Abstract
In this work, 80TeO2–(20 − x) Na2O–xTiO2 (x = 0, 5, 10 and 20) mol% glasses by melting quenching technique were obtained. Density (ρ) and molar volume (Vm) of these glasses have been investigated. The characterization was performed by X-ray diffraction (XRD), differential scanning calorimetry (DSC), Fourier transform infrared (FTIR) and ultraviolet–visible (UV–Vis) spectroscopy. As expected, the Vm values decreased with x increasing, exhibiting a density opposite behavior. DSC, FTIR and UV–Vis results indicated that, with the increasing the TiO2 content, the role of this oxide changes from network modifier to former. In addition, more covalent bonds are formed, reinforcing the network connectivity by increasing the Te–O–Ti inter-chain bounds. These features improved the glassy system thermal and optical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
TeO2-based glasses have increasing scientific and technological interest due to wide optical transmission window (0.350 to 5.0 µm), consistent vitreous stability (ΔT > 100 cm−1) and low phonon energy (between 600 to 850 cm−1) [1,2,3]. Additionally, these materials behold density over 4 g/cm−3 and linear refractive index higher than 2 [4,5,6]. These properties allows application for photonics and electronics, such as: optical fiber, amplifiers and sensors [2, 7, 8].
The TeO2-glassy is obtained only with ultra-rapid cooling rates [9]. However, the addition of modifier oxides, such as alkali, alkaline earth or transition metal oxides drops the cooling rates to 1 kJ/s, making it possible to obtain these glasses by conventional melt-quenching technique [10,11,12,13].
The addition of Na2O in TeO2-based glasses was first reported by Mochida et al. with a range of glass formation between 10 ≤ x ≤ 46.5 mol% [11]. The Na2O oxide plays the role of network modifier, causing the transformation of the basic structural unit TeO4 to TeO3+1, breaking the Te–O–Te linkages [14]. Nishida et al. [15] proposed that this transformation occurs due to the change of the glass matrix from a three- or two-dimensional network structure to a lower dimensional one. These structural changes gives rise to consequences on the thermal properties decreasing the vitreous transition temperature (Tg) [3, 16].
On the other hand, TiO2 is effective in reinforce TeO2 glass network by the substitutional positioning of Te to Ti site [6, 17, 18]. In addition, the incorporation of this oxide increases third-order optical non-linearities, linear refractive index and thermal stability for systems like TeO2–Li2O–TiO2. These features have been attributed to the change in coordination from TeO3 to TeO4 units by Te–O–Ti linkages [4, 19,20,21,22].
In this sense, the correlation between structural with thermal and optical properties of TeO2–Na2O–TiO2 glassy system were investigated, in order to understand the role of TiO2 into the glass as Na2O was exchanged for TiO2.
2 Materials and methods
The 80TeO2–(20 − x) Na2O–xTiO2 (x = 0, 5, 10, 15 and 20 mol%) samples were prepared by means of melt-quenching technique in air atmosphere. The precursors reagents: TeO2 (Alfa Aesar 99.99%), Na2O (Sigma-Aldrich 99.5%) and TiO2 (Synth 99.5%) were weighted and mixed with pistil and agate mortar during 40 min. The mixtures were melted in Pt crucible at a temperature of 840 °C for 30 min. The melt was quenched in a preheated brass mold at a temperature 20 °C lower than Tg and submitted to an annealing treatment for 240 min.
The density measurements were carried out by means the Archimedes’s Principle with xylene (ρ = 0.88 g/cm3) using a balance (AY220, Shimadzu) with 10−4 g precision. The diffractograms were recorded on X-ray Automatic Diffractometer (Ultima IV, Rigaku) at a scan rate of 2º/min (CuKα radiation λ = 1.5418 Å, 30 kV, 40 mA).
Differential Scanning Calorimetry (DSC) data were obtained with a 4 °C precision in calorimeter (Labsys Evo, Setaram Instrumentation). Such measures were realized under Argon atmosphere with 20 mL/min flux and heating rate of 10 °C/min, from ambient temperature to 600 °C using alumina crucible. In order to apply DRX and DSC, the samples were powdered and sieved (≤ 53 µm).
