Abstract
Poly(3,4-ethylenedioxythiophene) (PEDOT):poly(styrene sulfonate) (PSS) complex is of considerable interest for a variety of applications such as the hole-collecting electrode in solar cells. The conductivity of PEDOT:PSS is low, therefore it is doped or treated with solvents having high boiling points and high dielectric constants resulting in enhancement of conductivity and improvement of efficiency of devises fabricated from this polymer. In this work, to enhance the conductivity of PEDOT:PSS films, N-methylpyrrolidone (NMP) was added into aqueous dispersion of PEDOT:PSS and the conductivity of PEDOT:PSS films prepared on glass was investigated as a function of annealing temperature. The electrochemical properties of untreated and solvent-treated PEDOT:PSS films were studied through cyclic voltammetry. To study the impact of preparation conditions, the films were prepared at different coating speeds and coating times. To investigate the effect of annealing conditions on the conductivity, the films were annealed in an inert environment (N2) and air. Surface topography images obtained by atomic force microscopy showed that addition of NMP into PEDOT:PSS resulted in a uniform distribution of PEDOT domains and smoother phase edges, which could enhance the charge transfer. The conductivity of films increased with lowering the coating speed, which resulted in a higher thickness. Slower coating speeds providing enough time for PEDOT to crystallize led to a uniform distribution of PEDOT regions as compared to faster speeds. Conductivity of the treated films decreased with thermal annealing in both air and N2 indicating that the O2 molecules had no negative effect on the conductivity of PEDOT:PSS films. Hence, the decreased conductivity could be ascribed to interruption of the chains, which changed the morphology and conductivity. The encapsulated films stored in a normal laboratory atmosphere for 30 days exhibited a ~50–60 % decrease in conductivity. However, with applying a second thermal annealing step, the original value of the conductivity was recovered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Poly(3,4-ethylenedioxythiophene) (PEDOT) is one of the best known π-conjugated polymers. It is one of the most successful new conducting polymers because of its many interesting properties such as excellent transparency in the visible light range, good electrochemical, high conductivity, and good thermal stability of its electrical properties as compared to that of other polythiophenes [1–3].
PEDOT is synthesized by polymerization of 3,4-ethylenedioxythiophene (EDOT) monomer; but, it is unstable in neutral state because of its rapid oxidation in air. To improve its processability, a polyelectrolyte solution [poly(styrene sulfonate)] (PSS) can be added [1, 4]. When PEDOT, which is insoluble in many common solvents, is synthesized in the presence of PSS, an aqueous dispersion is obtained that can be cast into thin films [5, 6]. The PSS serves not only as a dispersant of PEDOT but also as a hole transporting layer, because of its suitable work function and acceptable transparency in visible light range [7, 8].
The PEDOT:PSS films can be readily fabricated through conventional solution processing such as spin coating. These films have a high transparency in visible light, high mechanical flexibility, and excellent thermal stability, which enables them to smooth the surface of indium tin oxide (ITO) anode, enhance the adhesion to the organic layer, and decrease the hole injection barrier due to its higher work function (5.17 ± 0.1 eV) [9] in comparison with that of ITO [10].
The PEDOT:PSS systems have extensively been used as a buffer layer in organic optoelectronics and organic solar cells applications. Consequently, the degradation of PEDOT:PSS especially under atmospheric air containing oxygen and moisture, which influence the structure of this polymer, is crucial for the performance of organic electronic devices and it is still under investigation [11].
PEDOT:PSS dispersions, generally used as antistatic coatings and as a hole injection layer in organic devices due to a high work function, high hole affinity, and good transparency suffers a problem of low conductivity [12]. Different factors have been reported to increase the conductivity of PEDOT:PSS complex. One of the most important factors is the addition of polar organic compounds having high boiling points and high dielectric constants [13–15]. Besides using solvents, conductivities higher than 1100 S/cm have been reported for vapor phase polymerized PEDOT. The polymerization of EDOT using a derivative monomer (EDOT–CH2OH) with an oxidant [iron(III)-tosylate] and a weak base (imidazole) yielded conductivities as high as 900 S/cm. On the other hand, solvent-added PEDOT:PSS showed somewhat lower conductivities compared to the alternative processes, because excess PSS present in the formulations, necessary for dispersion in water, can disturb the conduction path inside the polymer film [12, 16, 17]. Nowadays, the use of solvent to improve the conductivity of PEDOT:PSS electrodes is an important issue for ink-jet printed electrodes for plastic field effect transistors [18].
