Abstract
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is the most commonly investigated conductive polymer due to its unique thermoelectric properties. Several post treatments using various acids have been reported to improve the thermoelectric properties of PEDOT:PSS. In most articles, however, only the role of acids on the structure and thermoelectric properties of PEDOT:PSS were reported. In this article, we report the major role of the pressure of nitrogen gas (N2) on the structure and thermoelectric properties of PEDOT:PSS after treatment with various acids at optimized temperatures. After treatment with acids followed by N2 gas-passing, electrical conductivity enhances significantly. Therefore, N2 gas-passing after acid treatments results in higher thermoelectric properties than other traditional acid treatment methods. The enhancement in electrical conductivity is attributed to the removal of insulating PSS and the conformational change of the PEDOT chain. Furthermore, the pressure of N2 is responsible for the additional conformation of PEDOT chain, favoring the linear orientation of the PEDOT structure and resulting in an improvement of the electrical conductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The major portion of today’s energy supply originates from fossil fuels and, which induce pollution and environmental problems [1,2,3]. However, energy is wasted as heat from automobile engines, industries, motors and power systems during their operation every day. Energy harvesting devices enable the conversion of wasted heat to energy for various applications [4, 5]. Thermoelectric (TE) material which can convert heat to electrical energy and vice versa, could help to address this issue by recycling the waste heat [6, 7]. The efficiency of the thermoelectric material is defined by dimensionless figure of merit, ZT = S2σT/κ, where S is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature and κ is the thermal conductivity [8,9,10].
Owing to the discovery and development of conductive polymers by Heeger, Macdiarmid and Shirakawa, it has been used in a wide range of electronic applications [11, 12]. Poly (3,4-ethylenedioxythiophene)(PEDOT): poly styrene sulfonate (PSS) has advantages over other conductive polymers due to its outstanding properties such as easy processing, high electrical conductivity, stability and flexibility [13, 14]. On the other hand, TE performance of PEDOT:PSS are still lower for its practical uses due to the excess insulating PSS in the PEDOT:PSS dispersion. Moreover, PSS molecules play an important role in PEDOT:PSS as counter ions as well as dispersion agent for PEDOT. Therefore, it is necessary to selectively remove the excess PSS to improve electrical conductivity of PEDOT:PSS [15, 16]. Previous works have employed the post-treatment of PEDOT:PSS films with various acids to improve the TE properties by controlling the doping level. Nevertheless, only the effect of acids or organic solvents on the structure and thermoelectric properties of the PEDOT:PSS are focused in their reports [10, 16,17,18,19]. In this article, we report the post-treatment of PEDOT:PSS films with various acids followed by N2 gas-passing. The influence of the pressure of N2 gas on the structure and thermoelectric properties of PEDOT:PSS at various pressures are also discussed. The electrical conductivity significantly improved from less than 1 Scm−1 to 1810 ± 200 Scm−1 for H2SO4, 1175 ± 23 Scm−1 for HNO3 and 846 ± 72 Scm−1 for HCl treatments followed by N2 gas-passing with the pressure of 0.1 MPa. Corresponding power factor of 97 ± 8 µWm−1K−2, 121 ± 10 µWm−1K−2 and 32 ± 1 µWm−1K−2 were observed for H2SO4, HNO3, and HCl treatments, respectively. Based on our results, acids are mainly responsible for the removal of insulating PSS resulting in the conformational change of the PEDOT polymer chain while N2 pressure is responsible for the additional conformation of PEDOT polymer favoring the linearly oriented structure. Therefore, mechanical force such as N2 pressure after post-treatment of PEDOT:PSS with various acids can lead to additional conformation of the polymer and can result in higher thermoelectric performance.
2 Experimental details
PEDOT:PSS (Clevios PH 1000) was purchased from Heraeus Deutschland GmbH &Co. KG (Germany). Sulfuric acid (95%), nitric acid (69%) and hydrochloric acid (37%) from Wako Pure Chemical Industries Ltd. (Japan) were used as received in our experiment.