The structural analysis was carried out by means of Fourier transform infrared (FTIR) spectroscopy. For FTIR it was used a spectrometer, (IR-Prestige 21, Shimadzu). The glassy powdered samples were pulverized in an agate mortar with 100 mg of KBr in the 1:50 proportion. The resulting spectra were obtained in the absorbance model in the interval between 4000 and 400 cm−1 using 64 scans with 4 cm−1 resolution.
The optical absorption spectra were acquired using a spectrometer (Carry 50, Varian) in the wavelength range 300–700 nm. The samples thickness were 300 µm.
3 Results and discussion
3.1 Density, molar volume and oxygen density packing
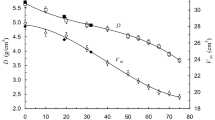
The physical parameters density (ρ), molar volume (Vm) and oxygen density package (OPD) are presented in Table 1. The ρ values observed in samples for x = 0 and x = 20 corroborate with the previously reported [23, 24]. The exchange of Na2O by TiO2 increases the ρ values, which is due to the substitution of a lower molecular weight substance (Na2O 61.98 g/mol) with a higher molecular weight one (TiO2 79.87 g/mol), resulting in an increase in total molecular weight. Besides, the density increase is due to the structural modifications occurring in the amorphous network. In sample with x = 0, Na2O plays the role of network modifier changing the Te atom coordination from TeO4 → TeO3. With TiO2 addition, more covalent bonds are formed, promoting a continuous glassy network by the Te–O–Ti linkages [17].
Other physical parameters which can contribute with valuable information about the changes in glass structure are molar volume and the oxygen packing density. Vm was calculated by using \(V_{m} = \frac{M}{\rho }\), where M is the glass sample molecular weight. The OPD was determined using the equation: \(OPD = 1000C_{0} \times\frac{\rho }{M}\), where C0 is the number of oxygen atoms per formula unit. As expected, the Vm values (Table 1) decrease from 29.1 to 27.26 cm3/mol, with increasing x, exhibiting density opposite behavior. The increase of OPD values (Table 1) with x is an indicative of close packing glass network structure. This packing degree is dependent of cation field intensity and the cation radius [25]. Since the ionic radius of Na+ and Ti4+ are 0.098 nm and 0.064 nm, respectively [26]. Therefore, the average of the cation field intensity increases with x, consequently increasing the OPD.
3.2 X-ray diffraction (XRD) results
Figure 1 presents the XRD results of the studied samples. We can notice that the sample in the composition for x = 0 is mostly amorphous, except for two sharp peaks at 13.3 and 26.5° superimposed on broad peaks due to the majority glassy phase. In fact, during the experimental studies, this sample was observed to be hygroscopic, such behavior is in accordance with literature for (100 − x)TeO2–xNa2O binary system. This feature can be attributed to the hydrated compound presence such as Na2Te2O5·2H2O phase [23]. For x = 5 we notice the predominant amorphous character with some sharp peaks superimposed at 13.3 and 26.5°, showing the same Na2Te2O5 phase presence. The peaks at 26.1, 29.8 and 32.0° are attributed to the TiTe3O8 phase [27]. The αTeO2 phase presents peaks at 28.7 and 48.5°. For x = 10 and x = 15 the samples presented amorphous character. The sample with x = 20 contained significant amount of crystalline phases and presented several peaks attributed αTeO2 (19.7, 28.7 and 48.5°) and TiTe3O8 (26.1, 29.8, 32.0, 34.6, 46.7 and 55.4°) [28].
Figure 2 shows, the all sample photo that were characterized in Fig. 1. These samples change from yellowish to brownish with increasing x, being the glassy sample (x = 10 and x = 15) and partially crystallized (x = 0, 5 and 20).
