Abstract
BaTiO3/(ZnO)x ceramics (x = 0, 2, 5 and 10 wt%) were produced via solid state reaction by using high energy ball milling. The morphological, structural, spectral, optical, electrical and dielectric properties were systematically investigated. X-ray diffraction indicated that all ceramics crystallize in the tetragonal structure. The grains size increases with ZnO additions. The optical band gap energy (Eg) was also evaluated and found to reduce with increasing ZnO concentration. The dielectric and electric properties revealed that an optimal ZnO content lead to obtain ceramic with high dielectric constant and low tangent loss, which are encouraging for radio frequencies and microwaves applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The spinel structure is adopted by various materials, including catalysts, superconductors, magnetic materials, semiconductors and microwave dielectric ceramics at millimeter region [1, 2]. They are promising candidates for millimeter-wave region application. It is required to possess low dielectric constant, high quality factor and near zero temperature coefficient. Furthermore, the perovskite metal oxides with ABO3 structure have received a great attention due to their ferroelectric, dielectric, photoelectric, pyroelectric, piezoelectric, and catalytic responses [3, 4]. These properties lead them to be invested in several technological applications such as electronics, electro-mechanical and electro-optical applications. Barium titanate BaTiO3 (BTO for brevity) exhibits important dielectric constant and resistivity and low dielectric loss. It has a tetragonal ferroelectric structure at room temperature (RT) with lattice parameters a = 3.994 Å and c = 4.034 Å [5]. Numerous electronic devices are based on the use of BTO ceramics such as capacitors, passive memory storage devices, thermistors, piezoelectric transducers, sensors and many electro-optical devices [6, 7]. Several routes including hydrothermal method, sol–gel process, solid-state reaction method, chemical coprecipitation, etc. have been used to synthesize BTO ceramics with nanoscale size [8].
Many researchers over wide the world seek to improve the dielectric and electrical properties of BTO ceramics. To achieve this objective, a control of the microstructure is required. The dielectric properties could be altered by adding dopants. Various kinds of additives have been used and greatly affected the dielectric properties of perovskites ceramics. For instance, Paunović et al. [9] investigated the impacts of rare-earth (RE = Dy, Sm and La) additions on the microstructural and dielectric properties of BTO ceramics produced by the solid-state reaction. An improvement of dielectric permittivity as well as a reduction of dissipation factor was obtained for low doping additive. BaTiO3–TiO2 nanosized ceramics were produced by several researchers with the purpose be used for dye-sensitized solar cell applications [10, 11]. The different findings showed that the BaTiO3–TiO2 nanosized ceramics could be potential for photocatalytic activity and photovoltaic systems. Zhang et al. [12] have explored the influence of SiO2 doping on the microstructural and dielectric properties of BTO product produced by microwave sintering method. It is found that such content of SiO2 added to BTO ceramics lead to improve the dielectric properties, particularly the permittivity increased and the dielectric loss decreased. Numerous other doping in BTO ceramics were reported in previous studies, such as NiO [13], MgO [14], CaO [15] and Y2O3 [16] doped BaTiO3. It is showed that NiO additions reduces the relative permittivity and temperature sensitivity of the doped BTO ceramics.