The glass substrates were washed with deionized (DI) water, ethanol, and acetone and finally cleaned in the Filgen UV ozone cleaner for 20 min. PEDOT:PSS film (100 µl) was prepared by spin-coating at a spin speed of 600 rpm for 30 s followed by 2000 rpm for 20 s.
- H2SO4 treatment:
-
PEDOT:PSS film was put on the hot plate at a temperature of 160 °C, then 100 µl of H2SO4 was dropped onto it. After 10 min, N2 gas was passed with a pressure of 0.1 MPa using gun. Then, the films were thoroughly rinsed with DI water and dried on the hot plate at a temperature of 120 °C for 15 min
- HNO3 treatment:
-
HNO3 treatment was done as in our previous report [20]
- HCl treatment:
-
HCl treatment was done the same procedure as the H2SO4 treatment except the treatment temperature was at 70 °C
The films were fixed at 20 mm away from the gun for all experiments. All treatments were done at their optimized treatment temperatures and conditions. Figure 1 shows the process of N2 gas-passing on the PEDOT:PSS films after treatment with various acids.
Electrical conductivity, Seebeck coefficient (thermo power) and power factor of the films were measured in a helium environment by ZEM-3M8 ULVAC (ADVANCED RIKO, Inc.), which is attached to the sample holder for the thin film measurement. Surface of the films was observed by digital microscope (OLYMPUS BX53M) at a magnification of 7.5×. Carrier concentration and carrier mobility were measured to examine the effect of N2 on the improvement of electrical conductivity using Lake Shore Model 8403, AC/DC Hall effect measurement system. The change in bonding condition before and after treatment was analyzed by Raman spectroscopy (JASCO, NRS 450 NMDS) with a green laser (wavelength 532.21 nm). The morphology and surface roughness of the PEDOT:PSS films were observed by Scanning Probe Microscopy (SPM, Nano Navi SII). The removal of insulating PSS was confirmed by X-ray photoelectron spectroscopy (XPS, JEOL JPS 9030, Japan). UV–Vis-NIR (JASCO V-670 spectrophotometer) was performed to observe the doping states of the PEDOT. The change in crystallinity of PEDOT:PSS films after treatment at various pressures of N2 gas was characterized by RIGAKU SmartLab X-ray diffractometer with CuKα radiation (λ = 1.5418° A) at a scanning rate of 1 degree per minute.
3 Results and discussion
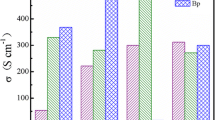
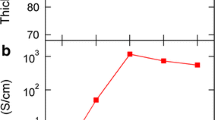
Electrical conductivity, Seebeck coefficient and power factor of pristine PEDOT:PSS are around 1.7 ± 0.2 Scm−1, 20 ± 1 µVK−1 and 0.07 ± 0.005 µWm−1K−2 at a measurement temperature of 423 K. Electrical conductivity of H2SO4, HNO3 and HCl treated PEDOT:PSS films are 1400 ± 210 Scm−1, 656 ± 75 Scm−1 and 632 ± 27 Scm−1 respectively after washing with DI water. Electrical conductivity increased to 1810 ± 200 Scm−1 for H2SO4 treated, 1175 ± 23 Scm−1 for HNO3 treated and 846 ± 72 Scm−1 for HCl treated PEDOT:PSS films after N2 gas-passing with a pressure of 0.1 MPa as illustrated in Fig. 2a. All the measurements for electrical conductivity, Seebeck coefficient and power factor are carried out at 423 K in our experiments. The improvement in electrical conductivity after acids treatment followed by DI water-washing can be attributed to the removal of the insulating PSS, also resulting in the conformational change of the PEDOT polymer chain from benzoid (coiled) to quinoid (linear) structure [21,22,23]. In contrast, the improvement in the electrical conductivity after acids treatment followed by N2 gas-passing can be ascribed as the additional conformational change of the PEDOT due to the pressure of N2 gas. It is confirmed by Raman spectroscopy as shown in Fig. 4. Seebeck coefficient does