crystal phases can be detected, which indicates the formation of Ag/AgCl/PbBiO2B composites.
3.2 XPS analysis
The elemental valence states of the as-synthesized Ag/AgCl/PbBiO2Br (20.4%) were detected by X-ray photoelectron spectroscopy (XPS) technology, and the obtained results are illustrated in Fig 1. The main peaks in the XPS survey spectrum of Ag/AgCl/PbBiO2Br (20.4%) composite (Fig 1b) correspond to Br 3d, Pb 4f, Bi 4f, Cl 2p, Ag 3d, and O 1s. Figure 1c displays the XPS spectrum of Ag 3d, where the peaks at 367.93 and 373.60 eV are assigned to Ag0, and the other two strong peaks at 367.69 and 373.87 eV are ascribed to Ag 3d5/2 and Ag 3d3/2 of Ag+ in Ag/AgCl, respectively. This result is consistent with other reports in literatures [28]. In Fig. 1d, the binding energy peaks at 138.27 and 143.02 eV are corresponding to Pb 4f7/2 and Pb 4f5/2, respectively [29]. Figure 1e shows that the XPS spectrum of Bi element, where the peaks at 157.09 and 164.32 eV are attributed to Bi 4f7/2 and Bi 4f5/2, respectively [30,31,32,33,34], indicating that Bi3+ ions exist in PbBiO2Br. Furthermore, in Fig 1f, two typical peaks at 199.61 and 197.99 eV can be attributed to Cl 2p3/2 and Cl 2p1/2, indicating that Cl− ions exist in AgCl phase [34]. Figure 1g shows the XPS spectrum of Br 3d, where the binding energy peaks at 68.5 and 69.4 eV are corresponding to Br 3d3/2 and Br 3d5/2, respectively [35]. The O 1s XPS spectrum of Ag/AgCl/PbBiO2Br (20.4%) composite (Fig. 1h) is composed of fitted peaks at 529.66 and 531.04 eV, which could be attributed to the lattice oxygen of PbBiO2Br and surface-adsorbed oxygen species, respectively [36, 37]. From the XPS analysis, it is clear that Ag/AgCl/PbBiO2Br is a composite sample composed of Ag/AgCl and PbBiO2Br.
3.3 Scanning electron microscope (SEM) and EDX analysis
Figure 2 shows the morphologies of the PbBiO2Br, Ag/AgCl, and Ag/AgCl/PbBiO2Br (20.4%) composite. Figure 2a reveals that the AgCl consists of cubic-like NPs with grain size of 300–500 nm and Ag NPs are dispersed on the surfaces of AgCl cubes. Figure 2b shows that the as-prepared PbBiO2Br has a sheet-like morphology with thickness about 30 nm. As seen from Fig. 2c, the Ag/AgCl NPs are attached on the surface of PbBiO2Br nanosheets. Moreover, we also notice that compared with the pure Ag/AgCl, the particle size of Ag/AgCl in the Ag/AgCl/PbBiO2Br (20.4%) composite undergoes significant change, which could be due to the fact that PbBiO2Br could influence the surface energy of Ag/AgCl and thus impede their growth. Additionally, energy-disperse X-ray (EDX) spectroscopy analysis of Ag/AgCl/PbBiO2Br (20.4%) composite was carried out, and the obtained result is illustrated in Fig. 2d. From the EDX spectrum, the peaks belonging to Ag, Cl, Pb, Bi, O, C, Au, and Br are observed (C and Au element come from the test instrument). The atomic ratio of Ag/Pb equals to 1:1, which is in good agreement with the Ag/Pb atomic ratio of Ag/AgCl/PbBiO2Br (20.4%) composite.
3.4 TEM analysis
To further obtain more detailed structure information of Ag/AgCl/PbBiO2Br (20.4%) composite, field emission transmission electron microscopy (TEM) and high resolution TEM (HRTEM) images were carried out. As shown in Fig. 2e, the Ag/AgCl NPs are formed on the surface of PbBiO2Br nanosheets. From Fig. 2f, it is clearly seen that the lattice fringes of 0.235 and 0.277 nm are corresponding to the (111) and (220) planes of Ag and AgCl, respectively [38]. The lattice fringes of 0.291 nm are correlated with the (103) plane of PbBiO2Br [39].
3.5 Optical properties of the photocatalysts
The optical properties of photocatalysts are very important for their photocatalytic application in the degradation of the antibiotics. Therefore, the optical properties of as-obtained bare PbBiO2Br, Ag/AgCl, and different Ag/AgCl/PbBiO2Br composites were investigated via ultraviolet–visible diffuse reflectance spectra (UV–Vis DRS) measurement, as shown in Fig. 3a. The bare PbBiO2Br exhibits the absorption edge at 500 nm, which is in agreement with the previous results in literatures [17, 40]. It can be seen that Ag/AgCl exhibits a strong absorption in the visible-light region. It is also obvious that the absorption intensities of Ag/AgCl/PbBiO2Br composites are stronger than that of bare PbBiO2Br in the visible-light regions, which can be attributed to the Ag SPR strategy [41].