3.3 Differential scanning calorimetry (DSC) results
Thermal behavior of each sample was determined by DSC analysis. The obtained thermograms are presented in Fig. 3. From these data, the glass transition (Tg), the onset crystallization (Tx), the first exothermic peak at the crystallization (Tc) temperatures and the thermal stability were determined. The last one was determined using two criteria: \(\Delta T = T_{x} - T_{g}\), which is frequently used for glass materials, and the Weinberg parameter \(H_{w} = \frac{{T_{x} - T_{g} }}{{T_{g} }}\) [29, 30]. These results are presented in Table 1. We can notice an increase in Tg from 245 to 367 °C with the exchange of Na2O for TiO2. This increase can be related to the reinforcement of the glass network due to the high coordination number of Ti4+ (4 or 6) [31]. Besides, the asymmetric crystallization peak for x = 0, 5 and 20 suggests different distinct phase crystallizations. The shift in Tc from 370 to 340 °C with x = 5 is an indicator of the increasing concentration of nuclei in the glass and consequently the probability of crystallization inside the glass matrix increases, this is a direct consequence of the αTeO2 presence, as observed in XRD results [32].
In binary glasses, excessive addition of a network modifier decreases vitrification behavior, due to the decreasing stability of TeO3 units [23], increasing Tg values and decreasing glass-forming ability. So, the addition of 5% mol of TiO2 makes this oxide plays a role of modifier network. However, for 10 and 15% mol the TiO2 assumes the role of former network, rearranging the breaking caused by Na2O which creates TeO3 and TeO3+1 and does not allow the formation of the Na2Te2O5 phase, facilitating the amorphization of the glass network. This directly impacts the shape of the peak Tc which is narrower for the samples x = 10 and 15 and is shifted to higher temperatures, indicating a lower concentration of nuclei that induce the crystallization.
In Table 1, ΔT decreases from x = 0 to x = 5 and slightly increases for x = 10 and 15. This behavior is in accordance with XRD results, since the samples x = 10 and 15 presented an amorphous behavior showing that the melting quenching technique was successfully performed for these two compositions. This result endorses the change of the role of TiO2 oxide from network modifier to former. Besides, Hw (Table 1) decreases with the exchange of Na2O for TiO2, that suggest an increase in the crystallization tendency [33] which is an indicator of the substitution of Te–O–Te for Te–O–Ti bounds favoring the covalent character of the network [34]. These statements are reinforced by ρ, Vm and OPD results.
3.4 FTIR analysis
In order to obtain some insights about the structural change with composition, FTIR experiments were performed and the results are shown in Fig. 4. According to the literature, in the region from 400 to 1000 cm−1, there are, at least, eight characteristic regions corresponding to the different structural units present in tellurite glasses with TiO2: ~ 450, 580, 620, 660, 710, 750, 780, 800–830, 852–890. [22, 23, 27, 32, 35,36,37,38].
The region at ~ 450 cm−1 can be related to Te–O–Te and/or Te–O–Ti stretching vibrations. Besides, for x = 0 we notice a shoulder at 451 cm−1 and for x = 20 at 440 cm−1. This shoulder has been related to symmetric stretching of O–Te–O in α-TeO2 structure. The 467 cm−1 peak for x = 0 is related to Te–O–Te stretching vibrations. For x = 20, Te–O–Ti stretching vibrations appear at 486 cm−1. For x = 5, 10 and 15 these modes were superimposed and presented a broad band in this region [27, 32, 35].
The region at 2900 - 3500 cm−1 is attributed to the vibration of the elongation of the hydroxyl group and hydrogen. This high intensity region decreases with increasing x. In this case the high Na2O content confirm the sample tendency to be hygroscopic [36].
FTIR results in the region from 500 to 950 cm−1 were detailed into Gaussian fitting as presented in Fig. 5. The band centered at 580 cm−1, which is attributed to Te–O stretching vibrations of TeO4 and TeO3+1 units, remains at the same position but decreases in area from x = 10 to x = 20. According to Dimitriev [38], the addition of modifier network stimulates TeO3+1 units formation. Our results showed that the TiO2 acts as network modifier for the sample with x = 5. On the other hand, for x = 10, 15 and 20 its role changed from network modifier to former reinforcing the DSC results as shown in Fig. 3.