not change significantly for all acids except for HNO3 from 27 ± 1 to 32 ± 1 µVK−1 (Fig. 2b) after DI water-washing and N2 gas-passing respectively, but which is still unclear why. The power factor improved from 72 ± 6 to 97 ± 8 µWm−1 K−2 for H2SO4, from 46 ± 3 to 121 ± 10 µWm−1 K−2 for HNO3 and 26 ± 1 to 32 ± 1 µWm−1 K−2 for HCl treated PEDOT:PSS films (Fig. 2c) after corresponding acid treatments followed by DI water-washing and N2 gas-passing at a pressure of 0.1 MPa. Therefore, N2 gas-passing after acid treatments can give the higher thermoelectric power factor values rather than DI water- washing after acid treatments. Electrical conductivity, Seebeck coefficient and power factor of HNO3 treated PEDOT:PSS films at different pressures of N2 gas are shown in Fig. 2d. The electrical conductivity increases at the pressure range of 0.1 to 0.2 MPa and then decreases beyond 0.2 MPa. This is probably due to the film damage or some defects on the surface of the films because of the high pressure of N2 gas. These surface defects are observed by digital microscope as in Fig. 3. Smooth surface are observed in pristine PEDOT:PSS film and the film treated with a pressure of 0.1 MPa. Some pinholes or micropores are observed on the surface of the films treated at pressure of 0.15 and 0.2 MPa while big scratches are observed on the film treated with a pressure of 0.25 MPa.
a Electrical conductivity, b Seebeck coefficient and c power factor of the PEDOT:PSS films treated with various acids followed by DI water-washing and passing with the pressure of N2 gas (0.1 MPa). d Electrical conductivity, Seebeck coefficient and power factor of the HNO3 treated PEDOT:PSS films followed by passing with different pressures of N2 gas such as 0.05 MPa, 0.1 MPa, 0.15 MPa, 0.2 MPa, 0.25 MPa and 0.3 MPa
The optimum Seebeck coefficient of 32 ± 1 µVK−1 was obtained at a pressure of 0.1 MPa. Seebeck coefficient is defined by
where σ(E) is the conductivity of the material at Femi energy Ef, kβ is the Boltzmann constant and e is the charge of the electron [24, 25]. Based on this equation, Seebeck coefficient also depends on the local band structure of the material. The removal of PSS may transform the local band structure of PEDOT:PSS and such a probable change in the band structure may be responsible for the improvement in the Seebeck coefficient [26]. Unfortunately, we failed to do experiment at a pressure of 0.1 MPa in our previous report [20]. Nevertheless, the optimum power factor of 121 ± 10 µWm−1K−2 was obtained by the treatment with HNO3 at a pressure of 0.1 MPa due to the high Seebeck coefficient.
We observed that the pressure of N2 gas favors the phase segregation of PSS as well as the conformation of the PEDOT polymer chain resulting in the enhancement of the carrier concentration, especially the carrier mobility. It is confirmed by the Hall measurement as shown in Table (1). Pristine PEDOT:PSS film has hole mobility of around 2.4 ± 0.3 cm2V−1s−1 and hole concentration of an order of 17 cm−3. After treatment with HNO3and passing with N2 gas at various pressures, the hole concentration enhanced to an order of 21 and the hole mobilities are also improved. The optimum carrier mobility of 7 ± 1.4 cm2V−1s−1 is obtained for the sample treated at a pressure of 0.1 MPa while the carrier concentrations are not too much different at various pressures of N2 gas. Therefore, the pressure of the N2 gas can lead to the linear orientation of the polymer structure. The carrier mobility decreases again beyond the pressure of 0.1 MPa and this may be attributed to the defects on the surface of the films such as pin holes and damages, which is also consistent with the digital micrographs.
