Abstract
BaTiO3/(WO3)x ceramics (where x = 0, 0.5, 1, 2 and 5 wt%) were prepared by solid state reaction. X-ray powder diffraction, scanning electron microscope, Fourier transform-infrared spectroscopy and ultraviolet–visible diffuse reflectance spectrophotometry were used to investigate the structure, morphology and optical properties, respectively. The electrical and dielectric properties were also performed for different synthesized ceramics. A pure phase was obtained for x = 0.5 wt% ceramic, nevertheless a secondary phase was detected for x ≥ 1 wt% ceramics. The grains size increases for x = 0.5 wt% ceramic and then reduces abruptly with further increasing WO3 content. The increase of grains size and the absence of impurities were all efficient to enhance the dielectric properties. A suitable WO3 content leads to obtain ceramics having high dielectric constant and low tangent loss, which is encouraging for radio frequencies and microwaves applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to their ferroelectric, piezoelectric, photoelectric, dielectric, pyroelectric and catalytic responses, the perovskite oxides having ABO3 structure has attracted a great attention [1,2,3]. These properties lead them to be used in various applications including electro-optical and electronics. One of the most common ferroelectric material is BaTiO3 (barium titanate; noted BTO) that exhibits important dielectric constant and resistivity and a low dielectric loss. BTO has a tetragonal ferroelectric structure at room temperature (RT) with lattice parameters a = 3.994 Å and c = 4.034 Å [4, 5]. This material is extensively used for electronic devices such as storage devices, capacitors, thermistors, piezoelectric transducers, sensors, etc. [6, 7]. The synthesis of nanosized BTO can be realized through numerous routes, including hydrothermal method, sol–gel process, solid-state reaction method, chemical coprecipitation, etc. [8].
To be performant in the various mentioned applications, it is necessary to control the microstructure of such material. The dielectric properties could be altered by adding dopants. Many types of additives have been included to perovskites ceramics and influenced their dielectric properties. Previous works have been done on BTO-SiO2 [9,10,11,12,13,14,15,16]. Luo [13] have explored the impact of SiO2 on the microstructural and dielectric properties of BTO product prepared by microwave sintering method. It was found that an optimal SiO2 content lead to improve the dielectric properties of BTO, particularly the permittivity increased and the dielectric loss decreased. Bi et al. [14] produced ultrafine nanostructures of BTO@SiO2 core–shell. Experimental results showed an optimization of the microstructure and dielectric responses. Furthermore, the ultrafine nanostructures provide high polarization that could efficiently enhance the electrical displacement and the breakdown strength simultaneously. The researchers confirmed the advantage of the ultrafine structures in improving the energy density of these ceramics, which provide an innovative technological method for the fabrication of high energy–density ceramics. Contrarily, Yan et al. [15] synthesized SiO2 added BaTiO3 ceramics and they found that with increasing the SiO2 content, an impurity phase was detected. This leads to decrease gradually the dielectric constant however an improvement of the electrical breakdown strengths was noticed. Furthermore, Lu et al. [16] synthesized series BTO-SiO2 composites through a combined route of conventional ceramic method and wet chemical coating. It is observed also a decrease of the dielectric constant on increasing the SiO2 content. BTO particles coated with Al2O3 were synthesized by Zhao et al. [17]. It is showed that the coating with Al2O3 leads to reduce the grains size and to narrow distribution. Furthermore, the synthesized products with Al2O3 coating showed an enhancement of dielectric properties, breakdown strength, energy efficiency and discharge energy density at room temperature compared to that produced without Al2O3 coating. Several groups synthesized BaTiO3–TiO2 ceramics for dye-sensitized solar cell applications [18,19,20,21,22]. An improvement in the photoelectronic conversion efficiency (PCE) and charge carrier separation ability of the composites was attained. This provides that the BaTiO3–TiO2 ceramics could be considered as promising for photocatalytic activity and photovoltaic systems. Paunović et al. [23] investigated the effects of rare-earth (RE = Dy, Sm and La) additions on the microstructural and dielectric properties of BTO ceramics. A low doping additive reveals an improvement of dielectric permittivity value together with low dissipation factor at RT. The impact of NiO inclusion on the microstructural and electrical properties of BTO was studied [24]. It is showed that NiO additions reduces the relative permittivity and temperature sensitivity of the doped BTO ceramics. Numerous other doping in BTO ceramics were reported in previous studies, such as MgO [25], CaO [26], Y2O3 [27], Cu [28], Sr–Ca [29] and RE = Lu, Yb, Er, Ho, Dy, Tb, Gd, Pr, La, Eu [30, 31] doped BaTiO3.