ZnO is an n-type semiconductig with a wide band gap (3.2 eV) and large exciron binding energy. This makes it promising for catalytic applications. Recently, some reports focused on V2O5-ZnO nanocomposites and evaluated their performances on photocatalytic application [17]. Al-syadi et al. [18] prepared (80 − x)V2O5/20Bi2O3/xBaTiO3 composites and studied their dielctric properties. On the other hand, with low melting point and high ionic polarizability, ZnO is an important additive in dielectric ceramics, which can be utilized to improve densification and electrical properties [19]. As a prominent additive, ZnO has been broadly utilized to engineer highly efficient ceramic capacitors. A plenty of investigations revealed that ZnO has an impact on the dielectric characteristics, the sintering behavior and the microstructural improvement of perovskite ceramics [20,21,22]. Recently, researchers have found that the composite formed by ZnNb2O6 was very effective to enhance the densification [20]. Zn and Nb co-doped BTO ceramics prepared by microwave sintering was not only useful to enhance the densification, but also helpful to decrease the dielectric loss. Iqbal and Jamal [23] were investigated the phase and microstructure of Ba1-2xTa2xTi1-xZnxO3 (0.005 ≤ x ≤ 0.1). They found that the dielectric constant decreased with an increase in the dopant concentration due to second phase formation. As well, ZnO is helpful in controlling grain growth and it improves densification during sintering process [22]. Caballero et al. [24] reported the effect of ZnO added to BaTiO3 on the sintering and microstructure development. They found that ZnO is helpful for grains growth of BTO ceramics. Therefore, owing to its effectiveness in controlling grain growth, ZnO appears to be an attractive additive for BTO dielectrics. With this potential in mind, we propose in this work to investigate deeply the effect of various ZnO contents inclusion on the structure, morphology, spectral, optical and dielectric properties of BTO-based products. Series of BaTiO3/(ZnO)x ceramics, with x = 0, 2, 5 and 10 wt%, were synthesized via solid state reaction.
2 Experimental
Raw material powders of BaCO3, TiO2 and ZnO with high purity 99.99% were purchased from Sigma Aldrich company. Series of BaTiO3/(ZnO)x ceramics, where x = 0, 2, 5 and 10 wt% were produced via solid state reaction by using planetary high energy ball milling (HEBM; Ball Mill PM 200 from Retsch). The different powders were mixed stoichiometrically in accordance to the designed compositions in ethanol. After, the mixtures were ball-milled in the planetary HEBM as a grinding media for 12 h. After drying, the grinded powders were pressed into discs with 13 mm in diameter. The obtained pellets were calcined at 1100 °C for 12 h. The steps of ball-milling, pressing into discs and calcination were repeated twice in order to achieve the desired nano-sized ceramics.
The phase identification was examined by X-ray powder diffraction (XRD; Rigaku Benchtop Miniflex) using Cu-kα radiation. The morphology was investigated by scanning electron microscope (SEM; FEI Quanta FEG). Fourier transform-infrared (FT-IR) spectra were collected using Bruker alpha-II FT-IR spectrometer in wavenumbers of 4000–400 cm−1. The diffuse reflectance spectra (DRS) were registered using UV–Vis spectrophotometer (JASCO V-780). The electrical and dielectric measurements were done by using Novocontrol Technologies (Alpha-N high-resolution analyzer).
3 Results and discussion
3.1 Structural analysis
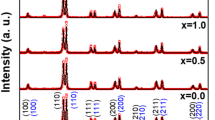
XRD patterns for various BaTiO3/(ZnO)x ceramics with x = 0–10 wt% are presented in Fig. 1. The different XRD patterns were refined through Rietveld method by using Match! program. The Rietveld method was successfully applied to determine quantitatively phase abundances of the different crystallographic phases in the compound, as well as the lattice parameters. Typical examples of XRD refinements for ceramics with x = 0 and 5 wt% are illustrated in Fig. 2. The Bragg RB factor and the goodness of fit χ2 were used as the numerical criteria of fitting. The deduced phase fractions and lattice parameters are summarized in Table 1. All the XRD patterns implied that the various produced ceramics crystallized in tetragonal structure (space group P4mm) according to the ICCD No. 96-210-0860. It is obvious that the lattice parameters a and b decrease with increasing ZnO doping concentration in the prepared ceramics. In the current study, the development of tetragonal structure for all ceramics is approved by the splitting peaks of (200)/(002) reflections (inset of Fig. 1) [25]. The overlapping of these reflections and the disappearance of (200) peak are an indication to a transition from tetragonal to cubic structure. In our case, the tetragonality was maintained for different synthesized BaTiO3/(ZnO)x ceramics with various doping levels. Furthermore, the values of c/a ratio are practically constant, indicating the preserving of tetragonality structure. It is also visible from the Fig. 1 that no impurity was found for pristine BTO sample. However, additional peaks that are corresponding to the ZnO phase (ICCD No. 96-152-7205) are identified. The concentration of secondary ZnO phase increases gradually with increasing the ‘x’ doping content.