Raman spectroscopy was also performed to confirm the effect of the pressure of N2 gas on the conformation of the PEDOT polymer chain. Calibration was performed using Si substrate before and after sample measurement in order to investigate the potential heating effect on the spectrum. No significant peak shift was found for the calibration results, indicating that there was no potential heating effect on the spectral peak position. The peaks assigned at 1227 cm−1 in Fig. 4 come from Cα‒Cα inter-ring stretching, 1337 cm−1 from Cβ‒Cβ inter-ring stretching, 1401 cm−1 from Cα=Cβ symmetric stretching, 1506 cm−1from Cα=Cβ asymmetric stretching, respectively [21,22,23, 27, 28]. The peak at 1401 cm−1shifts to 1404 cm−1, 1406 cm−1, 1407 cm−1, 1408 cm−1 and 1409 cm−1, respectively after treatment with HNO3 and the pressure of N2 gas at 0.05 MPa, 0.1 MPa, 0.15 MPa, 0.2 MPa and 0.25 MPa respectively. These peaks shift reveal the conformation of the PEDOT from benzoid (coiled) to quinoid (linear) structure [21,22,23]. Therefore, the pressure of the N2 gas after acid treatment can favor the linearly oriented structure of the PEDOT, resulting in the enhancement of the electrical conductivity which is also consistent with the Hall measurement. It was observed in the previous reports that oriented mechanical forces induced by directional film deposition methods [29, 30], mechanical rubbing [31] and capillary force [32] makes the polymer films highly aligned and causes anisotropic charge transport in the film. Similar to prior reports, N2 blown from one side of the sample might include oriented mechanical force, creating the anisotropic alignment in the PEDOT:PSS and leading to increase in electrical conductivity.
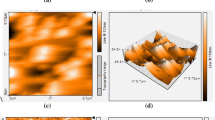
In order to observe the morphological changes of PEDOT:PSS at various pressures of N2 gas, the SPM images were scanned for the pristine PEDOT:PSS films and the HNO3 treated films. Figure 5 represents the topography and phase images of the pristine and HNO3-treated PEDOT:PSS films at various pressures of N2 gas at 0.05 MPa, 0.1 MPa, 0.15 MPa, 0.2 MPa and 0.25 MPa respectively. The bright region represents the PEDOT and the dark brown region represents the PSS in the phase images of the films. We can see clearly that some dark colored regions disappear after the treatment in the phase images. It can be attributed to the partial removal of PSS ions [33]. In the topographic images, the root mean square (rms) roughness of the pristine PEDOT:PSS film is 1.2 nm. However, the rms roughness of the treated films become 1.3 nm, 1.5 nm, 1.4 nm, 1.7 nm and 2.0 nm at the N2 pressures of 0.05 MPa, 0.1 MPa, 0.15 MPa, 0.2 MPa and 0.25 MPa respectively. It is an evidence of the phase separation of PSS from PEDOT indicating the removal of PSS phase [34]. Interestingly, the rms of the pristine and the HNO3 treated samples at N2 pressures of 0.05 MPa, 0.1 MPa and 0.15 MPa are not too much different, nevertheless, those of the treated samples at 0.2 MPa and 0.25 MPa significantly increase to 1.7 nm and 2.0 nm. Therefore, the pressure of N2 gas favors the smoother surface morphology even though there is phase separation between the PEDOT and PSS grains. On the other hand, the higher pressure of N2 gas leads to the surface defects like pin holes, resulting in the increase of rms values.