3.6 FT-IR analysis
Figure 3b shows the Fourier transform infrared spectroscopy (FT-IR) spectra of the samples. For the pristine Ag/AgCl sample, the peak at 1044.1 cm−1 is attributed to the stretching vibration of Ag–Cl [42]. Furthermore, the stretching vibration of Ag NPs bond can be also observed at 2790.1 and 2908.7 cm−1 [43]. For pure PbBiO2Br, the peaks at 1388.4 and 1600.1 cm−1 are attributed to the bending vibrations of the Pb–O bond and the Bi–O bond, respectively [44, 45]. The broad absorption bands on the right side from 3250 to 3425 cm−1 are corresponding to the stretching vibration O–H band by the absorbed H2O [46, 47]. As for the Ag/AgCl/PbBiO2Br composites, all the absorption peaks are from Ag/AgCl and PbBiO2Br. The analysis results indicate that Ag/AgCl/PbBiO2Br are successfully fabricated.
3.7 Nitrogen adsorption analysis
According to the previously reported literature [47,48,49], the photocatalytic efficiency of the catalyst is largely dependent on its specific surface area, so the Brunauer–Emmett–Teller (BET) specific surface areas of the as-prepared samples were measured using nitrogen adsorption–desorption measurements. The BET specific surface areas of pure PbBiO2Br, Ag/AgCl, and Ag/AgCl/PbBiO2Br composites are summarized in Table 1. It is found that the BET specific surface area of the Ag/AgCl/PbBiO2Br (20.4%) is measured to be 37.16 m2/g, which is 3.06 times higher than that of pure PbBiO2Br (12.12 m2/g). The much larger surface area facilitates the contaminant contact with the catalyst and enhances the photocatalytic performance.
3.8 Photocatalytic activity
The removal of OTC was used to evaluate the photocatalytic properties of the obtained photocatalysts under visible-light irradiation, and the attained results are given in Fig. 4a. No apparent OTC degradation is detected without photocatalyst under visible-light irradiation, indicating that the direct photolysis of OTC can be almost neglected. It can be observed that 44% and 51% of OTC solution is removed within 80 min visible-light irradiation for bare PbBiO2Br and Ag/AgCl, respectively. However, the Ag/AgCl/PbBiO2Br composites exhibit enhanced photocatalytic activity in comparison to pure PbBiO2Br and Ag/AgCl under identical experimental conditions. The degradation percentage of OTC solution reaches 72%, 93.2%, and 84% for Ag/AgCl/PbBiO2Br (13.6%), Ag/AgCl/PbBiO2Br (20.4%), and Ag/AgCl/PbBiO2Br (40.8%) composites within 80 min visible-light irradiation, respectively. It is worth noting that the Ag/AgCl/PbBiO2Br (40.8%) photocatalyst has a higher mass ratio of Ag than the Ag/AgCl/PbBiO2Br (20.4%) photocatalyst, however, the photocatalytic activity of the former is lower than that of the latter. The reason may be that Ag NPs are loaded on the surface of the photocatalyst, which not only motivate the SPR, but also promote separation of electrons and holes. However, excessive Ag NPs covering on surface of the AgCl could inhibit the light absorption and decrease the separation efficiency of e−/h+ pairs, thus leading to decreased photocatalytic activity. In addition, to get further insight into the reaction kinetic behaviors, the photocatalytic degradation rates are calculated using the following equation [50,51,52]:
where, kapp stands for degradation rates constant [53]. The results are drawn and displayed in Fig. 4b. The obtained rate constants kapp are 1.06 × 10–2, 7.25 × 10–3, 1.71 × 10–2, 3.25 × 10–1 and 2.21 × 10–1 min−1 for Ag/AgCl, PbBiO2Br, Ag/AgCl/PbBiO2Br (13.6%), Ag/AgCl/PbBiO2Br (20.4%), and Ag/AgCl/PbBiO2Br(40.8%), respectively. It is clear that the rate constant kapp of Ag/AgCl/PbBiO2Br (20.4%) is 3.04 and 4.48 times higher than that of Ag/AgCl and PbBiO2Br, respectively. These results confirm that Ag/AgCl/PbBiO2Br composites accelerate the degradation of OTC in photocatalytic progress.