Particularly, as a transition metal oxide (TMO), the tungsten oxide (WO3) is widely exploited in diverse technological applications such as optical recording devices, photocatalytics, sensors and electro-chromic devices [32, 33]. WO3, n-type semiconductor, is the most promising TMO that display electrochromic behavior in the infrared and visible region and exhibit better electrical properties and optical modulation regarding the other TMO [34]. Besides, WO3 possesses the performance of ferroelectric materials, yielding it to be utilized in numerous fields including anti-dazing devices, air condition, house building solar energy, automotive glasses, and smart windows [34]. Recently, thin films of WO3 have been explored for their sensing properties [35]. The former properties will lead to various applications in industrial pollution monitoring and environmental. Very recent, Slimani et al. [36, 37] investigated the influences of nano-sized WO3 entities on the performances of high-temperature YBa2Cu3Oy superconductor. The obtained results revealed an improvements in electrical and magnetic properties, particularly critical current densities, flux pinning capacities and critical magnetic fields. It is concluded that the inclusion of appropriate content of WO3 nanoentities into the high-temperature superconductor materials is an efficient route for improving their performances. Up today, the utilization of WO3 as additive in the BTO ceramics has not been yet studied in the literature. Accordingly, it is very important to investigate the influences of various WO3 amounts on the dielectric properties of BTO material. Therefore, series of BaTiO3/(WO3)x ceramics, where x = 0, 0.5, 1, 2 and 5 wt%, were prepared. The effect of various WO3 contents inclusion on the structure, morphology, spectral, optical and dielectric properties of BTO-based products was deeply discussed.
2 Experimental
2.1 Materials and instrumentations
High purity (99.99%) powders of BaCO3, TiO2 and WO3 were obtained from Sigma Aldrich. The planetary Ball Mill PM 200 from Retsch was employed to grind samples. X-ray powder diffraction (XRD; Rigaku Benchtop Miniflex) with Cu-Kα radiation was used to examine the phase identification. Bruker alpha-II FT-IR spectrometer was employed to perform Fourier transform-infrared (FT-IR) spectra. The morphological observation was done by FEI Quanta FEG scanning electron microscope (SEM). UV–Vis spectrophotometer (JASCO V-780) was used to collect the diffuse reflectance spectra (DRS). The dielectric and electrical measurements were carried out by means of Alpha-N high-resolution analyzer from Novocontrol Technologies.
2.2 Synthesis
Various series of BaTiO3/(WO3)x ceramics, where x = 0, 0.5, 1, 2 and 5 wt% were prepared via solid state reaction by using high energy ball milling (HEBM). The different powders were mixed stoichiometrically with respect to the desired compositions in ethanol. After, the mixtures were ball-milled in the planetary HEBM as a grinding media for 14 h. After drying, the grinded powders were pressed into discs with 13 mm in diameter. The obtained pellets were calcined at 1100 °C for 12 h. The steps of ball-milling, pressing into discs and calcination were repeated twice in order to achieve the desired nano-sized composites.
3 Results and discussion
3.1 Structural analysis
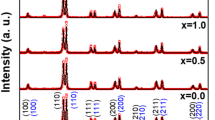
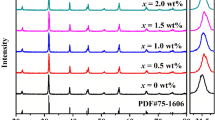
XRD patterns of the synthesized BaTiO3/(WO3)x ceramics shown in Fig. 1 are analyzed using Match program in order to determine the different structural parameters. The deduced phase fractions and lattice parameters are listed in Table 1. The goodness of the fit was evaluated through the Bragg RB factor and χ2 (Table 1). The data of pure BTO sample is found to match very well with the tetragonal phase (ICCD no. 96-210-0860) and space group P4mm. Figure 2 shows an enlarged view of the (002) and (200) refection peaks. The presence of splitting in (002)/(200) reflections for x = 0.0 and 0.5 samples is an indication of the tetragonal structure [38]. The lattice parameters a = b = 3.9987 Å and c = 4.0283 for pristine BTO product and its c/a = 1.0074 ratio are thoroughly comparable to those reported in the literature [38, 39]. As seen from Table 1, a and c decrease slightly with small WO3 content addition (i.e. x = 0.5%). This is mostly ascribed to the doping of W ions having ionic radii of 0.6 Å, which is smaller than those of Ba2+ (1.61 Å) and Ti4+ (0.62 Å) ions. The c/a ratio is almost constant for x = 0.0 (c/a = 1.0074) and x = 0.5 (c/a = 1.0073) ceramics. The decrease in the tetragonality of the structure could be approved by investigating the splitting of the (200) and (002) peaks. Nevertheless, the x = 0.5 ceramic exhibit well-splitted (200)/(002) peaks, confirming that the tetragonality was maintained for low doping content. The c/a ratio for x ≥ 1 wt% samples is equal to 1, indicating the cubic structure (ICCD no. 96-150-7758) with Pm3m space group in these products. This can be confirmed by the vanishing of the peak (002) plane in x ≥ 1 wt% samples, which is already exist in pure BTO sample along with the peak of (200) plane [40]. The merging of the two (002)/(200) reflections is a clear indication of the cubic structure.