The band area at 620 cm−1 increases with x content. This mode, which is attributed to Te–O bonds of deformed TeO4 units, increases from x = 0 to x = 5, remains constant from x = 5 to x = 15 and increases to x = 20. This behavior is an indicative that TiO2 inhibits the Na2O action in creates more TeO3+1 and TeO3 from TeO4 units [3, 23]. The 660 cm−1 band is associated to asymmetric stretching vibration of Te–O bonds in TeO3 pyramidal units [3]. The intensity of this band remains approximately constant up to x = 15 and decreases for x = 20. The 710 cm−1 band increases up to x = 10 and decreases with increasing x, this band can be attributed to TeO3 units [38]. The band at 750 cm−1 is attributed to TeO3+1 unit and increases with x content. The band at 773 cm−1 is well resolved for x = 0 and x = 5 samples and it is attributed to αTeO2 [36]. For the other samples this mode vibration is superimposed with the 800 cm−1 which it is attributed to TeO3 units [23]. This band decrease in intensity increasing x content in the samples. In Fig. 5, shifts from 800 to 852 cm−1 and from 831 to 889 cm−1, with x increasing from 5 to 20 can be perceived. These bands are related to stretching vibration of Ti–O–Ti bridges in TiO4 units [36]. FTIR analyses indicate that when Ti is incorporated in Te–O–Te interchain, Te–O–Ti bonds are formed, and for relative high TiO2 composition and after a saturation of Te–O–Ti interchains, occurs the TiO4 units formation [22, 23, 27, 35, 36].
3.5 Optical absorption
The analysis of the optical absorption edge is useful to obtain some insights about the optical transition and optical bandgap of materials. According to the literature [39], in glasses, the UV absorption edge shifts to longer wavelengths by increasing the molecular weight of their precursor oxides. The yellow color of the glasses, as observed in Fig. 2, is due to a variable shift of the ultraviolet absorption edge towards the visible range, which depends on the glass composition. For the analyzed samples, it was not possible to analyze the x = 20 sample UV edge, since it was completely opaque even for 300 µm thickness. For the other samples, the optical band gap and Urbach´s energy were determined.
Davis and Mott [40] presented an expression for the absorption coefficient (α) as a function of photon energy (hν), as follow:
where α is the absorption coefficient, (hν) is the energy of the photon, Eg is the bandgap energy and m indicates the type of electronic transition involved in the absorption process. The values for m depend on the bandgap characteristic: m = 1/2 for direct allowed transition; m = 3/2 for direct forbidden transition, m = 2 for indirect allowed transition and m = 3 for indirect forbidden transition. The absorption spectra presented in Fig. 6a were used to determine the optical bandgap (Eg) of samples using Eq. 1. Figure 6b presents the curves of (α.hν)1/m versus photon energy, with the Eg values being estimated by extrapolation of the linear portion of the curves, where (α.hν)1/m = 0. The best fit was obtained for m = 2, indicating an indirect allowed transition [41]. Another method to determine the bandgap is the intersection point between lines that extrapolate absorption coefficient values in the small hν range and at the linear absorption edge \(\left( {E^{\prime}_{\text{g}} } \right)\) [42]. Table 2 presents the values of Eg and \(E^{\prime}_{\text{g}}\) in eV. The Eg values changes from 3.394 to 2.845 eV with increasing x content. In principle, this behavior would be related to the increase in defects of glassy network, however from FTIR and DSC results we noticed that the exchange of Na2O for TiO2 is reinforcing the network. In fact, this behavior can be explained by means of ion oxide polarizability and it will be discussed later.
The optical absorption coefficient near the fundamental absorption band has an exponential dependency, which is given by the Urbach´s rule [41]:
in which α0 is a constant and ΔE is the Urbach’s energy. ΔE corresponds to the optical transitions between localized tail states adjacent to the valence band and extended states in the conduction band above the mobility edge [43]. The absorption coefficient values (in log scale) were plotted against the photon energies. The ΔE values were obtained by linear fitting from those plots and are presented in Table 2. It can be notice that the ΔE values decrease and then increase from x = 5 to x = 15. According to Mott and Davis [40] model, this energy depends on the degree of the both disorder and defects of the amorphous structure. If the glass has a more polymerized network, Urbach´s energy is lower.