XPS was conducted to analyze the influence of the pressure of N2 gas on the removal of extra PSS phase of the HNO3 treated PEDOT:PSS films. XPS bands at 169.1 eV and 167.7 eV are the S2p bands of the sulfur atoms in PSS and the bands at 164.3 eV and 163.1 eV are the S2p bands of the sulfur atoms in PEDOT [35] as in Fig. 6a. The S2p peaks of PSS shifts around 1 eV to the lower binding energy after the treatment with HNO3 followed by passing at various pressures of N2 gas. The atomic ratio of PEDOT:PSS was calculated from the area under the peaks using full width at half maximum (FWHM) of curve fitting. The ratio of PEDOT to PSS before treatment is 1:2.52. These ratios significantly increase to 1:0.41, 1:0.44, 1:0.58, 1:0.59 and 1:0.4 after treatment with HNO3 followed by the pressure of N2 gas at 0.05 MPa, 0.1 MPa, 0.15 MPa, 0.2 MPa and 0.25 MPa respectively. Nevertheless, the ratios are not significantly different at different pressures of N2 gas. Therefore, HNO3 treatment is essentially responsible for the removal of insulating PSS and, on the other words, the pressure of N2 gas after HNO3 treatment does not play a major role in removing the extra PSS. The peaks at 285.7 eV and 284 eV in C1 s spectra come from the C–O bond in PEDOT and C–C bond in PSS chain (Fig. 6b) [36]. After treatment with HNO3 and passing with various pressures of N2 gas, the intensities of the peaks at 285.7 eV increase while the intensities of the peaks at 284 eV decrease, indicating the removal of thiophene group of PSS. The peaks at 531.4 eV and 532.5 eV in Fig. 6c come from the oxygen atom which is attached to the sulfur atom in PSS and the oxygen atom in PEDOT. After treatment, the intensities of the peaks at higher binding energy increases while the intensities of the peaks at lower binding energy decreases. It can be attributed to the phase segregation of PSS from the PEDOT:PSS resulting in the formation of PSSH [34, 35]. However, by comparing the XPS results of the treated films at different pressures of N2 gas, the intensities of the peaks are very similar to each other. It means that passing with N2 gas after acid treatment does not play a major role in the removal of PSS.
UV–vis-NIR absorption spectra were recorded to confirm the above XPS analysis as illustrated in Fig. 7a and b. The decrease in the intensities of the two absorption bands at 196 nm and 225.8 nm in the UV region (Fig. 7a) after acid treatment followed by passing with N2 gas at various pressures indicates the removal of extra PSS from PEDOT:PSS, hence both bands come from the PSS moiety [36]. However, all the bands stack one upon another after HNO3 treatment at various pressures of N2 gas show that the amount of PSS removal is similar at different treatment pressures. The absorption bands at near infrared region were also recorded to examine the influence of N2 pressure after treatment. The absorption bands around 600 nm, 900 nm and 1200 nm come from the neutral, polaron and bipolaron states of the PEDOT, which have reported in the previous literature [36]. The improvement of the absorption intensities at ≈ 900 nm after HNO3 treatment and at various pressures of N2 gas reveals an increase in its doping state to polaronic state. Therefore, the results of UV–vis-NIR measurement agree well with XPS measurement.
To investigate the mechanism of significantly increasing the electrical conductivity after acid treatment followed by N2 gas-passing, X-ray diffraction (XRD) was performed as depicted in Fig. 8. The pristine PEDOT:PSS film shows only the amorphous halo inter-chain stacking at 2θ value of 23.8°. After treatment with HNO3 and at various pressures of N2 gas, the new peaks appear at around 2.5° and 6.8°, which are attributed to the lamellae stacking of the alternating structure of PEDOT and PSS. Therefore, after acid treatment, PEDOT crystallites incline to a linear structure resulting in more lamellae stacking [37,38,39], which is consistent with the Raman spectroscopy results. Moreover, no significant peaks shifts are observed for the positions of both peaks resulting in no obvious changes of lattice spacing although different pressures of N2 gas are used. Therefore, it can be ascribed as the pressure of N2 gas has no significant influences on the removal of extra PSS.
