Abstract
The core-shell g-C3N4@Zn0.5Cd0.5S heterojunction photocatalysts with excellent photocatalytic performance were synthesized via a simple co-precipitation hydrothermal method. The photocatalytic performance of the g-C3N4@Zn0.5Cd0.5S was significantly increased by the heterojunction structure, which improved the separation and mobility of the photogenerated carriers. The highest degradation efficiency was for the 50% g-C3N4@Zn0.5Cd0.5S (99%), and its degradation rate was 15.7 which was 3.7 times those of the pure g-C3N4 and Zn0.5Cd0.5S, respectively. The catalyst band gap gradually decreased with the increasing content of g-C3N4. The capture agent experiments revealed that the main active substances in the degradation process are h+ and O2−·. The photocatalytic activity remained at 95% after 4 cycles showing good light stabilities. This study provided a simple method for synthesizing the g-C3N4/ZnCdS heterojunctions with an excellent photocatalytic property.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor photocatalytic degradation is a new type wastewater treatment technology for solving environmental pollution. Metal sulfides (ZnS, CdS, et al.) are a type of photocatalyst showing a superior performance. Their main disadvantages are a wide energy band, easy photo-corrosion decomposition and poor catalytic stability [1, 2]. For example, the bandgap of ZnS is 3.77 eV [3], which is too high to effectively utilize visible light [4]. Nonetheless, the band gap can be adjusted by doping, such as a large content of Cd2+ can clearly decrease the band gap of ZnxCd1−xS [5, 6]. The ZnxCd1−xS as a typical alloyed chalcogenide semiconductor, shows good and adjustable absorption characteristics in the visible region [7, 8]. Wang et al. [9]. reported the photocatalytic degradation efficiency of Zn0.2Cd0.8S to RhB of 60%, which was significantly higher than that of ZnS and CdS. However, the low stability and photocatalytic efficiency of ZnxCd1−xS still need to be improved [10]. Generally, the heterojunction structure is considered to be an efficient method. The heterojunction structure can effectively reduce the photo-generated electron–hole recombination probability, enlarge the photo-response range, and improve the photo-corrosion performance of metal sulfides [11,12,13]. For example, Zhao et al. [14] synthesized a ZnxCd1−xS/Zn–Al layered double hydroxide (LDH). The photocatalytic efficiency of the 2Zn0.2Cd0.8S/Zn–Al was 82%, which was 89.5% and 50.32% higher than that of Zn0.2Cd0.8S and Zn–Al LDH, respectively. Wu et al. [15] reported that the photocatalytic degradation of the Zn0.5Cd0.5S-MWCNT heterojunction was 85% and the photocatalyst still had about an 80% efficiency after three recoveries.
Most of the valence bands (VBs) and conduction bands (CBs) of the metal sulfides match with g-C3N4 (The VB and CB potentials are 1.5 eV and − 1.2 eV, respectively), and they can be coupled to form the heterojunction structure catalysts [5]. It was found that the g-C3N4/CdS and g-C3N4/ZnS heterojunctions can enhance the visible light response range, reduce the photo-generated carries recombination and improve the photocatalytic efficiency [16, 17]. The photocatalytic degradation efficiencies of the CdS/g-C3N4 and ZnS/g-C3N4 heterojunctions to Methyl Orange (MO) were higher than those of the pure CdS, ZnS and g-C3N4. Usually, the flaky g-C3N4 is significantly stacked, inducing the nanoparticles of the metal sulfides to only disperse on the surface of the bulk g-C3N4 [18, 19]. This stacked structure results in a smaller contact area between the metal sulfide and g-C3N4, while the core–shell structure can form a coating, thus increasing the contact area between the metal sulfide and g-C3N4, which benefits the hole (h+) of Zn0.5Cd0.5S more easily transfer to g-C3N4. The core–shell structuring is beneficial to increasing the separation and migration of the photogenerated carriers, reducing the photocorrosion effect and enhancing the light stability.
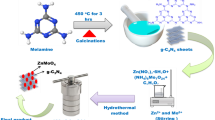
In this study, we synthesized the core–shell structure of g-C3N4@Zn0.5Cd0.5S. The hole (h+) of Zn0.5Cd0.5S more easily transfered to g-C3N4 due to g-C3N4 with a lower potential and more stable chemical property. The nanoblocks of the g-C3N4 surface displayed a negatively charged nature [20, 21], thus Zn2+ and Cd2+ could be adsorbed on the surface and coat the g-C3N4 forming the core–shell heterojunction via a simple co-precipitation-hydrothermal method. The g-C3N4@Zn0.5Cd0.5S reduced the disadvantages of the low visible light response range and high photoelectron-hole recombination probability.
2 Experiment
2.1 Preparation of g-C3N4
The pyrolysis was used to prepare g-C3N4. Weighed 6 g of melamine, calcined at 500 °C for 4 h with the heating rate was 5 °C/min, and then g-C3N4 was fully grinded.
