Abstract
It is highly desired to simplify the technology of electroless plating on inert polymer. Herein we developed a new kind of catalytic solution (mixture of H2O, AgNO3 and 3-aminopropyltriethoxysilane), which can write onto inert polymer directly with good stability, resolution and catalytic performance. Then, a facile method combined with catalyst solution printing and electroless plating to fabricate selective metal (copper and nickel) coating on polymer (polypropylene and polyethylene terephthalate) surface. The growth behavior, electrical, and structural properties of plated coating were investigated. The minimum width of copper coating is 250 μm, thickness is 2.2 μm, resistivity is 2.6 × 10−6 Ω cm, adhesion of 2.2 μm thick coating maintain 5B. In addition, the selective metal coating have excellent bending and fatigue resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electroless deposition process is an interesting approach for low-cost, simple, efficient and large-scale development of variety of compounds ranging from single metals, metal oxides to metal hydroxide/oxyhydroxides.
The method combined with inkjet (catalyst solution) printing and electroless deposition to fabricate selective metal coating on inert polymer have been employed as technology in the fabrication of selective metalization of polymer [1,2,3,4,5]. However, the metal catalysts hold incompact structure and easy to break off in the plating solution [6]. Pretreatment can improve the interfacial adhesion between metal catalysts and polymer surface [1,2,3,4,5,6]. Research efforts were focused on modifying a intermediate layer with some activated groups (hydroxyl, carboxyl, amino) which could adsorb catalytic [6,7,8]. There is great adhesion (catalysts/intermediate layer and intermediate layer/catalytic), so that the one can’t be dissolved in plating solution. The intermediate layer was generally fabricated using silane [6, 7] and primer [9,10,11]. The catalyst solution was the mixture of metal ion (Ag+, Pd2+, Au2+), ethanol, ethanediol, water, propanol and glycerol [6, 7, 9,10,11]. The intermediate layer can adsorb metal ions when the catalyst solution printed on its surface. The adsorbed metal ions will be reduced by a reductant in the plating solution. As a result, selectively activated polymer was fabricated.
In order to simplify the electroless plating process, some researches attempt to eliminate the process of surface modification. Zhang et al. [5] reported that selectively metallized photopaper was prepared with printing catalyst solution and then electroless plating. The catalyst solution ((NH4)2PdCl4 solution) could bond with amine group along the photopaper fiber. Sun et al. [4] reported that silver iron in solvent (ethanol and dichloroethane) was written on polyethylene terephthalate (PET) surface. The solvent would cause swelling effect or partial dissolution of the polymer surface. The catalytic particle was loaded onto the polymer surface. In these researches, the catalyst solution printed onto substrate directly, meanwhile, the adhesion force to substrate was strong. But these technologies demand very particular substrate (high activity) and activated solution (corrosiveness). Its particularity cumbers its widely use in selective electroless plating on inert polymer.
In this work, we have developed a new kind of catalytic solution. The catalytic solution can realize to modify and activate the inert polymer simultaneously. The mechanism of modify inert polymer and catalytic performance were discussed. Then, a facile method combined with printing catalyst solution and electroless deposition to fabricate selective metal coating. Finally, this facile technique can prepare high-resolution, high-adhesion, through-hole and double-sided metal pattern on various substrates, which is low-cost and suitability for printed electronics. The growth behavior, electrical, and structural properties of plated coating were investigated.
2 Experimental details
2.1 Preparation of catalyst coating
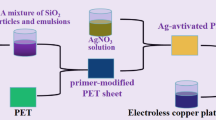
The 3-aminopropyltriethoxysilane (KH550) (10 g) and mixing solution (H2O (1 g) and ethanol (70 g)) were added in a four-neck flask equipped. 10 g AgNO3 solution (3 g/L) dropped into the system at 333 K for 2 h. The surface tension was adjusted to about 28.0 mN/m by adding ethanol (1 g), glycerol (3 g) and ethanediol (2 g). Then the catalyst solution was then obtained. The procedure for preparing catalyst solution was schematically illustrated in Fig. 1.