Both x = 0.0 and 0.5 samples does not contain any impurity. Nevertheless, BaWO4 secondary phase (marked as ‘*’; ICCD no. 96-152-7205) begins to appear for x ≥ 1 wt%. The concentration of impurity phase increases remarkably with rising the WO3 content. The densities were determined and values are listed in Table 1. With increasing WO3 doping content from 0.0 to 0.5 wt%, the density increased from 4.96 to 5.47 g/cm3. The addition of WO3 with lower content was found to be very helpful for the densification of BTO. The enhancement of densification of x = 0.5 wt% ceramic could contribute to further enhanced dielectric and electrical properties. Nevertheless, with further increasing WO3 content (i.e. x ≥ 1 wt%), the density decreased continuously. That is mostly due to presence of BaWO4 secondary phase [15]. The average crystallites size (DXRD) is estimated for all synthesized ceramics by using Scherrer equation [41]:
where k is a constant close to unity (k = 0.9), λ = 1.5418 Å for Cu Kα radiations, θ is Bragg’s angle and β is full width at half maximum. The estimated DXRD values for various BaTiO3/(WO3)x ceramics are listed in the Table 1 and are found to be in the range of 78.14–51.27 nm. It is obvious that the DXRD increases firstly for x = 0.5 wt% and then diminishes as the WO3 content increase. In previous investigations, it is reported that the tetragonality of the products reduces with reducing the grains size and can attain the cubic structure [42, 43]. This accords with the present obtained findings in which both the DXRD and tetragonality decrease with increasing WO3 doping content.
3.2 Morphological examination
SEM micrographs of BaTiO3/(WO3)x ceramics are showed in Fig. 3. Pure BTO (Fig. 2a) demonstrated a morphology with homogeneous nano-sized grains. Compared to pure sample, the grains size increase for lower WO3 doping content (i.e. x = 0.5). As seen from Fig. 3b–d, the samples with WO3 doping showed similar microstructures with pure BTO product, but with smaller grains size. The grains size decreases gradually with increasing WO3 doping content. This involves that the sintering temperatures of ceramics is decreased with the WO3 addition [15].
3.3 FT-IR investigation
The FT-IR spectra of the prepared BaTiO3/(WO3)x ceramics are showed in Fig. 4. All the products revealed characteristic band at about 500 cm−1 which correspond to the Ti–O vibration [44]. Another characteristic band with small intensity is observed around 1450 cm−1 for all composites, which is mostly attributed to the Ba–Ti–O vibration [44]. Except these two characteristic bands, a change is noticed in the composites prepared with high x content. In fact, an additional characteristic band is appeared between 820 and 850 cm−1 for the two composites synthesized with x = 2 and 5. This band is most probably a result of the signals of the W–O stretching vibration [45]. The intensity of this typical peak is higher in x = 2 wt% composite compared to x = 5 wt%. The FT-IR results prove the formation of the desired BaTiO3/(WO3)x ceramics.
3.4 Optical investigation
The UV–Visible DRS analysis was utilized to explore the optical properties of BaTiO3/(WO3)x ceramics. When a matter is irradiated by a light beam, it is known that the incident light will be reflected, transmitted and/or scattered inside the matter. Generally, the beam part that will scatter inside the matter and return to the surface is known as the diffused reflectance. Figure 5 presents the UV–Vis DRS of various synthesized BaTiO3/(WO3)x ceramics in the range of 200–800 nm. The spectra revealed an absorption of around 80% of light for various BaTiO3/(WO3)x ceramics in the UV range. Tauc model was employed to deduce the optical band gap energy (Eg) according to the following expression [46, 47]:
where \( h\nu \) is the photon energy and α is a constant independent on photon energy. The exponent r display four numeric values: r = 3/2 for forbidden indirect, r = 2 for allowed indirect, r = 3 for forbidden direct, and r = 1/2 for allowed direct optical transitions. Since BTO has a direct band gap [48], hence by plotting \( (\alpha h\nu )^{2} \) as a function of \( h\nu \), its Eg can be deduced. The direct band gap is deduced by extrapolating the linear portion to the low energies. The Eg values of BaTiO3/(WO3)x ceramics is deduced from the UV–Visible DRS by converting the diffused reflectance (R) values according to the Kubelka–Munk equation [46, 47]:
Figure 6 presents plots of \( (\alpha h\nu )^{2} \) against \( h\nu \). The values of Eg of various prepared ceramics were deduced by extrapolating the linear portions as presented in the inset of Fig. 6. The pristine BTO sample display Eg value of about 3.31 eV. The obtained value is lower than that of bulk BTO (Eg = 3.23 eV [49]) because of quantum size effect. The Eg values for 0.5, 1, 2 and 5 wt% ceramics were found to be equal to 3.24, 3.38, 3.41 and 3.42 eV, respectively. Compared to x = 0 wt% sample, a reduction of Eg from 3.31 to 3.24 eV was noticed in 0.5 wt% ceramic. The reduction of Eg in this ceramic could be ascribed to the increase in grains size and crystallinity as shown above in XRD and SEM analyses [50]. For ceramics with x ≥ 1 wt%, the Eg values show an increase, which might be accredited to the reduction in grains size. Furthermore, the dependence of Eg on the grains size may be described using the electron confinement effect. When the particles size is being smaller, quantum confinement effect will occur and the motion of electrons will be confined in discrete states, which led to an increase in the band gap energy [50, 51]. Another possible reason for the change in Eg values is the presence of BaWO4 impurities in the BaTiO3/(WO3)x ceramics [52].