In addition, PEDOT:PSS complex has been doped with a material of redox nature. In both cases of doping and solvent treatment, electrons and holes act as charge carriers in the redox process. Due to the existence of alternating double bonds in the conjugated polymer chains, the carriers can move along the carbon chain, which leads to an increased conductivity [13, 15].
Photoactive materials including PEDOT:PSS complex are sensitive to oxygen and moisture, which can degrade the conjugated polymers, alter the band gap, and reduce the conductivity. Therefore, aging of thin films consisting from conjugated polymers is very important. In this work, after solvent and thermal treatments, the effect of aging on the conductivity of PEDOT:PSS films was studied. PEDOT:PSS thin films were treated with N-methylpyrrolidone (NMP) and deposited by spin coating on glass substrates at various annealing temperatures (293, 393, and 453 K) and annealing times (10, 30, and 90 s), and different thicknesses (29.7, 55.3, and 84 nm) under air and an inert atmosphere (N2). The effect of annealing temperature and addition of solvent on surface topography was studied using atomic force microscopy (AFM). In addition, the effects of film thickness and aging on the conductivity were also studied. The electrochemical characterizations of the untreated and solvent-treated PEDOT:PSS samples were performed by cyclic voltammetry.
2 Experimental
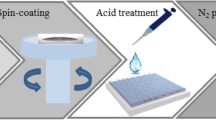
To prepare the aqueous solution of PEDOT:PSS, 4.3 g of PSS (18 wt%, Sigma-Aldrich) was mixed with 65 g deionized water using a magnetic stirrer until PSS well dissolved in aqueous medium. 0.52 g of EDOT monomer (Sigma-Aldrich), 15 ml of aqueous solution containing 1.07 g Na2S2O8 (Merck), and 0.007 g Fe2(SO4)3.5.9H2O (Merck) were added to the solution to initiate the reaction; and, the solution was stirred for 24 h at room temperature. Purification was carried out using dialysis membrane [MW cutoff = 3500, flat width = 47 mm, Membrane Filtration Product Incorporation (MFPI)]. To enhance the conductivity, 0.5 g of NMP (5 wt%, Merck) was added into 10 g of aqueous solution of PEDOT:PSS. Prior to spin coating, to improve the wettability of the glass substrates by PEDOT:PSS and formation of an uniform layer, 0.1 wt% TritonX-100 was added to PEDOT:PSS dispersion. The solution was sonicated at a power of 40 W for 15 min and then filtered using a PVDF syringe filter having a 0.45 µm pore size.
To remove the impurities from the glass substrates, first, the substrates were cleaned with water and a liquid detergent and then washed several times with distilled water. Then the substrates were placed in heated piranha (a 3:1 mixture of sulfuric acid 98 % and hydrogen peroxide 35 %). After 45 min, the substrates were rinsed with distilled water to remove the sediment from the glass surface. After that, the substrates were cleaned in an ultrasonic bath in a methanol/acetone mixture for 10 min and then dried with a nitrogen stream. Finally, the substrates were exposed to UV-O3 irradiation for 20 min.
PEDOT:PSS was spin-coated on the substrates at different coating speeds. Then the samples were heated up to 453 K to evaporate the water and anneal the PEDOT:PSS material. To study the effect of annealing conditions on the conductivity, the films were annealed in an inert environment (N2) and air. The nitrogen pressure of the glove box was 5–25 mbar. Sheet resistance of the thin films formed on the substrates was determined by a four-point-probe resistivity system (Jandel Engineering Ltd., UK). The effect of annealing process on surface topography was studied using an atomic force microscope (AFM) (Nanosurf Mobile S). Cyclic voltammetry (CV) measurements were carried out with a three-electrode electrochemical system, using PEDOT:PSS as the working electrode, Pt wire as the counter-electrode, and a Ag/AgCl electrode as the reference electrode in an acetonitrile solution, containing 10 mM I−, 1 mM I2, and 0.1 M LiClO4. The measurements were performed between −0.9 V and +1.2 V at scan rate of 30 mV/s.
3 Results and discussion
The NMP-treated PEDOT:PSS films were formed on the glass substrates by a varying speed spin coating method, which allowed one to obtain samples of various thicknesses. The thinner and thicker samples having thicknesses of 29.7 and 84 nm, respectively, were studied to find out the influence of thickness and roughness on the electrical properties.