2.2 Preparation of selective electroless copper plating
The commercial PET and polypropylene substrate was bought from Hefei Lucky & Technology Industry Co. Ltd (Hefei, China). Through-hole sample was mechanically drilled. The substrate was cleaned by NaOH solution (5%), and then deionized water. A fountain pen and writing brush were used to write the catalyst solution on through hole and two sides. The substrate was dried at 100 °C for 10 min and then catalyst coating was selectively formed on the substrate surface (ASP substrate). After that, the substrate was immersed in electroless plating bath. The components of the plating solution reported in previous work [9]. After deposition, the sample was annealed firstly at 323 K for 30 min, and then at 363 K for 20 min. Then, selective metal pattern established on the substrate surface (ECP substrate). The procedure for preparing catalyst solution was schematically illustrated in Fig. 2.
2.3 Characterization
Surface morphology was observed by scanning electron microscopy (SEM, JEOL, JSM-5600LV). Chemical structure was measured by X-ray diffraction (XRD, Rigaku D/max-2550 V) and X-ray photoelectron spectroscopy (XPS, Shimazu, AXIS ULTRADLD). The XPS spectrawere calibrated by using the C 1 s peak (284.5 eV). Room-temperature surface electrical resistivity was measured by a Hall system (Nanometrics, HL5500PC).
3 Results and discussion
Figure 3 represents FT-IR patterns of pristine and ASP PET. Upon printing catalyst solution, the –N–H2 (1595 cm−1) and -OH (about 3500 cm−1) appear in FT-IR patterns [9, 12], which demonstrates silane film successfully form on ASP PET. Surface analysis of ASP and ECP PET after ultrasonic treatment at 80 °C for 20 min were carried out using XPS (Fig. 4). Si 2p (113 eV), Si 2 s (154 eV) and Ag 3d3/2 (368.1 eV) peaks are observed in the XPS spectrum of ASP PET, Cu 2p3 (932.9 eV) peaks is observed in the XPS spectrum of ECP PET, respectively [5, 9, 12, 13]. The presence of the Ag 3d3/2 peak indicates that catalytic site form on ASP PET surface, which can catalyze plating reaction. The mapping energy-dispersive X-ray for elemental analysis further reveals the Si, C, O and Cu elements distribute uniformly throughout the plated coating (Fig. 5). It was indicated that copper particle form on ECP PET surface. In addition, the Ag 3d5/2 peak position below standard values (371 eV), infer a decrease in electron density in the Ag nucleus due to the formation of N-Ag coordinate bond [9, 14]. This will ensure the catalytic site stabilize in the plating solution. Figure 6 represents photographic images of ECP PET. As displayed in Fig. 6, selective all-region (Fig. 6a, b and c) and through-hole (Fig. 6c and d) copper coating were prepared successfully on PET surface through this approach. The minimum width of copper line is 250 μm (the precision of the pen (gel pen, BLLH-20C4) was about 250 μm, Fig. 6a), the adhesion is 5B. The plated PET sheet samples showed high conductivity (Fig. 6c), adhesion and flexibility (Fig. 6b).
Figure 7 represents photographic images patterns of selective ECP PET. Selective coating grew on the PET surface after electroless plating. The pattern for 43.3o and 50.4o has distinct peaks that can be well-indexed to Bragg peaks of face-centered Cu (111) and (200), as displayed in Fig. 8, full width at half maximum of the peaks became narrower with the increase of plated time, which revealed that crystallization characteristic of copper coating is enhanced with the increase of plated time. When the plated time increase from 5 to 20 min, the coating thickness is enhanced from 0.58 to 2.2 μm, and the electrical resistivity is decreased from 7.2 × 10−3 to 2.6 × 10−6 Ω cm (see Fig. 9). The adhesion of plated coating is 5B.
Flexibility of the metal coating is important for printed electronics. The way to evaluate the bending test is depicted in Ref 9. The sample compresses or stretches to circular profile of radius R (about 0.5 cm). As shown in Fig. 10, the fluctuation of volume resistivity is less than 0.35 × 10−5 Ω cm and the one exhibited irregularly change, following compressing and stretching test, which is due to random contacting errors produced during process and ECP PET structural differences. As the same time, the adhesion of bending ECP PET also can maintain 5B. It can be implied that there is no metal fatigue in the bending copper coating. It was indicated that the ECP PET can stay reliable in high quality and low resistances even after 1000 times of daily bending test.