3.5 Dielectric and electrical properties
3.5.1 Dielectric studies
The loss tangent (tgδ) and dielectric permittivity (ε′) responses versus frequency (10 Hz to 10 MHz) of the various BaTiO3/(WO3)x ceramics at RT are presented in Figs. 7a and b, respectively. It can be observed that the dielectric constant ε′ of all ceramics reduces on increasing the frequency and reaches a constant magnitude at higher frequencies, exhibiting dispersion behavior. The reduction of ε′ at lower frequencies originates from the polarization effect that does not happen immediately on applying the electric field due to inertia. The dipoles that are induced will follow the electric field at very low frequencies, and fully participate to the total polarization of the dielectric. At higher frequency, the dipoles are not able to align themselves due to very rapid variations in the field. As consequence, their influence on the polarization as well as on the ε′ become insignificant [53]. The observed high values of ε′ at lower frequency for all ceramics could be attributed to charges accumulation within grain boundaries, and at the interfaces among the electrode and the sample, i.e. Maxwell–Wagner polarization and interfacial polarization [54]. As seen in Fig. 7b, the dispersion behavior observed in dielectric permittivity is followed by a peak of relaxation in loss tangent (tgδ). The position of the tgδ peak shifts to higher frequencies with increasing the WO3 content. The dependence of loss peak on frequency indicates that the charge carriers hopping has a significant impact on their transport processes [55]. At high frequencies (f > 10 kHz), the losses show a decrease for all ceramics, which is encouraging for microwaves and radio frequencies applications.
The dielectric properties strongly depended on the WO3 concentration (see Figs. 7 and 8). Firstly, with increasing WO3 concentration from 0.0 to 0.5 wt%, the dielectric constant shows a significant improvement. This change in permittivity may be attributed to the microstructure of x = 0.5 ceramic. The increase of ε′ could be described by the improvement of the microstructure. In fact, the addition of WO3 can increase the grain size and subsequently the dielectric permittivity. Several authors have shown that the ε′ increases with grains size [55, 56]. In addition, the increase of ε′ is mostly qualified to the WO3 doping effect. The increase of doping content could strengthen the interaction among adjacent dipoles, which is beneficial to obtain high dielectric constant [57]. With further increasing the concentration of WO3, the dielectric permittivity shows a strong decrease. This change in permittivity with the increase of the doped concentration is not surprising and can be attributed to the microstructure of ceramics with x ≥ 1 wt%. Indeed, in agreement with the SEM images, the ceramic products with x ≥ 1 wt% show smaller grains size in comparison with the pure BTO and x = 0.5 samples. Moreover, the highly possible substitution of larger number of Ti4+ ions by W4+ (due to nearest ionic radii) could lead to narrowing the space between oxygen octahedrons and lowering the spontaneous polarization, or even partially losing the ferroelectricity, ultimately conducting to a reduction in ε′ [57]. Furthermore, the excessive increase of non-ferroelectric secondary phases can weak the dielectric constant [57]. Higher inclusion of WO3 resulted in a microstructure consisting of two-phases (see XRD results), and therefore, the ε′ of the ceramics reduces with rising WO3 content above 1 wt%. For x ≥ 1 wt%, the variations in the crystallinity provoked by W4+ ions along with changes of the grains size have larger influences on the dielectric constant [56]. Therefore, the x = 0.5 wt% with greater crystallinity, bigger grains size and absence of non-ferroelectric secondary phases has the maximum ε′ value. The dielectric loss (tan δ) is also strongly influenced by the WO3 doping concentration and is very related to the sample microstructure and presence/absence of secondary phases.
The x = 0.5 wt% ceramic exhibits the maximum permittivity and the lowest dielectric loss (tan δ) at RT, indicating that it is more suitable for use in tunable devices at RT. The dielectric and ferroelectric properties at microwave frequencies and in a broad range of temperature of x ≤ 0.5 products will be described in detail in a future article.