3.9 Cyclic experiments
In order to investigate the structural stability and practical application of Ag/AgCl/PbBiO2Br (20.4%) composite, recycling experiments were performed under the same condition, as shown in Fig. 4c. It can be observed that after the 4th run recycle experiment, the removal efficiency of Ag/AgCl/PbBiO2Br (20.4%) photocatalyst decreases from 93.2 to 92.8%. This implies that the decrease of the degradation efficiency can be negligible. Furthermore, Fig. 4d exhibits the XRD patterns of Ag/AgCl/PbBiO2Br (20.4%) photocatalyst before and after photodegradation recycling. It is clearly observed that all diffraction peaks undergo no change, indicating no any change in crystalline structure. Above results further confirm the stability of the Ag/AgCl/PbBiO2Br composites during photocatalytic process.
3.10 Possible photocatalytic mechanism
As we all know, superoxide radicals (\({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\)), hydroxyl radicals (·OH) and holes (h+) are involved in the photocatalytic reaction system as the main radical species [54]. To explore the role of the active species, the radical trapping experiments was implemented by separately adding 10 mM ethylene diaminetetraacetic acid disodium salt (EDTA-2Na), 10 mM isopropanol (IPA) and 1 mM benzoquinone (BQ) into the photocatalytic reaction system, which act as the h+, ·OH and \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\) scavengers, respectively. As depicted in Fig. 5, it is evident that with adding IPA, the degradation rate of Ag/AgCl/PbBiO2Br (20.4%) decreases slightly to 90.5%, demonstrating that there are almost no ·OH radicals generated in the degradation process. However, when adding BQ or EDTA-2Na, the degradation efficiency sharply decreases from 93.2 to 17 and 26%, respectively, demonstrating that \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\) and h+ play very important role in the degradation process. Furthermore, in this study, considering that the Cl− could be oxidized by holes to Cl0 atoms and the antibiotics OTC could be oxidized [55]. Cl0 atoms are considered to be another actual active species in the photocatalytic degradation process [56].
Many researches indicate that photoluminescence (PL) emission spectra can be induced by the recombination between photogenerated electrons and holes [57]. The lower the PL emission peaks, the less the recombination of photoexcited charge carriers. Therefore, the charge transfer and recombination processes in the photodegradation experiment can be investigated by PL spectra. Figure 6a shows the PL spectra of bare PbBiO2Br, Ag/AgCl, and different Ag/AgCl/PbBiO2Br composites in the range of 420–620 nm under excitation at 325 nm, which arise due to the recombination of photogenerated electrons and holes. It is observed that the emission spectrum intensity of bare PbBiO2Br is the strongest. However, after the coupling of Ag/AgCl NPs with PbBiO2Br nanosheets, the intensity of the PL emission spectra is decreased, indicating that the charge separation rate of Ag/AgCl/PbBiO2Br composites is more efficient than that of bare PbBiO2Br and Ag/AgCl nanoplates. It is noteworthy that the Ag/AgCl/PbBiO2Br (20.4%) composite exhibits the weakest intensity, suggesting that it has the highest separation efficiency of photoexcited charge carriers [58]. In order to further understand the charge transfer in the photocatalytic process. Electrochemical impedance spectroscopy (EIS) measurement was also carried out for the bare PbBiO2Br, Ag/AgCl, and Ag/AgCl/PbBiO2Br (20.4%) composite. As shown in Fig. 6b, it is found that Ag/AgCl/PbBiO2Br (20.4%) composite owns the smallest semicircle radius. It is commonly recognized that the curvature radius serves as an indicator of charge-transfer resistance, and a smaller semicircle radius implies higher charge transfer efficiency [59, 60].
The positions of the conduction band (CB) and valence band (VB) of obtained PbBiO2Br are about − 1.0 and 1.5 eV (vs.NHE), respectively, according to our previously reported results [17, 18]. Furthermore, in the light of the literature, the positions of the CB and VB of the AgCl are located at − 0.09 and 3.16 eV (vs. NHE), respectively [23, 25].