According to the literature [43], the behavior of the polarizability of the electronic ion are calculated by the following Eq. 3:
were γ is a polarizability of the cation, Vm is the molar volume, p denotes the number of cations and q oxide ions respectively, in de chemical formula Ap Oq. Figure 7 presents the bandgap values (Eg), Urbach’s energy (ΔE), and the polarizability (γ 2O ) versus TiO2 content.
The polarizability behavior should be inverse to the bandgap; however, this behavior does not occur to our system. The substitution of Na2O by TiO2 generated a decrease in the bandgap, but there is a polarizability atypical behavior, which does not accompany inversely the bandgap. Therefore, this same behavior which decreases the polarizability is related to the increase of mean simple bonding [43, 44]. This increase in average simple bonding is observed when a certain amount of TiO2 added, suggesting that there is an onset of composition which allow the formation of strong chemical bonds in the glass network. This statement is reinforced by the DSC results, which show a decrease in thermal stability for the samples. Finally, the feature of changing the role of TiO2 from network modifier to former drastically influences the thermal and optical properties in TeO2–Na2O–TiO2 glassy system.
4 Conclusion
We conclude that the structural of the TeO2–Na2O–TiO2 system investigated by FTIR analysis, with the equimolar substitution of Na2O by TiO2 showed a structural transformation in the TeO2 network. The Na2O performs the role of network modifier, changing the coordination of the atom of TeO4 → TeO3. With the addition of TiO2, more covalent bonds are formed, promoting a continuous glass network through the Te–O–Ti bond. The XRD results corroborate with FTIR, and the presence of the hydrated compound, such as the Na2Te2O5·2H2O phase and the TiTe3O8 phase.
In addition, the decrease in polarization is related to the increase in the mean simple bonding. This strong bond increase, which is related to decreases of the bandgap and polarizability values, indicates the increase of Te–O–Ti bonds (band at 450 cm−1). This increase also agrees with the DSC results, which show a decrease in the thermal stability for the samples. Finally, we conclude that TiO2 changes its role from network modifier to former after 5 mol%, which drastically influences the thermal and optical properties through increasing the network connectivity.
References
H. Bürger, W. Vogel, V. Kozhukharov, IR transmission and properties of glasses in the TeO2–RnOm, RnXm, Rn (SO4) m, Rn (PO3) mandB2O3] systems. Infrared Phys. 25, 395–409 (1985)
J.S. Wang, E.M. Vogel, E. Snitzer, Tellurite glass: a new candidate for fiber devices. Opt. Mater. (Amst) 3, 187–203 (1994)
J. Heo, D. Lam, G.H. Sigel Jr., E.A. Mendoza, D.A. Hensley, Spectroscopic analysis of the structure and properties of alkali tellurite glasses. J. Am. Ceram. Soc. 75, 277–281 (1992)
F. Chen, T. Xu, S. Dai, Q. Nie, X. Shen, J. Zhang, X. Wang, Linear and non-linear characteristics of tellurite glasses within TeO2–Bi2O3–TiO2 ternary system. Opt. Mater. (Amst) 32, 868–872 (2010)
H. Takebe, S. Pujino, K. Morinaga, Refractive-index dispersion of tellurite glasses in the region from 0.40 to 1.71 μm. J. Am. Ceram. Soc. 77, 2455–2457 (1994)
M. Udovic, P. Thomas, A. Mirgorodsky, O. Durand, M. Soulis, O. Masson, T. Merle-Méjean, J.-C. Champarnaud-Mesjard, Thermal characteristics, Raman spectra and structural properties of new tellurite glasses within the Bi2O3–TiO2–TeO2 system. J. Solid State Chem. 179, 3252–3259 (2006)