4 Conclusion
In summary, we have reported the influence of the pressure of N2 gas after acid treatment on the structure and thermoelectric properties of PEDOT:PSS. The improvement of electrical conductivity is due to the removal of insulating extra PSS from PEDOT:PSS and the conformation of PEDOT polymer structure from benzoid (coiled) to quinoid (linear) structure. In our experiment, there are two reasons for the conformation of PEDOT: (1) the removal of PSS by acid treatments and (2) the pressure of N2 gas. Acid treatment plays a major role in the removal of the insulating PSS, resulting in the conformation of PEDOT while the pressure of N2 gas is mainly responsible for the additional conformation of polymer favoring the linear orientation of the structure. Therefore, mechanical forces such as the pressure of N2 gas can affect the structure of acid-treated PEDOT: PSS regardless of the types of acids. Applying mechanical forces after acid treatment can give higher thermoelectric performance rather than traditional DI water-washing for PEDOT:PSS films.
References
R. Toshima, Recent progress of organic and hybrid thermoelectric materials. Synth. Metal 225, 3–21 (2017)
K. Prashantha, S. Wango, Smart power generation from waste heat by thermoelectric generator. Int. J. Mech. Prod. Eng., Special Issue (2016)
M. Culebras, A.M. Igual-Muñoz, C. Rodriguez-Fernández, M.I. Gómez-Gómez, C. Gómez, A. Cantarero, Manufacturing Te/PEDOT films for thermoelectric application. ACS Appl. Mater. Interfaces 9(24), 20826–20832 (2017)
E. Jang, A. Poosapati, D. Madan, Enhanced thermoelectric properties of F4TCNQ doped P3HT and its use as a binder for Sb2Te3 based printed thermoelectric films. ACS Appl. Energy Mater. 1(4), 1455–1462 (2018)
G. Chen, W. Xu, D. Zhu, Recent advances in organic polymer thermoelectric composites. J. Mater. Chem. C 5, 4350–4360 (2017)
M. Culebras, C. Cho, M. Krecker, R. Smith, Y. Song, M. Clara, C.M. Gomez, A. Cantarero, J.C. Grunlan, High thermoelectric power factor organic thin films through combination of nanotube multilayer assembly and electrochemical polymerization. ACS Appl. Mater. Interfaces 9(7), 6306–6313 (2017)
J. Li, Y. Du, R. Jia, J. Xu, S.Z. Shen, Thermoelectric properties of flexible PEDOT:PSS/polypyrrole/paper nanocomposite films. Mater. (Basel) 10(7), 780 (2017)
Z. Zhu, C. Liu, F. Jiang, J. Xu, E. Liu, Effective treatment methods on PEDOT:PSS to enhance its thermoelectric performance. Synth. Metals 225, 31–40 (2017)
J. Luo, D. Billep, T. Waechtler, T. Otto, M. Toader, O. Gordan, E. Sheremet, J. Martin, M. Hietschold, D.R.T. Zahn, T. Gessner, Enhancement of the thermoelectric properties of PEDOT:PSS thin films by post-treatment. J. Mater. Chem. A 1, 7576–7583 (2013)
J.S. Kim, W. Jang, D. Wand, The investigation of the seebeck effect of the poly (3,4-Ethylenedioxythiophene)-tosylate with the various concentrations of an oxidant. Polymers 11(1), 21 (2019)
V. Andrei, K. Bethke, F. Madzharova, S. Beeg, A. Knop-Gericke, J. Kneipp, K. Rademann, Size dependence of electrical conductivity and thermoelectric enhancements in spin-coated PEDOT:PSS single and multiple layers. Adv. Electron. Mater. 3, 2 (2017)