The average crystallites size (DXRD) is estimated for all synthesized nanocomposites by using Scherrer equation:
where k = 0.94 is a constant, λ = 1.5418 Å for Cu-Kα radiations, β is full width at half maximum, θ is Bragg’s angle. The estimated DXRD values for various BaTiO3/(ZnO)x ceramics are listed in the Table 1 and are found to be in the range of 51.99–69.36 nm. Lather et al. [26] reported that when the grains size reduces, the tetragonality of the products diminishes as (c/a-1) < 1% and achieve a pseudo-cubic structure with c/a ~ 1. Compared to pristine BTO product, it is obvious that the calculated DXRD increased with ZnO doping confirming the maintaining of the tetragonality.
3.2 FT-IR examination
FT-IR spectra of the prepared BaTiO3/(ZnO)x ceramics are showed in Fig. 3. All the products revealed characteristic band at about 498 cm−1 that is associated to Ti–O vibration [27]. Another characteristic band with very small intensity is observed around 1450 cm−1 for all composites, which is mostly attributed to the signals of the Ba–Ti–O vibration [27]. No such change is noticed in the various prepared nanocomposites.
3.3 Morphological investigation
SEM micrographs of BaTiO3/(ZnO)x ceramics are showed in Fig. 4. Pure BTO sample (Fig. 4a) illustrated a morphology with homogeneous nanosized grains. The samples with ZnO doping showed an increase in the grains size in comparison to pure BTO product, but with lesser homogeneity. That is mostly due to the presence of secondary phase and the increment of sintering temperature with ZnO doping [28].
3.4 Optical investigation
Figure 5 presents the UV–Vis DRS of various synthesized BaTiO3/(ZnO)x ceramics performed in the range of 200–800 nm. Tauc model was employed to deduce the optical band gap energy (Eg) according to the following expression [29]:
where hν is the photon energy and α is a constant independent on photon energy. Since BTO has a direct band gap [30], the exponent r will take value of 1/2 and hence by plotting (αhν)2 as a function of hν, the Eg can be deduced. The Eg is deduced by extrapolating the linear portion to the low energies. The Eg values of BaTiO3/(ZnO)x ceramics is deduced by converting the diffused reflectance values according to the Kubelka–Munk equation [31]:
Figure 6 presents plots of (αhν)2 against hν where Eg values are deduced by extrapolating the linear portions. The deduced Eg values are listed in Table 2. The pristine BTO sample (x = 0 wt%) display Eg value of about 3.32 eV. The Eg value decreased with increasing the ZnO content. This reduction in the BaTiO3/(ZnO) ceramics could be ascribed to the increase in grains size and crystallinity [32]. This is supported by the XRD and SEM analyses. Another possible reason for the change in Eg values is the presence of impurities in the BaTiO3/(ZnO)x ceramics [33].