The AFM phase and topography images obtained for the pristine and solvent-treated PEDOT:PSS films of different thicknesses are shown in Fig. 1, which indicate that addition of solvent changed the surface topography and morphology. The images obtained for PEDOT:PSS confirmed previous studies suggesting a grain-like structure for PEDOT:PSS films with a non-homogeneous distribution of the (PEDOT:PSS core surrounded by a PSS-rich shell) before solvent treating [20, 22]. The solvent-treated PEDOT:PSS film was significantly rougher and distribution of PEDOT regions was uniform. By adding NMP, phase edges were smoother, and in thicker films, a noticeable increase in the PEDOT grain size of the surface could be observed. The solvent could swell the particles and the PEDOT-rich particles became bigger resulting in an increased roughness. This caused approaching the particles to each other separated by a thinner PSS barrier, which enhanced the charge transfer [19–22]. The root-mean square roughness (Rrms) values for NMP-treated PEDOT:PSS films of different thicknesses and annealed at various times are given in Table 1. The rms roughness of the surface, measured over 8.7 × 8.7 μm2, changed from 0.99 to 8.77 nm as a result of solvent treatment.
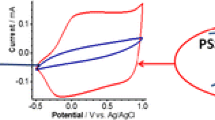
Figure 2 shows the cyclic voltammograms obtained for untreated as well as NMP-treated PEDOT:PSS complexes. The data were acquired from the sample treated with the highest tested potentials, V = + 1.2 V (oxidized) and V = −0.9 V (reduced). Oxidation peaks appeared at around 0.75 V corresponding to monomer oxidation leading to cation radical formation [23]. The oxidized waves were less defined which probably was due to a high electrical resistance [24, 25]. In this case, diffusion was not the limiting step of the electrochemical processes and thus that the whole film thickness was involved in the electrochemical reaction. Therefore, the charge flowing during the CV scans corresponded to the electrons that were needed to change the redox state of the whole PEDOT:PSS volume. Since the oxidation potential did not change substantially it could be concluded that the electronic structure of PEDOT was not changed with addition of NMP. This meant that the doping did not occur and the observed change in conductivity was due to changes in morphology. The different current values observed for two voltammograms might be due to different amounts of two samples used for voltammetry. The increased current was probably because of polystyrene oxidation whose oxidation peak was appeared after V = 1 V. Since the oxidation of polystyrene is irreversible this peak was gradually reduced (data are not shown here) in analyses with different scan rates, particularly with lower scan rates.
As shown in Fig. 3, at all annealing temperatures conductivity of the films increased with increasing thickness. From Table 1, the roughness of the films increased with increasing thickness suggesting that in thicker films, aggregation of PEDOT regions occurred (Fig. 1). This increase in the size of PEDOT regions at the surface indicated that the thickness of the insulating PSS “shell” surrounding the conducting PEDOT regions was reduced. The conductivity of PEDOT:PSS films increased when the average particle size increased. The decrease in the particle boundaries with the increasing average particle size would decrease the contact resistance and thereby enhance the conductivity of PEDOT:PSS films due to charge transfer increasing between PEDOT regions [22–27].
Another reason for the conductivity enhancement was the reduction of density of (localized) states N(E F) at the Fermi level by reducing the PSS layer thickness. Increasing conductivity was also attributed to narrowing of the localized states density resulting from reduced disorder in the NMP-treated films. The size of the PEDOT-rich particles was consistent with the observed, rather large, hopping length L. This indicated that the conductivity of NMP-treated PEDOT:PSS, like in the pristine material, took place by hopping of charge carriers between PEDOT-rich particles, rather than between single molecular sites [20].
Spinning time was another factor influencing the conductivity due to changing the PEDOT concentration. To investigate this effect, the films were prepared at different spinning times. Figure 4 indicates the variation of conductivity of NMP-treated PEDOT:PSS films against spinning time. As shown, the increase of spinning time reduced the conductivity. As mentioned above, conductivity is affected by the concentration of the PEDOT and ordered regions. In films dried for longer time periods (shorter spinning time), the aggregation of PEDOT with time occurred during drying of the films. The conductivity enhancement with decreasing PSS concentration suggested that the energy barrier for the charge transport across the conductive PEDOT chains was lowered and the localization length was increased [28]. In shorter spinning times, on the other hand, the solvent evaporation and drying of the film were carried out gradually as compared to longer spinning times, which provided enough times for PEDOT chains to crystallize. Both of these phenomena are key factors in hopping and transport of carriers. The presence of ordered regions in a material implies it has an inherent tendency to order that may be frustrated by the rapid drying during spin coating [20].