Further increase of plated time lead to the enhancement of crystallinity (see Fig. 8) and thickness (see Fig. 9). When the thickness of plating coatings exceed about 2.2 μm, pores, edge warp and shedding region form in the plated coatings, as shown in Fig. 7b and c. Interestingly, it was found that the formation of failure take place at substrate-coating interface (see Fig. 7a). We know that the activated groups absorbed Ag atoms, and then Cu atom deposited on Ag surface, that is copper coating is connected with substrate through Ag–N coordinated bonds. The result imply that increasing the coordinated bond energy and substrate-coating surface area would facilitate the improvement of the adhesion of plated coating.
Figure 11 shows photographic images of selectively ASP sheets and the corresponding ECP sheets, the amount of the catalyst solution were 0.66 mg/cm3, (b) 0.91 mg/cm3 and (c) 1.24 mg/cm3, (e) schematic diagram of drying process of catalyst solution on PET surface We write catalyst solution on PET substrates with 0.2 cm linewidth. The catalyst solution write with a writing brushes which stained with different amount of catalyst solution. As a result, the uniform-sized catalyst coating contained different amount of solution formed on the PET surface. As shown in Fig. 11 a, b and c, the amount of the catalyst solution were 0.66 mg/cm3 (a), 0.91 mg/cm3 (b) and 1.24 mg/cm3 (c), respectively. It is then dried to form a catalyst coating. It was found that the catalyst coating are heaved in the middle in the Fig. 11b and c, smooth coating is formed in the Fig. 11a. The plated coating have same phenomenon. Because of the surface tension effect of the catalyst solution with the evaporation of solvent, the thickness near the edge is smaller than that of the center region of the samples. In the dry course, the coating shrunken immediately, resulting in the phenomenon of uneven surface of the coating, as shown in Fig. 11d. The small amounts of activated solution had the advantage of short drying time, in addition, the viscosity of activated coating is increased in a short time with the evaporation of solvent. The surface tension is difficult to deform the film, as shown in Fig. 11d. The result indicate that the reasonable control of the amount of catalyst solution (write on PET) can facilitate the implementation of high-quality plated coating.
Overall, our studies confirm that the catalytic solution (mixture of H2O, AgNO3, KH550) can modify and activate the polymer surface simultaneously. Figure 12a shows the reaction mechanism of ASP and ECP polymer. At preparation stage, KH550 is hydrolyzed and formed the cross-linked polysiloxane with amino groups. The amino group is aimed to adsorb Ag+ via complexation. As the same time, polysiloxane molecule is full of Si–OH groups, and the hydrogen bond can form with substrate improve adhesive force. In addition, the polysiloxane molecule is linked through the dehydration condensation between the hydroxyl groups during drying. After coating substrate, selective catalytic site (Ag atom) form on the polymer surface, and then catalyze the electroless plating [14,15,16]. As a result, selectively ECP polymer was fabricated. The formation of pores, edge warp and shedding region will occur in the thick plated coating due to the discontinuous stress. Stress primarily results from hydrogen (generated in the plated reaction), defect and mismatch of lattice parameter coefficient and thermal expansion, the one enhanced with the increase of crystallization characteristic and thickness of plated coating [16,17,18].
In a word, a new kind activated solution can write onto inert polymer directly, meanwhile, the adhesion force to substrate is strong. The structure of activated coating would catalyze plating reaction. It is important to point out that the established method can potentially be adopted for the plating of metals such as copper, silver, cobalt, and nickel onto different types of substrate by changing the type of selective printing techniques (screen printing and inkjet printing). Figure 12b and c shows photographic images of copper-plated polypropylene sheet (a) and nickel-plated PET sheet (c). The plated polymer sheets has good workability and resolution.
4 Conclusion
In summary, selective metal coating has been successfully plated on surface of inert polymer. A new kind of catalytic solution (mixture of H2O, AgNO3, KH550) is prepared, which can write onto inert polymer directly with good stability and resolution and catalyst for following electroless plating process. The minimum width of copper coating is 250 μm, the thickness is 2.2 μm, the resistivity is 2.6 × 10−6 Ω cm, the adhesion of 2.2 μm thick coating can maintain 5B. In addition, no matter how the copper pattern are bent and stretched, conductivity and mechanical properties are not affected.