3.5 Dielectric analyses
The investigation of dielectric properties of materials is an essential tool to design passive devices for integrated circuits and microelectronic applications devices. The variation of dielectric constant (ε′) of BaTiO3/(ZnO)x ceramics (x = 0, 2, 5 and 10 wt%) is compared in Fig. 7a. It is found that, for various compositions, the dielectric constant decreases quickly in the region of low-frequency and then became more or less constant at high frequency representing the dielectric dispersion. The high ε′ and the dielectric dispersion behavior at low frequency could be ascribed to the Maxwell–Wagner interfacial polarization consistent with Koop’s phenomenological theory [34, 35]. Indeed, a dielectric compound having polycrystalline structure is supposed to be constituted of well conducting grains separated by highly resistive thin grain boundaries. The former is more efficient at lower frequencies whereas the grains are more efficient at high frequencies. Accordingly, the high ε′ at low frequency can be ascribed to the interfaces between the ferroelectric BaTiO3 and the nano-phases ZnO that display considerably different permittivities and conductivities. When the composite is subjected to an electrical field, the ZnO phase will provide space charges that accumulate at the interfaces of the two phases. Therefore, these interfaces provoke the additional polarization, i.e. interface polarization, that is inactive at higher frequencies because it is a typical slow polarization mechanism [36]. Consequently, it will lead to the dielectric dispersion at the lower frequencies. After the initial great values at low frequencies, the ε′ becomes approximately constant at frequencies higher than 10 kHz. One should note that the dielectric permittivity for x = 2, 5 and 10 wt% samples shows a good stability in the frequency range 100 Hz–10 MHz, whereas pure BTO (x = 0 wt%) shows a strong dispersion. This result shows that the addition of ZnO nanoparticles improves the stability of the dielectric constant. This is encouraging for radio frequencies and microwaves applications. The dielectric constant also depended on the ZnO concentration, the greatest magnitude of ε′ = 1880 was found for samples with x = 5 wt% of ZnO displaying a large and high density of granular microstructure and. The lowest magnitude of ε′ = 500 was found for x = 10 wt% ceramic, which shows the large impurity compared with the other samples.
The dispersion behavior observed in dielectric permittivity at low frequency is accompanied by a relaxation peak in loss tangent (tgδ = ε″/ε′) as displayed in Fig. 1b. The dependence of dielectric loss on frequency indicates that the charge carriers hopping plays a significant role in their transport progressions since a loss peak is a basic characteristic of the charge carriers hopping transport [37]. After the peak observed at low frequency, the loss tangent (tgδ) for all samples decreases rapidly when the frequency increases. This behavior is also encouraging for microwave applications (high frequency). The dielectric loss (tanδ) is influenced by the ZnO concentration and is related to the sample microstructure. The lowest value of the dielectric loss (tanδ = 0.016) was measured for x = 5 wt% ceramic. The x = 5 wt% ceramic exhibits the maximum permittivity and the lowest dielectric loss (tanδ) at room temperature, indicating that it is the most suitable concentration among the elaborated series. The high values and the good stability in a wide frequency range of dielectric permittivity combined with low dielectric loss (tgδ) make the BaTiO3/(ZnO) ceramics potential candidates for high frequency device applications, for decoupling capacitors, and for dynamic random-access memory. The dielectric, electric, mechanical properties over a large frequency and at various temperatures of the x = 5 wt% ceramic will be described in detail in a future investigation.
3.6 Electrical conductivity investigation
The ac conductivity (σac) of various BaTiO3/(ZnO)x ceramics is obtained using the following empirical formula:
where ε0 is permittivity in vacuum and ω = 2πf is angular frequency. The results of σac versus frequency were measured in the range 10 Hz ≤ F ≤ 107Hz at RT and presented in the Fig. 8. At low frequency, σac is almost independent on frequency and this may be attributed to the dc conductivity of samples. When frequency is further increased, σac is increased rapidly. The measured σac versus frequency could be explained by the Jonscher’s power law of the universal dielectric response (UDR) model [38]:
where σac is ac conductivity of the material, σdc is dc bulk conductivity, A is a thermally activated constant that determines the polarizability strength, and n is a constant that determines the degree of interaction among the ions and lattice. The experimentally observed values of the samples using Eq. (5) were fitted and the obtained parameters are reported in Table 3. At lower frequency range, the experimental and theoretical values of pristine sample are not in good agreement, which is mostly owing to the existence of space charge polarization at the interface of sample-electrode. However, in all frequency range, the curves of x = 2, 5 and 10 wt% ceramics are well fitted. At higher frequency (> 102 Hz), the rise of ac conductivity with frequency may be accredited to electrons hopping between two different valence states [39]. It should be noted that the high frequency conductivity remains almost the same for all the ZnO concentrations, while a slight difference in conductivity is obtained at low frequency.