In the light of above experimental results, a possible photocatalysis mechanism is proposed to explain the charge transfer behaviors of Ag/AgCl/PbBiO2Br composite in the photocatalytic process. As shown in Fig. 7, the PbBiO2Br and metallic Ag NPs are photoexcited to generate e−/h+ under visible-light irradiation (Eqs. 2, 3). The AgCl is difficult to be stimulated under visible-light irradiation due to its broad bandgap. Since the SPR of Ag NPs is energetic enough to the photoexcited electrons and can be easily injected into the ECB of AgCl or PbBiO2Br (Eq. 4). These accumulated electrons on the ECB of AgCl could not reduce oxygen to form \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\), due to the ECB potential of AgCl (− 0.09 eV) more positive than the standard reduction potential of \(E_{0} \left( {{\text{O}}_{2} /{\mathbf{ \cdot }}{\text{O}}_{2}^{ - } } \right) = - \,0.33\) eV vs. NHE [61,62,63,64]. These accumulated charges could react with O2 on the surface of PbBiO2Br to form \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\) due to the ECB potential of PbBiO2Br (− 1.0 eV) more negative than the standard reduction potential of \(E_{0} \left( {{{{\text{O}}_{2} } \mathord{\left/ {\vphantom {{{\text{O}}_{2} } {{\mathbf{ \cdot }}{\text{O}}_{2}^{ - } }}} \right. \kern-\nulldelimiterspace} {{\mathbf{ \cdot }}{\text{O}}_{2}^{ - } }}} \right) = - \,0.33\) eV vs. NHE (Eq. 5). Above radical trapping experimental results verify that the \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\) is one of the main active species in the photocatalytic process. Meanwhile, the residual h+ at Ag NPs migrates to the EVB of AgCl surface to oxidize the Cl− ion to form Cl0 atoms (Eqs. 6, 7). The Cl0 atoms are reactive radical species. After that, Cl0 atoms oxidize OTC and hence are reduced to Cl− again (Eq. 8) [65, 66]. Thus, the Ag/AgCl/PbBiO2Br can maintain good catalytic performance and stability. On the other hand, from a thermodynamic point of view, the photogenerated h+ cannot react with OH− or H2O to produce ·OH since the VB potential of PbBiO2Br is more negative than the redox potentials of \(E^{0} \left( {{{OH^{ - } } \mathord{\left/ {\vphantom {{OH^{ - } } {{\mathbf{ \cdot }}OH}}} \right. \kern-\nulldelimiterspace} {{\mathbf{ \cdot }}OH}}} \right)\) (1.99 eV vs. NHE) and \(E^{0} \left( {{{H_{2} O} \mathord{\left/ {\vphantom {{H_{2} O} {{\mathbf{ \cdot }}OH}}} \right. \kern-\nulldelimiterspace} {{\mathbf{ \cdot }}OH}}} \right)\) (2.38 eV vs. NHE), indicating that the h+ can directly oxide OTC [67,68,69]. The produced active species (h+,\({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\)) can efficiently decompose OTC into intermediate products and finally into H2O and CO2 (Eq. 9). The above discussion suggests that the Ag/AgCl/PbBiO2Br composites can improve the separation of photogenerated e−/h+, finally leading to the enhancement of photocatalytic activity.
4 Conclusions
In this study, visible-light-driven novel Ag/AgCl/PbBiO2Br composites were successfully synthesized through hydrothermal and in situ photoreaction method. The UV–Vis absorption spectra confirm that the as-obtained Ag/AgCl/PbBiO2Br composites exhibit remarkable photo-absorption property in the visible-light region as compared to PbBiO2Br nanosheets, which could be due to the surface Ag resonance. The Ag/AgCl/PbBiO2Br (20.4%) composite exhibits the strongest capacity for degradation of the antibiotic OTC under visible-light irradiation, which can be mainly attributed to strong visible-light absorbance and the efficiently separation of photoexcited charge. The recycling experiments demonstrate that the Ag/AgCl/PbBiO2Br composites possess good stability. In addition, active species trapping experiments confirm that \({\mathbf{ \cdot }}{\text{O}}_{2}^{ - }\), Cl0 and h+ play an very important role in the degradation process. This work provides a way to design an excellent environmental purification material.
Availability of data and materials
All data are fully available without restriction.