N. Dubey, M. Leclerc, Conducting polymers: efficient thermoelectric materials. J. Polym. Sci. 49(7), 467–475 (2011)
Q. Meng, Q. Jiang, K. Cai, L. Chen, Preparation and thermoelectric properties of PEDOT:PSS coated Te nanoroad/PEDOT:PSS composite films. Org. Electron. 64, 79–85 (2019)
H. Shi, C. Liu, Q. Jiang, J. Xu, Effective approach to improve the electrical conductivity of PEDOT:PSS: a review. Adv. Electron. Mater. 1, 4 (2015)
S.H. Lee, H. Park, W. Son, H.H. Choi, J.H. Kim, Novel solution-processable dedoped semiconductors for application in thermoelectric devices. J. Mater. Chem. A 2, 13380–13387 (2014)
X. Wang, A.K.K. Kyaw, C. Yin, F. Wang, Q. Zhu, T. Tang, P.I. Yee, J. Xu, Enhancement of thermoelectric performance of PEDOT:PSS films by post-treatment with a superacid. RSC Adv 8, 18334–18340 (2018)
Z. Fan, P. Li, D. Du, J. Ouyang, Significantly enhanced thermoelectric properties of PEDOT:PSS films through sequential post-treatments with common acids and bases. Adv. Energy Mater. 7, 8 (2017)
W. Meng, R. Ge, Z. Li, J. Tong, T. Liu, Q. Zhao, S. Xiong, F. Jiang, L. Mao, Y. Zhou, Conductivity enhancement of PEDOT:PSS films via phosphoric acid treatment for flexible all-plastic solar cells. ACS Appl. Mater. Interfaces. 7(25), 14089–14094 (2015)
A.K.K. Kyaw, T.A. Yemata, X. Wang, S.L. Lim, W.S. Chin, K. Hippalgaonkar, J. Xu, Enhanced thermoelectric properties of PEDOT:PSS films by sequential post-treatment with formamide. Macromol. Mater. Eng. 303, 1700429 (2018)
M.T. Myint, M. Hada, H. Inoue, T. Marui, T. Nishikawa, M. Umeno, S. Ichimura, Y. Nishina, A.K. Kyaw, Y. Hayashi, Simultaneous improvement in electrical conductivity and seebeck coefficient of PEDOT:PSS by N2 pressure-induced nitric acid treatment. RSC Adv. 8, 36563 (2018)
S.R.S. Kumar, N. Kurra, H.N. Alshareef, Enhanced high temperature thermoelectric response of sulfuric acid treated conducting polymer thin films. J. Mater. Chem. C 4, 215–221 (2016)
Y. Xia, J. Ouyang, Significant conductivity enhancement of conductive poly (3,4-ethylenedioxythiophene): poly (styrenesulfonate) films through a treatment with organic carboxylic acids and inorganic acids. ACS Appl. Mater. Interfaces 2(2), 474–483 (2010)
Z. Zhu, C. Liu, H. Shi, Q. Jiang, J. Xu, F. Jiang, J. Xiong, E. Liu, An effective approach to enhance thermoelectric properties of PEDOT:PSS films by a DES post-treatment. J. Polym. Sci. Part B 53(12), 885–892 (2015)
J. Kim, J.G. Jang, J.-I. Hong, S.H. Kim, J. Kwak, Sulfuric acid vapour treatment for enhancing the thermoelectric properties of PEDOT:PSS thin-film. J. Mater. Sci. 27, 6122–6127 (2016)
Y. Zheng, Y. Luo, C. Du, B. Zhu, Q. Liang, H.H. Hng, K. Hippalgaonkar, J. Xu, Q. Yan, Designing hybrid architectures for advanced thermoelectric materials. Mater. Chem. Front. 1, 2457–2473 (2017)
A. Mazaheripour, S. Majumdar, D. Hanemann-Rawlings, E.M. Thomas, C. MacGuiness, L. d’Alencon, M.L. Chabinyc, R.A. Segalman, Tailoring the seebeck coefficient of PEDOT:PSS by controlling ion stiochoimetry in ionic liquid additives. Chem. Mater. 30, 4816–4822 (2018)
J. Wang, K. Cai, S. Shen, Enhanced thermoelectric properties of poly (3,4-ethylenedioxythiophene) thin films treated with H2SO4. Org. Electron. 15(11), 3087–3095 (2014)