3.7 Electrical modulus analysis
For the composite structure, it is always better to know the effect of each phase (i.e., the dissociation between the bulk and the interface effect) and to highlight the effect of adding. The electric modulus (M*) could give important details about the relaxation mechanism. This formalism has the advantage of suppressing the electrode effects, the grain-boundary conduction process and the undesirable influences of extrinsic relaxation [30]. The representation of the electric modulus to analyze dielectric properties is discussed in various reports [39,40,41,42]. Based on the complex permittivity ε* = ε′ − jε″, M* is expressed as follow:
M′ and M″ are the real and imaginary parts of M*, respectively.
Figure 9a presents variation of M′ versus frequency for BaTiO3/(ZnO)x ceramics. At low frequency, the M’ values are very low and approaches to zero for all compositions. The M′ value increased with increasing frequency. At high frequency region, M′ shows a continuous increase and dispersion. M’ reached a maximum value corresponding to M∞ (the asymptotic value of M′ at higher frequencies). The continuous dispersion as frequency increases could be accredited to the process of conduction because of the short-range mobility of charge carriers [43]. Besides, this effect could be associated to the non-existence of a restorative force that governs the charge carriers’ mobility due to the induced electric field [43].
The evolution of M″ against frequency at RT is presented in Fig. 9b. The M″ plots exhibit clear characteristic peaks (at fmax) invented because of relaxation behavior and are in the center of the dispersion region of M′. The region of frequency below fmax gives the extent to which charge carriers are mobile on long distances (Jump conduction process). At the frequencies above fmax, the carriers are confined to potential wells and hence are mobile on short distances [44]. The variation of the position peak (fmax) for the different samples may be attributed to the microstructure change and to the occurrence of a distribution of relaxation times.
M* can be expressed as Fourier transform of a relaxation function ϕ(t) [45]:
where M* is the electrical modulus and M∞ represents the value of its real part at high frequencies. This equation used the Kohlrausch–Williams–Watts (KWW) function:
where β is an exponent indicating the Debye relaxation deviation (β = 1) and τ represents a characteristic relaxation time. The theoretical fits (solid lines) of the M′′ experimental data are depicted in the Fig. 9b. Obviously, the experimental data are well fitted excepting in the region of high frequencies. The different fitted parameters (M∞, β and τ) obtained by the KWW model for all compositions are reported in Table 4. The various fitting parameters are depending on the ZnO content. These values of β evidently imply the existence of non-ideal Debye-type relaxation phenomenon.
For the composite structure, it is always better to know the effect of each phase (i.e., the dissociation between the bulk and the interface effect. Figure 10 depicts M″ versus M′ for different BaTiO3/(ZnO)x ceramics. It is clearly seen that the pure BaTiO3 ceramics shows only one semi-circle, while two semi-circles are possibly traced for the x = 2, 5 and 10 wt% ceramics. This behavior suggests that the composite ceramics are composed of two phases which constituted of ZnO nanoparticles and BaTiO3 elements. The semi-circle observed at lower frequencies is ascribed to the contributions of grain boundaries and the interfacial effect specified by the interface between the BaTiO3 and the ZnO nanoparticles (interfacial properties), while the semi-circle occurred at higher frequency is associated to the grain (bulk properties).
4 Conclusion
In this study, BaTiO3/(ZnO)x ceramics (x = 0, 0.5, 1, 2 and 5 wt%) were successfully produced via solid state reaction. According to XRD analysis, pristine compound (i.e. x = 0 wt%) showed pure BTO phase with tetragonal structure. The tetragonality was maintained with ZnO additions. SEM observations showed an increase in grains size with increasing the ZnO concentration. Optical analyses revealed a reduction in Eg values, which is principally accredited to the increase of grains size. The investigation of electrical and dielectric properties indicated that the x = 5 wt% ceramic exhibits greatest dielectric constant and lowest tangent loss among various BaTiO3/(ZnO)x ceramics. The obtained results are encouraging to use the optimal ceramic in radio frequencies and microwaves applications.