To study the effect of environment type on the conductivity of PEDOT:PSS films, two samples were examined, which were annealed under different environments. Figure 5 indicates conductivity as a function of annealing time in an inert environment (N2) as well as in air. In both cases, the conductivity decreased with annealing time. This suggested that the O2 molecules existing in air did not have significant negative effect on the conductivity of PEDOT:PSS films at 393 K. The slight difference observed between the results obtained for thermal-treated films in both air and N2 could be attributed to the influence of oxygen, which can react with polythiophene chains resulting in partially converting the double (π) bonds to single (σ) bonds.
Figure 6 indicates the effect of annealing temperature on the conductivity of a NMP-treated PEDOT:PSS film in air. In this figure, the reduced conductivity σ/σ 0, where σ 0 is the conductivity at 293 K, is shown as a function of annealing temperature for three different annealing times, 10, 30 and 90 min in air. The electrical properties of conducting polymers are strongly dependent on their film morphology and structure. The alignment of molecules in the film often changes with temperature. Heat treatment may also affect the colloidal particle interactions in the PEDOT:PSS films. The reduced conductivity values can be attributed to degradation of the PEDOT:PSS chains, which cause decreasing the hopping length resulting in a decreased conductivity. This fact that conductivity was dependent on temperature and not on the surrounding atmosphere indicated that the damage in the polymer chains caused by the temperature rise was independent from the corresponding degradation imposed by oxygen.
Generally two mechanisms determine the overall electrical conductivity: first, the heat activated tunneling of carriers between the conductive grains (PEDOT) and enhanced crystallinity resulted from thermal annealing both of which increase conductivity. The other mechanism, the interruption of the chains because of the heating, decreases conductivity. From Fig. 6, it was implied that for T > T0 (T0 = 293 K), the latter mechanism prevailed. In this case, the conductivity decreased by increasing temperature. This behavior offered a “metallic” behavior (dσ/dT < 0). This behavior has been observed in many conductive polymers with a heterogeneous structure similar to that of PEDOT:PSS [29].
Finally, the encapsulated films were stored in ambient conditions for 30 days and then their conductivity was measured. Figure 7a indicates the film conductivity measured as a function of annealing time. From Fig. 7b, two films exhibited similar degradation trends with a ~50–60 % decrease in film conductivity. Following the 30 days environmental exposure, a reannealing process was performed in which the films were thermally annealed for 120 min at 393 K. The original conductivities were approximately recovered for both samples indicating that the domains distribution and morphological state in the films after aging in ambient conditions were improved as a result of second thermal annealing process.
Figure 8 shows the AFM phase and topography images obtained for the PEDOT:PSS films before and after thermal annealing and after storage in ambient conditions for 30 days. After thermal annealing, the roughness of the film increased slightly (Table 1) and the conductivity of the thermal annealed PEDOT:PSS films decreased. Phase image of the aged film (Fig. 8e) showed that after 30 days the homogenous distribution of PEDOT regions was decreased, which could decrease the charge transfer.
4 Conclusions
In this work, AFM analysis and four-point-probe resistivity measurements were used to investigate the conductivity of NMP-treated PEDOT:PSS films at different annealing temperatures and thicknesses under an inert environment and air. The results showed that the conductivity of PEDOT:PSS enhanced by solvent treatment. However, for solvent-treated films the conductivity decreased by thermal annealing. The conductivity enhancement of the treated PEDOT:PSS films was mainly discussed in term of the change in morphology as well as coalescence between PEDOT domains through solvent treatment. In this case, the distribution of PEDOT domains throughout PSS was uniform and phase edges were smoother, which would decrease the contact resistance and thereby enhance the charge transfer and conductivity of PEDOT:PSS films. Electrochemical analysis showed that the oxidized (i.e. positively biased) form of PEDOT:PSS was found around 0.75 V for both untreated and NMP-treated PEDOT:PSS films indicating that the electronic structure of PEDOT was not changed with addition of NMP. This meant that the doping did not occur and the observed change in conductivity was due to the changes in morphology. The conductivity of NMP-treated films decreased after thermal annealing under an inert environment and air. The decreased conductivity could be ascribed to the interruption of the chains because of the thermal annealing. The conductivity of PEDOT:PSS layers was improved gradually by increasing coating time. This suggested that the surface concentration of PSS barrier separating the PEDOT-rich grains was reduced with a decrease in coating time. Conductivity Losses about 50–60 % were observed for the films stored in ambient condition for 30 days. The original efficiency was recovered by applying a second thermal annealing process indicating that the degradation was primarily driven by changes in morphology.