References
Q.J. Yu, T. Ouyang, K.F. Zhou, C.T. Chang, Photocatalytic degradation of oxytetracycline by photosensitive materials and toxicological analysis by caenorhabditis elegans. J. Nanosci. Nanotechnol. 19, 6924–6932 (2019)
S.F. Wang, H.J. Gao, G. Sun, Y. Li, Y. Wang, H. Liu, C. Chen, L. Yang, Structure characterization, optical and photoluminescence properties of scheelite-type CaWO4 nanophosphors: effects of calcination temperature and carbon skeleton. Opt. Mater. 99, 109562 (2020)
S.J. Jiao, S.R. Meng, D.Q. Yin, L.H. Wang, L.Y. Chen, Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photoirradiation process. J. Environ. Sci. 7, 806–813 (2008)
Z.M. He, Y.M. Xia, B. Tang, J.B. Su, X.F. Jiang, Optimal co- catalytic effect of NiFe2O4/ZnO nanocomposites toward enhanced photodegradtion for dye MB. Z. Phys. Chem. 233, 347–359 (2017)
E. Guine, J.A. Garrido, R.M. Rodriguez, P.L. Cabot, C. Arias, F. Centellas, E. Brillas, Degradation of the fluoroquinolone enrofloxacin by electrochemical advanced oxidation processes based on hydrogen peroxide electrogeneration. Electrochim. Acta. 55, 2101–2115 (2010)
T. Xian, X.F. Sun, L.J. Di, Y.J. Zhou, J. Ma, H.Q. Li, H. Yang, Carbon quantum dots (CQDs) decorated Bi2O3-x hybrid photocatalysts with promising NIR-light-driven photodegradation activity for AO7. Catalysts 9, 1031 (2019)
T. Xian, L.J. Di, X.F. Sun, H.Q. Li, Y.J. Zhou, H. Yang, Photo-Fenton degradation of AO7 and photocatalytic reduction of Cr(VI) over CQD-decorated BiFeO3 nanoparticles under visible and NIR light irradiation. Nanoscale Res. Lett. 14, 397 (2019)
Y.L. Yu, S.L. Huang, Y. Gu, S. Yan, Z.J. Lan, W.J. Zheng, Y.A. Cao, Study of PbBiO2X (X = Cl, Br and I) square nanoplates with efficient visible photocatalytic performance. Appl. Surf. Sci. 428, 844–850 (2018)
F.Y. Liu, Y.R. Jiang, C.C. Chen, W.W. Lee, Novel synthesis of PbBiO2Cl/BiOCl nanocomposite with enhanced visible-driven-light photocatalytic activity. Catal. Today 300, 112–123 (2018)
B. Wang, J. Di, P.F. Zhang, J.X. Xia, S. Dai, H.M. Li, Ionic liquid-induced strategy for porous perovskite-like PbBiO2Brphotocatalysts with enhanced photocatalytic activity and mechanism insight. Appl. Catal. B 206, 127–135 (2017)
B. Wang, J. Di, L. Lu, S.C. Yan, G.P. Liu, Y.Z. Ye, H.T. Li, W.S. Zhu, H.M. Li, J.X. Xia, Sacrificing ionic liquid-assisted anchoring of carbonized polymer dots on perovskite-like pbBiO2Br for robust CO2 photoreduction. Appl. Catal. B 254, 551–559 (2019)
F.Y. Xiao, J. Xing, L. Wu, Z.P. Chen, X.L. Wang, H.G. Yang, Assembly of ultrathin PbBiO2Br nanosheets with enhanced visible light photocatalytic properties. RSC Adv. 3, 10687–10690 (2013)
C.K. Song, W.J. Feng, J.S. Zhao, X. Wang, Effect of drying temperature on properties of lithium-rich manganese-based materials in sol-gel method. Ionics 25, 4607–4614 (2019)
M. Li, W. Feng, W. Su, X. Wang, Complex hollow structures of cobalt(II) sulfide as a cathode for lithium–sulfur batteries. Int. J. Electrochem. Sci. 15, 526–534 (2020)
X. Li, J. Wang, D. Xu, Z. Sun, Q. Zhao, W. Peng, Y. Li, G. Zhang, F. Zhang, X. Fan, NbSe2 nanosheet supported PbBiO2Br as a high performance photocatalyst for the visible light-driven asymmetric alkylation of aldehyde. ACS Sustain. Chem. Eng. 3, 1017–1022 (2015)
S. Li, X. Wang, Y. Xu, H. Yang, F. Wei, X. Liu, The excellent photocatalytic synergism of PbBiO2Br/UiO-66-NH2 composites via multiple coupling effects. RSC Adv. 6, 89907–89915 (2016)
Y.M. Xia, Z.M. He, J.B. Su, K.J. Hu, Construction of novel Cu2O/PbBiO2Br composites with enhanced photocatalytic activity. J. Mater. Sci.: Mater Electron. 30, 9843–9854 (2019)
Z.M. He, J.B. Su, R. Chen, B. Tang, Fabrication of novel p-Ag2O/n-PbBiO2Br heterojunction photocatalysts with enhanced photocatalytic performance under visible-light irradiation. J. Mater. Sci.: Mater Electron. 30, 20870–20880 (2019)