M.S. Mahajan, D.M. Marathe, S.S. Ghosh, V. Ganesan, J.V. Sali, Changes in in-plane electrical conductivity of PEDOT:PSS thin films due to electric field induced dipolar reorientation. RSC Adv. 5, 86393–86401 (2015)
L. Li, P. Gao, K.C. Schuermann, S. Ostendorp, W. Wang, C. Du, Y. Lei, H. Fuchs, L.D. Cola, K. Müllen, L. Chi, Controllable growth and field-effect property of monolayer to multilayer microstripes of an organic semiconductor. J. Am. Chem. Soc. 132(26), 8807–8809 (2010)
A.K.K. Kyaw, L.S. Lay, G.W. Peng, J. Changyun, Z. Jie, A nanogrove-guided slot-die coating technique for highly ordered polymer films and high-mobility transistors. Chem. Commun. 52, 358–361 (2016)
C.Y. Yang, C. Soci, D. Moses, A.J. Heeger, Aligned rrP3HT film: structural order and transport properties. Synth. Met. 155, 639–642 (2005)
C. Luo, A.K.K. Kyaw, L.A. Perez, S. Patel, M. Wang, B. Grimm, G.C. Bazan, E.J. Kramer, A.J. Heeger, General strategy for self-assembly of highly oriented nanocrystalline semiconducting polymers with high mobility. Nano Lett. 14(5), 2764–2771 (2014)
T.-C. Tsai, H.-C. Chang, C.-H. Chen, Y.-C. Huang, W.-T. Whang, A facile dedoping approach for effectively tuning thermoelectricity and acidity of PEDOT:PSS films. Org. Electron. 15, 641–645 (2014)
C. Yeon, S.J. Yun, J. Kim, J.W. Lim, PEDOT:PSS films with greatly enhanced conductivity via nitric acid treatment at room temperature and their application as Pt/TCO-free counter electrodes in dye-sensitized solar cells. Adv. Electron. Mater. 1, 1500121 (2015)
S.H. Chang, C.H. Chiang, F.S. Kao, C.L. Tien, C.G. Wu, Unraveling the enhanced electrical conductivity of PEDOT:PSS thin films for ITO-free organic photovoltaics. IEEE Photon. 6(4), 8400 (2014)
D.A. Mengistie, M.A. Ibrahem, P.C. Wang, C.W. Chu, Highly conductive PEDOT:PSS treated with fomic acid for ITO-free polymer solar cell. ACS Appl. Mater. Interfaces 6(4), 2292–2299 (2014)
H. Park, S.H. Lee, F.S. Kim, H.H. Choi, I.W. Cheong, J.H. Kim, Enhanced thermoelectric properties of PEDOT:PSS nanofilms by a chemical dedoping process. J. Mater. Chem. A 2, 6532 (2014)
A.K. Sarker, J. Kim, B.-H. Wee, H.-J. Song, Y. Lee, J.-D. Hong, C. Lee, Hydroiodic acid treated PEDOT:PSS thin films as transparent electrodes: an approach towards ITO free organic photovoltaics. RSC Adv. 5, 52019 (2015)
A. Hu, L. Tan, X. Hu, L. Hu, Q. Ai, X. Meng, L. Chen, Y. Chen, Crystallization and conformation engineering of solution-processed polymer transparent electrodes with high conductivity. J. Mater. Chem. C 5, 382 (2017)
Acknowledgements
This work was partially supported by the JSPS KAKENHI (Grant Numbers 17K20065 and 18H01708). AKKK would like to thank financial support from Shenzhen Science, Technology and Innovation Commission (Basic Research Project (No. JCYJ20180305180645221) and Shenzhen Peacock Team Project (No. KQTD2016030111203005)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Myint, M.T.Z., Inoue, H., Ichimura, S. et al. Influence of pressure of nitrogen gas on structure and thermoelectric properties of acid-treated PEDOT:PSS films. J Mater Sci: Mater Electron 30, 13534–13542 (2019). https://doi.org/10.1007/s10854-019-01721-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01721-2