References
T.Y. Kim, J.E. Kim, Y.S. Kim, T.H. Lee, W.J. Kim, K.S. Suh, Preparation and characterization of poly(3,4-ethylenedioxythiophene) (PEDOT) using partially sulfonated poly(styrene–butadiene–styrene) triblock copolymer as a polyelectrolyte. Curr. Appl. Phys. 9, 120–125 (2009)
T.A. Skotheim, J. Reynolds (eds.), Handbook of Conducting Polymers, 3rd edn. (CRC, Boca Raton, 2007)
X. Crispin, S. Marciniak, W. Osikowicz, G. Zotti, A.W.D. Van Der Gon, F. Louwet, M. Fahlman, L. Groenendaal, F. De Schryver, W.R. Salaneck, Conductivity, morphology, interfacial chemistry, and stability of poly(3,4-ethylene dioxythiophene)-poly(styrene sulfonate): a photoelectron spectroscopy study. J. Polym. Sci. Part B Polym. Phys. 41, 2561–2583 (2003)
C.A. Cutler, M. Bouguettaya, J.R. Reynolds, PEDOT:PSS polyelectrolyte based electrochromic films via electrostatic adsorption. Adv. Mater. 14, 684–688 (2002)
A.M. Nardes, M. Kemerink, M.M. de Kok, E. Vinken, K. Maturova, R.A.J. Janssen, Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 9, 727–734 (2008)
Y.H. Kim, C. Sachse, M.L. Machala, C. May, L. Müller-Meskamp, K. Leo, Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 21, 1076–1081 (2011)
O.P. Dimitrev, Y.P. Pirytinski, A.A. Pud, Evidence of the controlled interaction between PEDOT and PSS in the PEDOT:PSS complex via concentration changes of the complex solution. Phys. Chem. 115, 1357–1362 (2011)
J. Yan, C. Sun, F. Tan, X. Hua, P. Chen, S. Qu, S. Zhou, J. Xu, Electropolymerized poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) film on ITO glass and its application in photovoltaic device. Sol. Energy Mater. Sol. Cells 94, 390–394 (2010)
T.M. Brown, J.S. Kim, R.H. Friend, F. Cacialli, R. Daik et al., Built-in field electroabsorption spectroscopy of polymer light-emitting diodes incorporating a doped poly(3,4-ethylene dioxythiophene) hole injection layer. Appl. Phys. Lett. 75, 1679–1681 (1999)
Y. Zhou, Y. Yuan, L. Cao, J. Zhang, H. Pang, J. Lian, X. Zhou, Improved stability of OLEDs with mild oxygen plasma treated PEDOT:PSS. J. Lumin. 122–123, 602–604 (2007)
S. Sakkopoulos, E. Vitoratos, Differentiation of the aging process of PEDOT:PSS films under inert Helium and ambient atmosphere for two different rates of thermal treatment. Open J Org Polym Mater 4, 1–5 (2014)
Y. Xia, H. Zhang, J. Ouyang, Highly conductive PEDOT:PSS films prepared through a treatment with zwitterions and their application in polymer photovoltaic cells. J. Mater. Chem. 20, 9740–9747 (2010)
J.Y. Kim, J.H. Jung, D.E. Lee, J. Joo, Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 126, 311–316 (2002)
C. Gong, H.B. Yang, Q.L. Song, Z.S. Lu, C.M. Li, Mechanism for dimethylformamide-treatment of poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) layer to enhance short circuit current of polymer solar cells. Sol. Energy Mater. Sol. Cells 100, 115–119 (2012)
J. Ouyang, Q. Xu, C.W. Chu, Y. Yang, G. Li, J. Shinar, On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 45, 8443–8450 (2004)
Y. Xia, J. Ouyang, PEDOT:PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 21, 4927–4936 (2011)
J. Huang, P.F. Miller, J.S. Wilson, A.J. de Mello, J.C. de Mello, D.D.C. Bradley, Investigation of the effect of doping and post-deposition treatments on the conductivity, morphology, and work function of poly(3,4-ethylenedioxythiophene)/poly(styrene sulfonate) films. Adv. Funct. Mater. 15, 290–296 (2005)