N. Boubata, A. Roula, I. Moussaoui, Thermodynamic and relative approach to compute glass-forming ability of oxides. Bull. Mater. Sci. 36, 457–460 (2013)
S. Manning, H. Ebendorff-Heidepriem, T.M. Monro, Ternary tellurite glasses for the fabrication of nonlinear optical fibres. Opt. Mater. Express. 2, 140–152 (2012)
P.T. Sarjeant, R. Roy, New glassy and polymorphic oxide phases using rapid quenching techniques. J. Am. Ceram. Soc. 50, 500–503 (1967)
R. El-Mallawany, The optical properties of tellurite glasses. J. Appl. Phys. 72, 1774–1777 (1992)
N. Mochida, K. Takahashi, K. Nakata, S. Shibusawa, Properties and structure of the binary tellurite glasses containing mono-and di-valent cations. J. Ceram. Assoc. Jpn. 86, 317–326 (1978)
H. Bürger, K. Kneipp, H. Hobert, W. Vogel, V. Kozhukharov, S. Neov, Glass formation, properties and structure of glasses in the TeO2–ZnO system. J. Non. Cryst. Solids. 151, 134–142 (1992)
D. Souri, Y. Shahmoradi, Calorimetric analysis of non-crystalline TeO2–V2O5–Sb2O3. J. Therm. Anal. Calorim. 129, 601–607 (2017)
T. Sekiya, N. Mochida, A. Ohtsuka, M. Tonokawa, Raman spectra of MO1/2TeO2 (M = Li, Na, K, Rb, Cs and Tl) glasses. J. Non. Cryst. Solids. 144, 128–144 (1992)
T. Nishida, S. Saruwatari, Y. Takashima, Structural study of Na2O–TeO2 glasses by Mössbauer spectroscopy and differential thermal analysis. Bull. Chem. Soc. Jpn 61, 4093–4097 (1988)
Y. Himei, A. Osaka, T. Nanba, Y. Miura, Coordination change of Te atoms in binary tellurite glasses. J. Non. Cryst. Solids. 177, 164–169 (1994)
T. Hayakawa, M. Koduka, M. Nogami, J.R. Duclère, A.P. Mirgorodsky, P. Thomas, Metal oxide doping effects on Raman spectra and third-order nonlinear susceptibilities of thallium–tellurite glasses. Scr. Mater. 62, 806–809 (2010)
M. Soulis, A.P. Mirgorodsky, T. Merle-Méjean, O. Masson, P. Thomas, M. Udovic, The role of modifier’s cation valence in structural properties of TeO2-based glasses. J. Non. Cryst. Solids. 354, 143–149 (2008)
S.M. Lima, W.F. Falco, E.S. Bannwart, L.H. Andrade, R.C. Oliveira, J.C.S. Moraes, K. Yukimitu, E.B. Araújo, E.A. Falcão, A. Steimacher et al., Thermo-optical characterization of tellurite glasses by thermal lens, thermal relaxation calorimetry and interferometric methods. J. Non. Cryst. Solids. 352, 3603–3607 (2006)
H. Nasu, O. Matsushita, K. Kamiya, H. Kobayashi, K. Kubodera, Third harmonic generation from Li2O–TiO2–TeO2 glasses. J. Non. Cryst. Solids. 124, 275–277 (1990)
W.A. Capanema, K. Yukimitu, J.C.S. Moraes, F.A. Santos, M.S. Figueiredo, S.M. Sidel, V.C.S. Reynoso, O.A. Sakai, A.N. Medina, The structure and optical dispersion of the refractive index of tellurite glass. Opt. Mater. (Amst) 33, 1569–1572 (2011)
W. Stambouli, H. Elhouichet, M. Ferid, Study of thermal, structural and optical properties of tellurite glass with different TiO2 composition. J. Mol. Struct. 1028, 39–43 (2012)
K.B. Kavaklioğlu, S. Aydin, M. Çelikbilek, A.E. Ersundu, The TeO2–Na2O system: thermal behavior, structural properties, and phase equilibria. Int. J. Appl. Glas. Sci. 6, 406–418 (2015). https://doi.org/10.1111/ijag.12103
G. Jayasinghe, D. Coppo, P. Bandaranayake, J.L. Souquet, Electrical properties of TeO2 glasses with Na2O as network modifier. Solid State Ionics 76, 297–300 (1995)
A. Bachvarova-Nedelcheva, R. Iordanova, S. Ganev, Y. Dimitriev, Glass formation and structural studies of glasses in the TeO2–ZnO–Bi2O3–Nb2O5 system. J. Non. Cryst. Solids. 503, 224–231 (2019)