References
L. Xiaochi, W. Bian, Y. Li, H. Zhu, F. Zhenxiao, Q. Zhang, Influence of inverse spinel structured CuGa2O4 on microwave dielectric properties of normal spinel ZnGa2O4 ceramics. J. Am. Ceram. Soc. 101, 1646–1654 (2018)
L. Xiaochi, W. Bian, C. Min, F. Zhenxiao, Q. Zhang, H. Zhu, Cation distribution of high-performance Mn-substituted ZnGa2O4 microwave dielectric ceramics. Ceram. Int. 44, 10028–10034 (2018)
C.D. Chandler, C. Roger, M.J. Hampdensmith, Chemical aspects of solution routes to perovskite-phase mixed-metal oxides from metal-organic precursors. Chem. Rev. 93, 1205–1241 (1993)
M.A. Pena, J.L.G. Fierro, Chemical structures and performance of perovskite oxides. Chem. Rev. 101, 1981–2017 (2001)
J. Harada, T. Pedersen, Z. Barnea, X-Ray and neutron diffraction study of tetragonal barium titanate. Acta Crystall. a-Crys. A 26, 336 (1970)
F.I.H. Rhouma, A. Dhahri, J. Dhahri, H. Belmabrouk, M.A. Valente, Structural and dielectric properties of Ba0.8La0.133Ti0.90Sn0.1O3. Solid State Commun. 152, 1874–1879 (2012)
M.Z.C. Hu, G.A. Miller, E.A. Payzant, C.J. Rawn, Homogeneous (co)precipitation of inorganic salts for synthesis of monodispersed barium titanate particles. J. Mater. Sci. 35, 2927–2936 (2000)
S. Dudley, T. Kalem, M. Akinc, Conversion of SiO2 diatom frustules to BaTiO3 and SrTiO3. J. Am. Ceram. Soc. 89, 2434–2439 (2006)
V. Paunovic, L. Zivkovic, Influence of Rare-earth additives (La, Sm and Dy) on the microstructure and dielectric properties of doped BaTiO3 ceramics. Sci. Sinter. 42, 69–79 (2010)
H.A. Moghaddam, M.R. Mohammadi, TiO2-BaTiO3 nanocomposite for electron capture in dye-sensitized solar cells. J. Am. Ceram. Soc. 100, 2144–2153 (2017)
H.A. Moghaddam, M.R. Mohammadi, S.M.S. Reyhani, Improved photon to current conversion in nanostructured TiO2 dye-sensitized solar cells by incorporating cubic BaTiO3 particles deliting incident. Sol. Energy 132, 1–14 (2016)
Y.M. Zhang, M.H. Cao, Z.H. Yao, Z.J. Wang, Z. Song, A. Ullah, H. Hao, H.X. Liu, Effects of silica coating on the microstructures and energy storage properties of BaTiO3 ceramics. Mater. Res. Bull. 67, 70–76 (2015)
W.H. Tzing, W.H. Tuan, H.L. Lin, The effect of microstructure on the electrical properties of NiO-doped BaTiO3. Ceram. Int. 25, 425–430 (1999)
T. Nagai, K. Iijima, H.J. Hwang, M. Sando, T. Sekino, K. Niihara, Effect of MgO doping on the phase transformations of BaTiO3. J. Am. Ceram. Soc. 83, 107–112 (2000)
Y. Sakabe, N. Wada, T. Hiramatsu, T. Tonogaki, Dielectric properties of fine-grained BaTiO3 ceramics doped with CaO. Jpn. J. Appl. Phys. 41, 6922–6925 (2002)
Y.H. Song, J.H. Hwang, Y.H. Han, Effects of Y2O3 on temperature stability of acceptor-doped BaTiO3. Jpn. J. Appl. Phys. 44, 1310–1313 (2005)
R. Saravanan, V.K. Gupta, E. Mosquera, F. Gracia, Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J. Mol. Liq. 198, 409–412 (2014)
A.M. Al-syadi, V.K. Gupta, E. Mosquera, M.M. El-Desoky, M.S. Al-Assiri, Impedance spectroscopy of V2O5–Bi2O3–BaTiO3 glass–ceramics. Solid State Sci. 26, 72–82 (2014)