H.P. Lin, W.W. Lee, S.T. Huang, L.W. Chen, T.W. Yeh, J.Y. Fu, C.C. Chen, Controlled hydrothermal synthesis of PbBiO2Br/BiOBr heterojunction with enhanced visible-driven-light photocatalytic activities. J. Mol. Catal. A 417, 168–183 (2016)
M.J. Islam, D.A. Reddy, R. Ma, Y. Kim, T.K. Kim, Reduced-graphene-oxide-wrapped BiOI-AgI heterostructured nanocomposite as a high-performance photocatalyst for dye degradation under solar light irradiation. Solid State Sci. 61, 32–39 (2016)
H.J. Gao, X.X. Zhao, H.M. Zhang, J.F. Chen, S.F. Wang, H. Yang, Construction of 2D/0D/2D face-to-face contact g-C3N4@Au@Bi4Ti3O12 heterojunction photocatalysts for degradation of rhodamine B. J. Electron. Mater. (2020). https://doi.org/10.1007/s11664-020-08243-2
Z.H. Shah, Y.Z. Ge, W.Y. Ye, X.J. Lin, S.F. Zhang, R.W. Lu, Visible light activation of SrTiO3 by loading Ag/AgX (X = Cl, Br) for highly efficient plasmon-enhanced photocatalysis. Mater. Chem. Phys. 198, 73–82 (2017)
D.B. Xu, W.D. Shi, C.J. Song, M. Chen, S.B. Yang, W.Q. Fan, B.Y. Chen, In-situ synthesis and enhanced photocatalytic activity of visible-light-driven plasmonic Ag/AgCl/NaTaO3 nanocubes photocatalysts. Appl. Catal. B 191, 28–234 (2016)
T.T. Sun, D.M. Cui, Q. Ma, X. Peng, L.J. Yuan, Synthesis of BiVO4/MWCNT/Ag@AgCl composite with enhanced photocatalytic performance. J. Phys. Chem. Solids 111, 190–198 (2017)
Z.M. He, J.B. Su, Y.M. Xia, B. Tang, Fabrication and photocatalytic performance of Bi24O31Br10 nanosphere by a polyacrylamide gel method. Micro Nano Lett. (2020). https://doi.org/10.1049/mnl.2020.0016
J.B. Zhou, W. Liu, W.Q. Cai, The synergistic effect of Ag/AgCl@ZIF-8 modified g-C3N4 composite and peroxymonosulfate for the enhanced visible-light photocatalytic degradation of levofloxacin. Sci. Total Environ. 696, 133962 (2019)
Y.F. Wang, M. Zhang, J. Li, H.C. Yang, J. Gao, G. He, Z.Q. Sun, Construction of Ag@AgCl decorated TiO2 nanorod array film with optimized photoelectrochemical and photocatalytic performance. Appl. Surf. Sci. 476, 84–93 (2019)
Y.X. Yan, H. Yang, Z. Yi, T. Xian, R.S. Li, X.X. Wang, Construction of Ag2S@CaTiO3 heterojunction photocatalysts for enhanced photocatalytic degradation of dyes. Desalin. Water Treat. 170, 349–360 (2019)
Y.M. Xia, Z.M. He, J.B. Su, K.J. Hu, Polyacrylamide gel synthesis and photocatalytic performanceof PbBiO2Br nanosheets. Mater. Lett. 241, 64–67 (2019)
Z.M. He, J.B. Su, B. Tang, Y.M. Xia, Fabrication of novel Cu2O/Bi24O31Br10 composites and excellent photocatalytic performance. J. Mater. Sci.: Mater Electron. 29, 19544–19553 (2018)
Z.M. He, Y.M. Xia, J.B. Su, Fabrication of novel AgBr/Bi24O31Br10 composites with excellent photocatalytic performance. RSC Adv. 8, 39187–39196 (2018)
N.M. Mahmoodi, A. Taghizadeh, M. Taghizadeh, J. Abdi, In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. J. Hazard. Mater. 378, 120741 (2019)
M.M. Sajid, N.A. Shad, A.M. Afzal, Y. Javed, S.B. Khan, N. Amin, A. Shah, I. Yousaf, H.F. Zhai, Generation of strong oxidizing radicals from plate-like morphology of BiVO4 for the fast degradation of crystal violet dye under visible light. Appl. Phys. A 126, 314 (2020)
Y.M. Xia, J.B. Su, Z.M. He, Z-scheme charge separation in Bi24O31Br10/SrTiO3 nanocomposites for degradation of methyl orange. J. Electron. Mater. 48, 3890–3899 (2019)
Y.X. Yan, H. Yang, Z. Yi, X.X. Wang, R.S. Li, T. Xian, Evolution of Bi nanowires from BiOBr nanoplates through a NaBH4 reduction method with enhanced photodegradation performance. Environ. Eng. Sci. 37, 64–77 (2020)
Y.M. Xia, Z.M. He, W. Yang, B. Tang, Y.L. Lu, K.J. Hu, J.B. Su, X.P. Li, Effective charge separation in BiOI/Cu2O composites with enhanced photocatalytic activity. Mater. Res. Express 5, 025504 (2018)