3.5.2 Electrical conductivity investigation
Using dielectric data, the ac electrical conductivity (σac) of BaTiO3/(WO3)x ceramics was calculated using the empirical formula:
ε0 is vacuum permittivity and ω = 2πf is angular frequency. Figure 9 displays the frequency variation of σac for diverse synthesized BaTiO3/(WO3)x ceramics where x = 0, 0.5, 1, 2 and 5 wt%. The study of the conductivity in alternative regime shows the same pace for all the samples; σac rises as the frequency increases due to the increment in carriers charge hopping. To explain its behavior, the σac data was evaluated by the Jonscher’s power law of the universal dielectric response model [58]:
Here A is a pre-exponential constant, σdc is the dc bulk conductivity, and n is the power-law exponent varying between 0 and 1. Generally, for perovskite oxides, there are two conductivity behaviors:
-
(i)
Low frequency region, in which the conductivity is almost independent on frequency (called σdc), the charge carriers make jumps long distance through the volume of the material to the electrodes.
-
(ii)
High frequencies region, where conductivity is frequency dependent and show a linear behavior. In this regime, the charge carriers trapped at the grain boundaries make short jumps. The conduction process is therefore related to a dielectric relaxation mechanism.
In our work, we observe that the dc conductivity behavior is not very clear and it can be visible for frequencies lower than 1 kHz, by against the asymptotic regime (Aωn) is dominant. This suggests that the short-range motions of carriers govern the relaxation processes. The conductivity remains almost the same for all the concentrations of WO3 at high frequency, while a slight difference in conductivity is obtained at low frequency. Finally, the high ε′, low tan δ, and low σ of the prepared ceramics make them promising candidates to be used in embedded devices in the micro-electronics industry.
3.5.3 Electrical modulus analysis
For the composite structure, it is always better to know the effect of each phase (i.e., the dissociation between the bulk and the interface effect) and to highlight the effect of adding. The electric modulus (M*) could give important details about the relaxation mechanism. This formalism has the benefit of suppressing the polarization of electrodes and phenomena of space charge injection and identify the effects of grains and grain boundaries contributions on the charge transport in the poly-crystalline compounds [59, 60]. Based on the complex permittivity ε* = ε′ − jε″, M* can be expressed as follow:
where M′ and M″ are the real and imaginary components of the M*, respectively.
Figure 10 depicts the imaginary part (M″) versus the real part (M′) of the electrical modulus over the same frequency range for different prepared BaTiO3/(WO3)x ceramics. The figure shows a clear effect of WO3 doping on the electric modulus behavior. It is clear seen that the pure BTO and x = 0.5 ceramics show only one semi-circle, while the ceramics prepared with x ≥ 1 wt% exhibit two semi-circles. This behavior suggests that the ceramics with x ≥ 1 wt% are composed of two phases. The representation of the imaginary part versus the real part of the electrical modulus at RT of the x ≥ 1 wt% ceramics shows the presence of two partially overlapping semi-circles. This behavior is occurred when the materials present a dual effect: the bulk and the interfacial effect. More clearly, the semi-circle observed at lower frequency is attributed to the interfacial effect specified by the interface between the BaTiO3 matrix and the WO3 nanoparticles, while the semi-circle occurred at higher frequency is due to the BaTiO3-bulk effect.
4 Conclusion
In this investigation, BaTiO3/(WO3)x ceramics (where x = 0, 0.5, 1, 2 and 5 wt%) were successfully prepared via solid state reaction route. According to XRD analysis, pristine compound (i.e. x = 0 wt%) showed pure BTO phase with tetragonal structure. When WO3 content is 0.5 wt%, no impurity was observed, and it exhibited larger grains size with homogeneous microstructure compared to pristine product. Nevertheless, when x ≥ 1 wt%, appearance of BaWO4 secondary phase, tetragonal-cubic structure transition and a reduction in grains size were noticed. A variation in Eg was remarked with WO3 additions in BTO, which might be ascribed to variations of grains size and existence of impurities. The investigation of electrical and dielectric properties indicated that x = 0.5 wt% ceramic exhibit great dielectric constant and low tangent loss. The obtained results are encouraging to use the obtained ceramic in radio frequencies and microwaves applications.