N. Zhang, L. Li, J. Chen, J. Yu, ZnO-doped BaTiO3-Na0.5Bi0.5TiO3-Nb2O5-based ceramics with temperature-stable high permittivity from − 55 °C to 375 °C. Mater. Lett. 138, 228–230 (2015)
T. Wang, X. Wei, Q. Hu, L. Jin, Z. Xu, Y. Feng, Effects of ZnNb2O6 addition on BaTiO3 ceramics for energy storage. Mater. Sci. Eng. B 178, 1081–1086 (2013)
Y. Yan, C. Ning, Z. Jin, H. Qin, W. Luo, G. Liu, The dielectric properties and microstructure of BaTiO3 ceramics with ZnO–Nb2O5 composite addition. J. Alloys. Compd. 646, 748–752 (2015)
Q.K. Muhammad, M. Waqar, M.A. Rafiq, M.N. Rafiq, M. Usman, M.S. Anwar, Structural, dielectric, and impedance study of ZnO doped barium zirconium titanate (BZT) ceramics. J. Mater. Sci. 51, 10048–10058 (2016)
Y. Iqbal, A. Jamal, The effect of Ta2O5- and ZnO-doping on the Curie temperature of BaTiO3. J. Phys 371, 012035 (2012)
A.C. Caballero, J.F. Fernández, C. Moure, P. Durán, Y.M. Chiang, Grain growth control and dopant distribution in ZnO-doped BaTiO3. J. Am. Ceram. Soc. 81, 939–944 (1998)
M. Atif, S. Ahmed, M. Nadeem, M.K. Ali, M. Idrees, R. Grossinger, R.S. Turtelli, Role of competing phases in the structural, magnetic and dielectric relaxation for (1 − x)CoFe2O4 + (x)BaTiO3 composites. Ceram. Int. 42, 14618–14626 (2016)
S. Lather, A. Gupta, J. Dalal, V. Verma, R. Tripathi, A. Ohlan, Effect of mechanical milling on structural, dielectric and magnetic properties of BaTiO3-Ni0.5Co0.5Fe2O4 multiferroic nanocomposites. Ceram. Int. 43, 3246–3251 (2016)
S. Kappadan, T.W. Gebreab, S. Thomas, N. Kalarikkal, Tetragonal BaTiO3 nanoparticles: an efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process 51, 42–47 (2016)
Y. Yan, L. Liu, C. Ning, Y. Yang, C.J. Xia, Y.T. Zou, S.Y. Liu, X.X. Wang, K.H. Liu, X.K. Liu, G. Liu, Improved electrical properties of SiO2-added BaTiO3 ceramics by microwave sintering. Mater. Lett. 165, 135–138 (2016)
Y. Slimani, H. Gungunes, M. Nawaz, A. Manikandan, H.S. El Sayed, M.A. Almessiere, H. Sozeri, S.E. Shirsath, I. Ercan, A. Baykal, Magneto-optical and microstructural properties of spinel cubic copper ferrites with Li-Al co-substitution. Ceram. Int. 44, 14242–14250 (2018)
P.G. Wang, C.M. Fan, Y.W. Wang, G.Y. Ding, P.H. Yuan, A dual chelating sol-gel synthesis of BaTiO3 nanoparticles with effective photocatalytic activity for removing humic acid from water. Mater. Res. Bull. 48, 869–877 (2013)
Z.A. Garmaroudi, M.R. Mohammadi, Design of TiO2 dye-sensitized solar cell photoanode electrodes with different microstructures and arrangement modes of the layers. J. Sol–Gel Sci. Techn. 76, 666–678 (2015)
Y.C. Teh, A.A. Saif, Influence of annealing temperature on structural and optical properties of sol-gel derived Ba0.9Gd0.1TiO3 thin films for optoelectronics. J. Alloys. Compd. 703, 407–413 (2015)
L.V. Maneeshya, P.V. Thomas, K. Joy, Effects of site substitutions and concentration on the structural, optical and visible photoluminescence properties of Er doped BaTiO3 thin films prepared by RF magnetron sputtering. Opt. Mater. 46, 304–309 (2015)