V. Dimitrov, T. Komatsu, An interpretation of optical properties of oxides and oxide glasses in terms of the electronic ion polarizability and average single bond strength. J. Univ. Chem. Technol. Met. 45, 219–250 (2010)
I. Shaltout, Crystallization kinetics and structure of (TeO2–TiO2–Fe2O3) glasses. J. Mater. Sci. 35, 323–329 (2000)
N. Elkhoshkhany, R. El-Mallawany, E. Syala, Mechanical and thermal properties of TeO2–Bi2O3–V2O5–Na2O–TiO2 glass system. Ceram. Int. 42, 19218–19224 (2016)
A.F. Kozmidis-Petrović, Theoretical analysis of relative changes of the Hruby, Weinberg, and Lu–Liu glass stability parameters with application on some oxide and chalcogenide glasses. Thermochim. Acta 499, 54–60 (2010)
A.F. Kozmidis-Petrović, Sensitivity of the Hruby, Lu–Liu, Fan, Yuan, and Long glass stability parameters to the change of the ratios of characteristic temperatures Tx/Tg and Tm/Tg. Thermochim. Acta 510, 137–143 (2010)
F. Chen, Q. Yu, B. Qiao, S. Dai, Q. Zhang, Influence of TiO2 on thermal stability and crystallization kinetics of tellurite glasses within TeO2–Bi2O3–Nb2O5 pseudo-ternary system. J. Non. Cryst. Solids. 404, 32–36 (2014)
E. Idalgo, E.B. Araújo, K. Yukimitu, J.C.S. Moraes, V.C.S. Reynoso, C.L. Carvalho, Effects of the particle size and nucleation temperature on tellurite 20Li2O–80TeO2 glass crystallization. Mater. Sci. Eng., A 434, 13–18 (2006)
M.C. Weinberg, Assessment of glass stability criteria. Phys. Chem. Glas. 35, 119–123 (1994)
J.-C. Sabadel, P. Armand, P.-E. Lippens, D. Cachau-Herreillat, E. Philippot, Mössbauer and XANES of TeO2–BaO–TiO2 glasses. J. Non Cryst. Solids. 244, 143–150 (1999)
M. Çelikbilek, A. Erçin Ersundu, S. Aydin, Glass formation and characterization studies in the TeO2–WO3–Na2O System. J. Am. Ceram. Soc. 96, 1470–1476 (2013)
I.N. Sapian, M.I.M. Yusof, A.K. Yahya, Elastic and structural properties of (95–x) TeO2-5La2O3-xTiO2 lanthanum tellurite glass system. Chalcogenide Lett. 11, 10 (2014)
S.Q. Mawlud, M.M. Ameena, M.R. Sahar, Z.A.S. Mahraz, K.F. Ahmed, Thermal stability and Judd-Ofelt analysis of optical properties of Sm3 + -doped sodium tellurite glasses, in: AIP Conf. Proc., 2017: p. 20032
Y. Dimitriev, V. Dimitrov, M. Arnaudov, IR spectra and structures of tellurite glasses. J. Mater. Sci. 18, 1353–1358 (1983)
M.A. Villegas, J.M.F. Navarro, Physical and structural properties of glasses in the TeO2–TiO2–Nb2O5 system. J. Eur. Ceram. Soc. 27, 2715–2723 (2007)
E.A. Davis, N.F. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22, 903–922 (1970)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324 (1953)
M. Nowak, B. Kauch, P. Szperlich, Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 80, 46107 (2009)
V. Dimitrov, S. Sakka, Electronic oxide polarizability and optical basicity of simple oxides. J. Appl. Phys. 79, 1736–1740 (1996)
V. Dimitrov, T. Komatsu, Classification of oxide glasses: a polarizability approach. J. Solid State Chem. 178, 831–846 (2005)
Acknowledgments
The authors thank to FINEP, CAPES, CNPQ and Fundação Araucária for the partial financial support. To LABMU-UEPG for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gunha, J.V., Gonçalves, A., Somer, A. et al. Thermal, structural and optical properties of TeO2–Na2O–TiO2 glassy system. J Mater Sci: Mater Electron 30, 16695–16701 (2019). https://doi.org/10.1007/s10854-019-01496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01496-6