X. Crispin, F.L.E. Jakobsson, A. Crispin, P.C.M. Grim, P. Andersson, A. Volodin, C. van Haesendonck, M. Van der Auweraer, W.R. Salaneck, M. Berggren, The origin of the high conductivity of poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT-PSS) plastic electrodes. Chem. Mater. 18, 4354–4360 (2006)
J.S. Yeo, J.M. Yun, D.Y. Kim, S. Park, S.S. Kim, M.H. Yoon, T.W. Kim, A.S.I. Na, Significant vertical phase separation in solvent-vapor-annealed poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) composite films leading to better conductivity and work function for high performance Indium tin oxide-free optoelectronics. Appl. Mater. Interfaces 4, 2551–2560 (2012)
A.M. Nardes, R.A.J. Janssen, M. Kemerink, A morphological model for the solvent-enhanced conductivity of PEDOT:PSS thin films. Adv. Funct. Mater. 18, 865–871 (2008)
J. Gasiorowski, R. Menon, K. Hingerl, M. Dachev, N.S. Sariciftci, Surface morphology optical properties and conductivity changes of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) by using additives. Thin Solid Films 536, 211–215 (2013)
S.K.M. Jonssona, J. Birgersonb, X. Crispinb, G. Greczynskib, W. Osikowiczb, A.W.D. van der Gon, W.R. Salaneckb, M. Fahlmana, The effects of solvents on the morphology and sheet resistance in poly(3,4-ethylenedioxythiophene)–polystyrenesulfonic acid (PEDOT–PSS) films. Synth. Met. 139, 1–10 (2003)
A.R. Gonçalves, M.E. Ghica, C.M.A. Brett, Preparation and characterisation of poly(3,4-ethylenedioxythiophene) and poly(3,4-ethylenedioxythiophene)/poly(neutral red) modified carbon film electrodes, and application as sensors for hydrogen peroxide. Electrochim. Acta 56, 3685–3692 (2011)
M. Marzocchi, I. Gualandi, M. Calienni, I. Zironi, E. Scavetta, G. Castellani, B. Fraboni, Physical and electrochemical properties of PEDOT:PSS as a tool for controlling cell growth. ACS Appl. Mater. Interfaces. doi:10.1021/acsami.5b04768
M.-H. Yeh, L.-Y. Lin, C.-P. Lee, H.-Y. Wei, C.-Y. Chen, C.-G. Wu, R. Vittalaand, K.-C. Ho, A composite catalytic film of PEDOT:PSS/TiN–NPs on a flexible counter-electrode substrate for a dye-sensitized solar cell. J. Mater. Chem. 21, 19021 (2011)
M. Weber, Terahertz Transmission Spectroscopy of the Organic Polymer: PEDOT:PSS. PhD Thesis, Free University Berlian, 2009
A. Elschner, S. Kirchmeyer, W. Lovenich, U. Merker, K. Reuter, PEDOT: Principles and Applications of an Intrinsically Conductive Polymer (CRC Press, Boca Raton, 2010)
B. Friedel, P.E. Keivanidis, T.J.K. Brenner, A. Abrusci, C.R. McNeill, R.H. Friend, N.C. Greenham, Effects of layer thickness and annealing of PEDOT:PSS layers in organic photodetectors. Macromolecules 42, 6741–6747 (2009)
E. Vitoratos, S. Sakkopoulos, N. Paliatsas, K. Emmanouil, S.A. Choulis, Conductivity degradation study of PEDOT:PSS films under heat treatment in Helium and atmospheric air. Org. Polym. Mater. 2, 7–11 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khodakarimi, S., Hekhmatshoar, M.H., Nasiri, M. et al. Effects of process and post-process treatments on the electrical conductivity of the PEDOT:PSS films. J Mater Sci: Mater Electron 27, 1278–1285 (2016). https://doi.org/10.1007/s10854-015-3886-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3886-2