T.G. Reddy, B.R. Kumar, T.S. Rao, J.A. Ahmad, Structural and dielectric properties of barium bismuth titanate (BaBi4Ti4O15) ceramics. Int. J. Appl. Eng. Res. 6, 571–580 (2011)
P. Jaita, A. Watcharapasorn, N. Kumar, D.P. Cann, S. Jiansirisomboon, Large electric field-induced strain and piezoelectric responses of lead-free Bi0.5(Na0.80K0.20)0.5TiO3-Ba(Ti0.90Sn0.10)O3 ceramics near morphotropic phase boundary. Electron. Mater. Lett. 11, 828–835 (2011)
X.X. Dong, H.W. Chen, M. Wei, K.T. Wu, J.H. Zhang, Structure, dielectric and energy storage properties of BaTiO3 ceramics doped with YNbO4. J. Alloys. Compd. 744, 721–727 (2018)
K. Kumari, A. Prasad, K. Prasad, Dielectric, Impedance/modulus and conductivity studies on [Bi0.5(Na1-xKx)0.5]0.94Ba0.06TiO3,[0.16 ≤ x ≤ 0.20] leadfree ceramics. Am. J. Mater. Sci. 6, 1–8 (2016)
A.K. Jonscher, The ‘universal’dielectric response. Nature 267, 673 (1977)
L. Singh, U.S. Rai, K. Mandal, B.C. Sin, S.I. Lee, Y. Lee, Dielectric, AC-impedance, modulus studies on 0.5BaTiO3· 0.5CaCu3Ti4O12 nano-composite ceramic synthesized by one-pot, glycine-assisted nitrate-gel route. Ceram. Int. 40, 10073–10083 (2014)
A. Rouahi, A. Kahouli, F. Challali, M.-P. Besland, C. Vallée, B. Yangui, S. Salimy, A. Goullet, A. Sylvestre, Impedance and electric modulus study of amorphous TiTaO thin films: highlight of the interphase effect. J. Phys. D 46, 065308 (2013)
A. Selmi, O. Khaldi, M. Mascot, F. Jomni, J. Carru, Dielectric relaxations in Ba0.85Sr0.15TiO3 thin films deposited on Pt/Ti/SiO2/Si substrates by sol–gel method. J. Mater. Sci. 27, 11299–11307 (2016)
M. Sahu, S.K. Pradhan, S. Hajra, B.K. Panigrahi, R. Choudhary, Studies of structural, electrical, and excitation performance of electronic material: europium substituted 0.9(Bi0.5Na0.5TiO3)–0.1(PbZr0.48Ti0.52O3). Appl. Phys. A 125, 183 (2019)
C. Rayssi, S.E. Kossi, J. Dhahri, K. Khirouni, Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1−xCo4x/3O3 (0 ≤ x ≤ 0.1). RSC Adv. 8, 17139–17150 (2018)
R. Schmidt, S. Pandey, P. Fiorenza, D.C. Sinclair, Non-stoichiometry in “CaCu3Ti4O12” (CCTO) ceramics. RSC Adv. 3, 14580–14589 (2013)
A. Rouahi, A. Kahouli, A. Sylvestre, E. Defay, B. Yangui, Impedance spectroscopic and dielectric analysis of Ba0.7Sr0.3TiO3 thin films. J. Alloys. Compd. 529, 84–88 (2012)
Acknowledgments
The authors highly acknowledged the supports of the Institute for Research & Medical Consultations (Projects application No. 2017-IRMC-S-3, No. 2017-576-IRMC and No. 2018-IRMC-S-2) of Imam Abdulrahman Bin Faisal University (IAU—Saudi Arabia).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Slimani, Y., Selmi, A., Hannachi, E. et al. Impact of ZnO addition on structural, morphological, optical, dielectric and electrical performances of BaTiO3 ceramics. J Mater Sci: Mater Electron 30, 9520–9530 (2019). https://doi.org/10.1007/s10854-019-01284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01284-2