References
C.D. Chandler, C. Roger, M.J. Hampden-Smith, Chemical aspects of solution routes to perovskite-phase mixed-metal. Chem. Rev. 93, 1205–1241 (1993)
M.A. Pena, J.L.G. Fierro, Chemical structures and performance of perovskite oxides. Chem. Rev. 101, 1981–2017 (2001)
S. Royer, D. Duprez, F. Can, X. Courtois, C. Batiot-Dupeyrat, S. Laassiri, H. Alamdari, Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality. Chem. Rev. 114, 10292–10368 (2014)
H.F. Kay, H.J. Vellard, P. Vousden, Atomic positions and optical properties of barium titanate. Nature 163, 636 (1949)
J. Harada, T. Pedersen, Z. Barnea, X-ray and neutron diffraction study of tetragonal barium titanate. Acta Cryst. B 26, 336 (1970)
M. Wongduan, K. Reinhard, B.H. Robert, P. Simon, Structural and dielectric properties of Ba0.8La0.133Ti0.90Sn0.1O3. J. Eur. Ceram. Soc. 23, 127 (2003)
M.Z.-C. Hu, G.A. Miller, E.A. Payzant, C.J. Rawn, Homogeneous (co)precipitation of inorganic salts for synthesis of monodispersed barium titanate particles. J. Mater. Sci. 35, 2927–2936 (2000)
S. Dudley, T. Kalem, M. Akinc, Conversion of SiO2 diatom frustules to BaTiO3 and SrTiO3. J. Am. Ceram. Soc. 89, 2434–2439 (2006)
Y. Luo, P. Yongping, P. Zhang, J. Zhao, W. Yurong, Y. Liu, Study on dielectric properties of SiO2-doped BaTiO3 ceramics. Ferroelectrics 492, 10–16 (2016)
E.P. Gorzkowski, M.J. Pan, B. Bender, C.C.M. Wu, Glass-ceramics of barium strontium titanate for high energy density capacitors. J. Electroceram. 18, 269–276 (2007)
J.S. Park, Y.H. Han, Nano size BaTiO3 powder coated with silica. Ceram. Int. 31, 777–782 (2005)
M. Cernea, B.S. Vasile, A. Boni, A. Iuga, Synthesis, structure characterization and dielectric properties of Nb doped BaTiO3/SiO2 core-shell heterostructure. J. Alloys Compd. 587, 553–559 (2014)
Y. Zhang, M. Cao, Z. Yao, Z. Wang, Z. Song, A. Ullah, H. Hao, H. Liu, Effects of silica coating on the microstructures and energy storage properties of BaTiO3 ceramics. Mater. Res. Bull. 67, 70–76 (2015)
K. Bi, M. Bi, Y. Hao, W. Luo, Z. Cai, X. Wang, Y. Huang, Ultrafine core-shell BaTiO3@SiO2 structures for nanocomposite capacitors with high energy density. Nano Energy 51, 513–523 (2018)
Y. Yan, L. Liu, C. Ning, Y. Yang, C. Xia, Y. Zou, S. Liu, X. Wang, K. Liu, X. Liu, G. Liu, Improved electrical properties of SiO2-added BaTiO3 ceramics by microwave sintering. Mater. Lett. 165, 135–138 (2016)
X. Lu, Y. Tong, H. Talebinezhad, J. Liu, Y. Cai, Z.Y. Cheng, L. Wang, Effects of SiO2 coating on the dielectric and ferroelectric properties of BaTiO3-SiO2 composites, in 2017 Joint IEEE International Symposium on the Applications of Ferroelectric (ISAF)/International Workshop on Acoustic Transduction Materials and Devices (IWATMD)/Piezoresponse Force Microscopy (PFM)
Q. Zhao, X. Wang, H. Gong, B. Liu, B. Luo, L. Li, The properties of Al2O3 coated fine-grain temperature stable BaTiO3-based ceramics sintered in reducing atmosphere. J. Am. Ceram. Soc. 101, 1245–1254 (2018)
H.A. Moghaddam, M.R. Mohammadi, TiO2–BaTiO3 nanocomposite for electron capture in dye-sensitized solar cells. J. Am. Ceram. Soc. 100, 2144–2153 (2017)
R. Li, Q. Li, L. Zong, X. Wang, J. Yang, BaTiO3/TiO2 heterostructure nanotube arrays for improved photoelectrochemical and photocatalytic activity. Electrochim. Acta 91, 30–35 (2013)
L. Zhang, Y. Shi, S. Peng, J. Liang, Z. Tao, J. Chen, Dye-sensitized solar cells made from BaTiO3-coated TiO2 nanoporous electrodes. J. Photochem. Photobiol., A 197, 260–265 (2008)
Z.A. Garmaroudi, M.R. Mohammadi, Design of TiO2 dye-sensitized solar cell photoanode electrodes with different microstructures and arrangement modes of the layers. J. Sol-Gel. Sci. Technol. 76, 666–678 (2015)