Y.M. Xia, Z.M. He, J.B. Su, B. Tang, K.J. Hu, Y.L. Lu, X.P. Li, Fabrication of n-SrTiO3/p-Cu2O heterojunction composites with enhanced photocatalytic performance. J. Alloys Compd. 753, 356–363 (2018)
M. Zhao, W. Zhou, M.M. Lu, Z.P. Guo, C.D. Li, W.J. Wang, Novel AgCl nanotubes/BiOCl nanosheets composite with improved adsorption capacity and photocatalytic performance. J. Alloys Compd. 773, 1146–1153 (2019)
Y.M. Xia, Z.M. He, J.B. Su, One-step construction of novel Ag3PO4/PbBiO2Br composite with enhanced photocatalytic activity. Mater. Res. Express 6, 085909 (2019)
M. Golkari, H. Shokrollahi, H. Yang, The influence of Eu cations on improving the magnetic properties and promoting the Ce solubility in the Eu Ce-substituted garnet synthesized by the solid state route. Ceram. Int. 46, 8553–8560 (2020)
C.D. Yu, P. Wang, X.F. Wang, F. Chen, H.G. Yu, Silver-melamine nanowire-assisted synthesis of net-like AgCl-Ag/g-C3N4 for highly efficient photocatalytic degradation ability. J. Alloys Compd. 806, 263–271 (2019)
S.F. Yang, C.G. Niu, D.W. Huang, H. Zhang, C. Liang, G.M. Zeng, SrTiO3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A. Environ. Sci-Nano 4, 585–595 (2017)
J.W. Pan, Z.M. He, J.B. Su, R. Chen, B. Tang, Preparation and optical properties of Ni-doped PbBiO2Br nanoparticles. Mater. Res. Express 6, 115042 (2019)
Y.M. Xia, Z.M. He, J.B. Su, X.P. Li, B. Tang, One-step construction of novel PbBiO2Br/ZnO heterojunction composites with enhanced photocatalytic activity. Phys. Status Solidi A 216, 1900406 (2019)
Y.M. Xia, Z.M. He, J.B. Su, S.Q. Zhu, B. Tang, Sustainable solar-light-driven SrTiO3/PbBiO2Br nanocomposites with enhanced photocatalytic activity. J. Electron. Mater. 249, 3259–3268 (2020)
O. Dehghani Dastjerdi, H. Shokrollahi, H. Yang, The enhancement of the Ce-solubility limit and saturation magnetization in the Ce0.25BixPryY2.75-x-yFe5O12 garnet synthesized by the conventional ceramic method. Ceram. Int. 46, 2709–2723 (2020)
M.M. Sajid, N. Amin, N.A. Shad, S.B. Khan, Y. Javed, Z.J. Zhang, Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater. Sci. Eng. B 242, 83–89 (2019)
M.M. Sajid, N.A. Shad, A.M. Afzal, Y. Javed, S.B. Khan, Z. Imran, S. Hassan, Z. Hussain, Z.J. Zhang, N. Amin, Fast surface charge transfer with reduced band gap energy of FeVO4/graphene nanocomposite and study of its electrochemical property and enhanced photocatalytic activity. Arab. J. Sci. Eng. 44, 6659–6667 (2019)
M.M. Sajid, N.A. Shad, Y. Javed, S.B. Khan, Z.J. Zhang, N. Amin, H.F. Zhai, Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surf. Interfaces 19, 100502 (2020)
Z.M. He, Y.M. Xi, J.B. Su, B. Tang, Fabrication of magnetically separable NiFe2O4/Bi24O31Br10 nanocomposites and excellent photocatalytic performance under visible light irradiation. Opt. Mater. 88, 195–203 (2019)
C.X. Zheng, H. Yang, Z.M. Cui, H.M. Zhang, X.X. Wang, A novel Bi4Ti3O12/Ag3PO4 heterojunction photocatalyst with enhanced photocatalytic performance. Nanoscale Res. Lett. 12, 608 (2017)
Y.M. Xia, Z.M. He, J.B. Su, B. Tang, K.J. Hu, Y.L. Lu, S.P. Sun, X.P. Li, Fabrication of magnetically separable NiFe2O4/BiOI nanocomposites with enhanced photocatalytic performance under visible-light irradiation. RSC Adv. 8, 4284–4294 (2018)
G.J. Gao, W.J. Feng, W.X. Su, S.J. Wang, L.J. Chen, M.M. Li, C.K. Song, Preparation and modification of MIL-101(Cr) metal organic framework and its application in lithium-sulfur batteries. Int. J. Electrochem. Sci. 15, 1426–1436 (2020)
S.F. Wang, H.J. Gao, C. Chen, Q. Li, C. Li, Y. Wei, L. Fang, Effect of phase transition on optical and photoluminescence properties of nano-MgWO4 phosphor prepared by a gamma-ray irradiation assisted polyacrylamide gel method. J. Mater. Sci.: Mater. Electron. 30, 15744–15753 (2019)