References
X. Zhao, F. Muench, S. Schaefer et al., Electroless decoration of macroscale foam with nickel nano-spikes: a scalable route toward efficient catalyst electrodes. Electrochem. Commun. 65, 39–43 (2016)
J.U. Yang, J.H. Cho, M.J. Yoo, Selective metallization on copper aluminate composite via laser direct structuring technology. Compos. Part B 110, 361–367 (2017)
X. Li, G. Wang, X. Wang et al., Flexible supercapacitor based on MnO2 nanoparticlesvia electrospinning. Mater. Chem. A 1(35), 10103–10106 (2013)
X. Sun, L. Zhang, S. Tao, S. Li, A general surface swelling-induced electroless deposition strategy for fast fabrication of copper circuits on various polymer substrates. Adv. Mater. Interfaces 4, 1700052 (2017)
T. Zhang, X. Wang, T. Li, Q. Guo, T. Yang, Fabrication of flexible copper-based electronics with high-resolution and high-conductivity on paper via inkjet printing. J. Mater. Chem. C 2(2), 286–294 (2014)
Y. Wang, Y. Wang, J.J. Chen et al., A facile process combined with inkjet printing, surface modification and electroless deposition to fabricate adhesion-enhanced copper patterns on flexible polymer substrates for functional flexible electronics. Electrochim. Acta 218, 24–31 (2016)
Y. Chang, Y. Tao, Q. Zhang, Z.G. Yang, Selective adsorption of catalyst and copper plating for additive fabrication of conductive patterns and through-holes. Electrochim. Acta 158, 7–12 (2015)
D. Chen, Y. Zhang, T. Bessho, Layer by layer electroless deposition: an efficient route for preparing adhesion-enhanced metallic coatings on plastic surfaces. Chem. Eng. J. 303, 100–108 (2016)
J.J. Huang, C.A. Tian, J. Wang et al., Fabrication of selective electroless copper plating on PET sheet: effect of PET surface structure on resolution and adhesion of copper coating. Appl. Surf. Sci. 458, 734–742 (2018)
Z.P. Sun, J.J. Huang, L.B. Wang et al., Method for electroless nickel plating on poly(ethylene terephthalate) substrate modified with primer and self-assembled monolayer. J. Mater. Sci. 26, 10132–10137 (2015)
J.J. Huang, Z.M. Chen, F. Zhou et al., High-adhesive electroless copper plating on polyethylene surface modified with primer. J. Solid State Electrochem. 21, 1559–1566 (2017)
A. Hashemi, A. Bahari, S. Ghasemi, Reduction the leakage current through povidone-SiO2 nano-composite as a promising gate dielectric of FETs. J. Mater. Sci. 28(18), 13313–13319 (2017)
L.X. Yi, J. Heitmann, R. Scholz, M. Zacharias, Si rings, Si clusters, and Si nanocrystals-different states of ultrathin SiOx, layers. Appl. Phys. Lett. 81(22), 4248–4250 (2002)
X. Wang, H. Hu, Y. Shen et al., Stretchable conductors with ultrahigh tensile strain and stable metallic conductance enabled by prestrained polyelectrolyte nanoplatforms. Adv. Mater. 23(27), 3090 (2011)
Z. Chen, X. Xu, C.C. Wong, S. Mhaisalkar, Effect of plating parameters on the intrinsic stress in electroless nickel plating. Surf. Coat Technol. 167(2–3), 170–176 (2003)
Y. Lu, S. Sathasivam, J. Song et al., Robust self-cleaning surfaces that function when exposed to either air or oil. Science 347(6226), 1132–1135 (2015)
M. Baloch, C.M. López, Effect of additives on the pulsed-galvanostatic electrodeposition of iron nanoparticles from formamide media. ChemElectroChem 3(6), 883–886 (2016)
Y.J. Yim, K.Y. Rhee, S.J. Park, Influence of electroless nickel-plating on fracture toughness of pitch-based carbon fibre reinforced composites. Compos. Part B 76, 286–291 (2015)
Acknowledgement
The authors thank the financial supports by the Research Foundation of Hefei University (No: 17ZR03ZDA), the State Key Laboratory of Rare Earth Permanent Magnetic Materials (No: SKLREPM17OF05) and the Opening project of Guangxi Key Laboratory of Calcium Carbonate Resources Comprehensive Utilization (HZXYKFKT201805).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, G., Zhang, Y., Yuan, M. et al. A facile method combined with catalyst solution printing and electroless plating to fabricate selective metal coating on inert polymer. J Mater Sci: Mater Electron 30, 9767–9774 (2019). https://doi.org/10.1007/s10854-019-01313-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01313-0