H. Asgari Moghaddam, M.R. Mohammadi, S.M. Seyed Reyhani, Improved photon to current conversion in nanostructured TiO2 dye-sensitized solar cells by incorporating cubic BaTiO3 particles deliting incident. Sol. Energy 132, 1–14 (2016)
V. Paunović, L.J. Živković, V. Mitić, Influence of rare-earth additives (La, Sm and Dy) on the microstructure and dielectric properties of doped BaTiO3 ceramics. Sci. Sinter. 42, 69–79 (2010)
W.H. Tzing, W.H. Tuan, H.L. Lin, The effect of microstructure on the electrical properties of NiO-doped BaTiO3. Ceram. Int. 25, 425–430 (1999)
T. Nagai, K. Iijima, H.J. Hwang, M. Sando, T. Sekino, K. Niihara, Effect of MgO doping on the phase transformations of BaTiO3. J. Am. Ceram. Soc. 83, 107–112 (2000)
Y. Sakabe, N. Wada, T. Hiramatsu, T. Tonogaki, Dielectric properties of fine-grained BaTiO3 ceramics doped with CaO. Jpn. J. Appl. Phys. 41, 6922 (2002)
Y.H. Song, J.H. Hwang, Y.H. Han, Effects of Y2O3 on temperature stability of acceptor-doped BaTiO3. Jpn. J. Appl. Phys. 44, 1310 (2005)
A. Shukla, R.N.P. Choudhary, A.K. Thakur, D.K. Pradhan, Structural, microstructural and electrical studies of La and Cu doped BaTiO3 ceramics. Physica B 405, 99–106 (2010)
X. Cheng, M. Shen, Enhanced spontaneous polarization in Sr and Ca co-doped BaTiO3 ceramics. Solid State Commun. 141, 587–590 (2007)
Y.-S. Jung, E.-S. Na, U. Paik, J. Lee, J. Kim, A study on the phase transition and characteristics of rare earth elements doped BaTiO3. Mater. Res. Bull. 37, 1633–1640 (2002)
J. Li, M. Kuwabara, Preparation and luminescent properties of Eu-doped BaTiO3 thin films by sol–gel process. Sci. Technol. Adv. Mater. 4, 143–148 (2003)
H. Zheng, J. Zhen-Ou, M.S. Strano, R.B. Kaner, A. Mitchell, K. Kalantar Zadeh, Nanostructured tungsten oxide—properties, synthesis, and applications. Adv. Funct. Mater. 21, 2175–2196 (2011)
W.J. Lee, Y.K. Fang, J.-J. Ho, W.T. Hsieh, S.F. Ting, D. Huang, F.C. Ho, Effects of surface porosity on tungsten trioxide (WO3) films’ electrochromic performance. J. Electron. Mater. 29, 183–187 (2000)
M.M. El-Nahass, H.A.M. Ali, M. Saadeldin, M. Zaghllol, AC conductivity and dielectric properties of bulk tungsten trioxide (WO3). Physica B 407, 4453–4457 (2012)
R.S. Vemuri, K. Kamala Bharathi, S.K. Gullapalli, C.V. Ramana, Effect of structure and size on the electrical properties of nanocrystalline WO3 films. ACS Appl. Mater. Interfaces. 2, 2623–2628 (2010)
Y. Slimani, M.A. Almessiere, E. Hannachi, A. Baykal, A. Manikandan, M. Mumtaz, F. Ben Azzouz, Influence of WO3 nanowires on structural, morphological and flux pinning ability of YBa2Cu3Oy superconductor. Ceram. Int. 45, 2621–2628 (2019)
Y. Slimani, M.A. Almessiere, E. Hannachi, M. Mumtaz, A. Manikandan, A. Baykal, F. Ben Azzouz, Improvement of flux pinning ability by tungsten oxide nanoparticles added in YBa2Cu3Oy superconductor. Ceram. Int. 45, 6828–6835 (2019)
S. Rajan, P.M. Mohammed-Gazzali, G. Chandrasekaran, Electrical and magnetic phase transition studies of Fe and Mn co-doped BaTiO3. J. Alloys Compd. 656, 98–109 (2016)
B. Deka, S. Ravi, A. Perumal, D. Pamu, Ferromagnetism and ferroelectricity in Fe doped BaTiO3. Physica B 448, 204–206 (2014)
B.D. Cullity, Elements of X-ray diffraction (Addison-Wesley, Boston, 1956)
E. Hannachi, Y. Slimani, A. Ekicibil, A. Manikandan, F. Ben Azzouz, Magneto-resistivity and magnetization investigations of YBCO superconductor added by nano-wires and nano-particles of titanium oxide. J. Mater. Sci. Mater. Electron. 30, 8805–8813 (2019)
S. Lather, A. Gupta, J. Dalal, V. Verma, R. Tripathi, A. Ohlan, Effect of mechanical milling on structural, dielectric and magnetic properties of BaTiO3–Ni0.5Co0.5Fe2O4 multiferroic ceramics. Ceram. Int. 43, 3246–3251 (2017)
G. Arlt, D. Hennings, G. de With, Dielectric properties of fine-grained barium titanate ceramics. J. Appl. Phys. 58, 1619–1625 (1985)