K.H. Wu, Y.C. Cheng, K.F. Cheng, J.C. Wang, Antibacterial activity of surface-modified fabric with Ag/AgCl-doped quaternary a mmonium modified silicate hybrid. J Nanosci Nanotechnol. 19, 7285–7293 (2019)
Q. Xiao, Z.C. Si, J. Zhang, C. Xiao, X.K. Tan, Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J. Hazard. Mater. 150, 62–67 (2008)
S.F. Wang, C. Chen, Y. Li, Q. Zhang, H. Gao, Synergistic effects of optical and photoluminescence properties, charge transfer, and photocatalytic activity in MgAl2O4: Ce and Mn-codoped MgAl2O4: Ce phosphors. J. Electron. Mater. 48, 6675–6685 (2019)
Y.M. Xia, Z.M. He, J.B. Su, Y. Liu, B. Tang, Fabrication and photocatalytic property of novel SrTiO3/Bi5O7I nanocomposites. Nanoscale Res. Lett. 13, 148 (2018)
Z.M. He, Y.M. Xia, B. Tang, J.B. Su, Fabrication and photocatalytic property of magnetic NiFe2O4/Cu2O composites. Mater. Res. Express. 4, 095501 (2017)
Y.M. Xia, Z.M. He, J.B. Su, B. Tang, Y. Liu, Enhanced photocatalytic performance of Z-scheme Cu2O/Bi5O7I nanocomposites. J. Mater. Sci.: Mater. Electron. 29, 15271–15281 (2018)
M. Li, W.J. Feng, W. Su, X. Wang, CoNi-embedded nitrogen-enriched porous carbon framework for long-life lithium–sulfur batteries. J. Solid State Electrochem. 23, 2317–2324 (2019)
Y.M. Xia, Z.M. He, J.B. Su, B. Tang, Y. Liu, X.P. Li, Fabrication of novel n-SrTiO3/p-BiOI heterojunction for degradation of crystal violet under simulated solar light irradiation. NANO 13, 1850070 (2018)
X.X. Yao, X.H. Liu, D. Zhu, C.B. Zhao, L.D. Lu, Synthesis of cube-like Ag/AgCl plasmonic photocatalyst with enhanced visible light photocatalytic activity. Catal. Commun. 59, 151–155 (2015)
Z.M. He, Y.M. Xia, B. Tang, X.F. Jiang, J.B. Su, Fabrication and photocatalytic property of ZnO/Cu2O core-shell nanocomposites. Mater. Lett. 184, 148–151 (2016)
S.F. Wang, H.J. Gao, Y. Wang, G. Sun, X. Zhao, H. Liu, C. Chen, L. Yang, Effect of the sintering process on the structure, colorimetric, optical and photoluminescence properties of SrWO4 phosphor powders. J. Electron. Mater. (2020). https://doi.org/10.1007/s11664-020-07941-1
S.F. Wang, H.J. Gao, Y. Wei, Y.W. Li, X.H. Yang, L.M. Fang, L. Lei, Insight into the optical, color, photoluminescence properties, and photocatalytic activity of the N-O and C-O functional groups decorating spinel type magnesium aluminate. CrystEngComm 21, 263–277 (2019)
Y.M. Xia, Z.M. He, Y.L. Lu, B. Tang, S.P. Sun, J.B. Su, X.P. Li, Fabrication and photocatalytic property of magnetic SrTiO3/NiFe2O4 heterojunction nanocomposites. RSC Adv. 8, 5441–5450 (2018)
M.M. Sajid, N.A. Shad, Y. Javed, S.B. Khan, N. Amin, Z.J. Zhang, Z. Imran, M.I. Yousuf, Facile synthesis of Zn3(VO4)2/FeVO4 heterojunction and study on its photocatalytic and electrochemical properties. Appl. Nanosci. (2018). https://doi.org/10.1007/s13204-019-01199-8
M.M. Sajid, N.A. Shad, Y. Javed, S.B. Khan, Z.J. Zhang, N. Amin, N. Amin, H.F. Zhai, Morphological effects on the photocatalytic performance of FeVO4 nanocomposite. Nano-Struct. Nano-Object. 22, 100431 (2020)
Funding
This research has been supported by the China National Key R&D Project during the 13th Five-year Plan Period (Grant No. 2017YFB0602500) and University Natural Science Research Program of Jiangsu Province (16KJA610002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Liu, Z., Song, W. et al. Novel AgCl nanoparticles coupling with PbBiO2Br nanosheets for green and efficient degradation of antibiotic oxytetracycline hydrochloride under visible-light irradiation. J Mater Sci: Mater Electron 31, 12137–12147 (2020). https://doi.org/10.1007/s10854-020-03760-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03760-6