S. Kappadan, T.W. Gebreab, S. Thomas, N. Kalarikkal, Tetragonal BaTiO3 nanoparticles: an efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process. 51, 42–47 (2016)
S. Adhikari, D. Sarkar, H.S. Maiti, Synthesis and characterization of WO3 spherical nanoparticles and nanorods. Mater. Res. Bull. 49, 325–330 (2014)
Y. Slimani, B. Unal, E. Hannachi, A. Selmi, M.A. Almessiere, M. Nawaz, A. Baykal, I. Ercan, M. Yildiz, Frequency and dc bias voltage dependent dielectric properties and electrical conductivity of BaTiO3-SrTiO3/(SiO2)x nanocomposites. Ceram. Int. 45, 11989–12000 (2019)
Y. Slimani, A. Selmi, E. Hannachi, M.A. Almessiere, A. Baykal, I. Ercan, Impact of ZnO addition on structural, morphological, optical, dielectric and electrical performances of BaTiO3 ceramics. J. Mater. Sci. Mater. Electron. 30, 9520–9530 (2019)
P. Wang, C. Fan, Y. Wang, G. Ding, P. Yuan, A dual chelating sol–gel synthesis of BaTiO3 nanoparticles with effective photocatalytic activity for removing humic acid from water. Mater. Res. Bull. 48, 869–877 (2013)
J.-i. Fujisawa, T. Eda, M. Hanaya, Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 685, 23–26 (2017)
Y.C. Teh, A.A. Saif, Influence of annealing temperature on structural and optical properties of sol-gel derived Ba0.9Gd0.1TiO3 thin films for optoelectronics. J. Alloys Compd. 703, 407–413 (2017)
M. Ozta, Influence of grain size on electrical and optical properties of InP films. Chin. Phys. Lett. 25, 4090–4092 (2008)
L.V. Maneeshya, P.V. Thomas, K. Joy, Effects of site substitutions and concentration on the structural, optical and visible photoluminescence properties of Er doped BaTiO3 thin films prepared by RF magnetron sputtering. Opt. Mater. 46, 304–309 (2015)
K. Karishma, P. Ashutosh, K. Prasad, Impedance/modulus and conductivity studies on [Bi0.5(Na1-xKx)0.5]0.94Ba0.06TiO3, (0.16 ≤ x ≤ 0.20) lead-free ceramics. Am. J. Mater. Sci. 6, 1–18 (2016)
A. Selmi, O. Khaldi, M. Mascot, F. Jomni, J.C. Carru, Dielectric relaxations in Ba0.85Sr0.15TiO3 thin films deposited on Pt/Ti/SiO2/Si substrates by sol–gel method. J. Mater. Sci. Mater. Electron. 27, 11299–11307 (2016)
T. Gopal Reddy, B. Rajesh Kumar, T. Subba Rao, J. Altaf Ahmad, Structural and dielectric properties of barium bismuth titanate (BaBi4Ti4O15) ceramics. Int. J. Appl. Eng. Res. 6, 571–580 (2011)
A. Jain, A.K. Panwar, A.K. Jha, Effect of ZnO doping on structural, dielectric, ferroelectric and piezoelectric properties of BaZr0.1Ti0.9O3 ceramics. Ceram. Int. 43, 1948–1955 (2017)
X. Dong, H. Chen, M. Wei, W. Kaituo, J. Zhang, Structure, dielectric and energy storage properties of BaTiO3 ceramics doped with YNbO4. J. Alloys Compd. 744, 721–727 (2018)
A.K. Jonscher, The ‘universal’ dielectric response. Nature 267, 673–679 (1977)
L. Singh, U.S. Rai, K. Mandal, B.C. Sin, S.-I. Leed, Y. Lee, Dielectric, AC-impedance, modulus studies on 0.5BaTiO3-0.5CaCu3Ti4O12 nano-composite ceramic synthesized by one-pot, glycine-assisted nitrate-gel route. Ceram. Int. 40, 10073–10083 (2014)
C.R. Cena, A.K. Behera, B. Behera, Structural, dielectric, and electrical properties of lithium niobate microfibers. J. Adv. Ceram. 5, 84–92 (2016)
Acknowledgements
This work was supported by the Institute for Research & Medical Consultations (Projects Nos. 2017-IRMC-S-3 and 2018-IRMC-S-2) and the Deanship for Scientific Research (Projects Nos. 2018-209-IRMC and 2017-576-IRMC) of Imam Abdulrahman Bin Faisal University (IAU – Saudi Arabia).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Slimani, Y., Selmi, A., Hannachi, E. et al. Study of tungsten oxide effect on the performance of BaTiO3 ceramics. J Mater Sci: Mater Electron 30, 13509–13518 (2019). https://doi.org/10.1007/s10854-019-01718-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01718-x