2.2 Preparation of Zn0.5Cd0.5S and g-C3N4@Zn0.5Cd0.5S
The g-C3N4 was prepared by heating 6 g of melamine at 550 °C for 2 h in a muffle furnace at the a heating rate of 5 °C/min. After natural cooling, the obtained sample was sufficiently ground. The Zn0.5Cd0.5S and g-C3N4@Zn0.5Cd0.5S were synthesized via the method according to Wu et al. [20]. The g-C3N4@Zn0.5Cd0.5S was synthesized as follows: 1.5 mmol Zn(Ac)2·2H2O and 1.5 mmol Cd(Ac)2·2H2O were mixed in 30 ml of deionized water and stirred for 30 min.
The obtained g-C3N4 (0.06 g, 0.18 g, 0.3 g, 0.42 g, 0.54 g) was added to the above solution according to the mass percentages, and ultrasonically treated for 50 min. Three mmol Na2S was dissolved in 30 ml of deionized water at a rate of 1 drop/s and stirred for 4 h. The mixed solution was then transferred to a Teflon-lined reactor and heated at 120 °C for 12 h. Finally, the precipitate was washed three times with deionized water and absolute ethanol, then dried at 70 °C for 12 h to obtain different compound ratios of g-C3N4@Zn0.5Cd0.5S, labelled as 10-CN@ZCS, 30-CN@ZCS, 50-CN@ZCS, 70-CN@ZCS and 90-CN@ZCS.
2.3 Characterization techniques
The crystal phase of the photocatalyst was analyzed by X-ray diffraction (XRD, D/MAX-3B, 2θ = 10–80, Cu-Kα, 40 kV, 30 mA).The surface morphologies of the samples were observed by a scanning electron microscope (SEM, FEI Sirion 200, operating voltage set at 20 KV) and a transmission electron microscope (TEM, JEOL JEM-2010, electron acceleration voltage of 200 KV).The recombination of the photo-generated carriers was tested using a photoluminescence spectrumeter (PL, RF-5301PC, Shimadzu, Japan). Test the chemical information of the sample using X-ray photoelectron spectroscopy (XPS XSAM-800). The fourier transform infrared (FT-IR) spectra of the samples were measured by a Nicolet IS10 infrared spectrometer. The UV–Vis DRS was a USB 4000 UV diffuse reflectance spectrometer from Ocean Optics USA. The three-electrode system was used to test the photocurrent and AC impedance. A 0.2 M Na2SO4 solution was used as the conductive solution, and a 300 W Xe lamp was used to intermittently irradiate the conductive surface of the ITO glasses. The electrochemical workstation model was the French Bioroy VMP3 workstation.
2.4 Photocatalytic experiment
Photocatalytic experiments were carried out in a self-assembled photoreactor at room temperature. A 0.1 g sample of the g-C3N4@Zn0.5Cd0.5S was added to the 10 mg/L MB solution, then the suspension stirred for 1 h in the dark, A sample was taken every 30 min, the absorbance measured, and its adsorption performance calculated. The solution was then subjected to photocatalytic methylene blue (MB) and tested 25 cm from a 500 W gx-2500 Xenon source, and samples were taken every 30 min to determine the absorbance. The absorption of the resulting clear solutions at λmax = 664 nm was evaluated using a 722 spectrophotometer and the decolorization rate of the dye was calculated by the following formula:
where C0 is the initial concentration of the dye and Ct is the concentration of the dye at a particular time during the experiment.
3 Results and discussion
3.1 XRD characterization
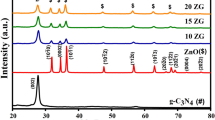
Figure 1 is the XRD pattern of the CN@ZCS heterojunction structure with different g-C3N4 composition ratios. Comparing the JCPDF cards, the composition consists of g-C3N4 and Zn0.5Cd0.5S. There are 27.17°, 44.68° and 53.15° diffraction peaks of Zn0.5Cd0.5S related to the standard cubic blende structure of CdS (PDF 42-1411), corresponding to its (111), (220) and (311) crystal faces, respectively. The three diffraction peaks all move toward a large angle, induced by the ionic radius of Zn (0.74 Å) being slightly smaller than that of Cd (0.97 Å). According to the Hume-Rothery rule [22], the ionic radius ratio of Zn2+ and Cd2+ is less than 15%, which means that the difference in electronegativity is low. Moreover, CdS and ZnS are both cubic sphalerite. Thus, Zn2+ is allowed to enter the main lattice of CdS, resulting in a lattice contraction with the diffraction peak shifting to a greater angle [8, 23].
The g-C3N4 has two characteristic diffraction peaks at 13.05° and 27.59°, corresponding to its (100) and (002) crystal faces, respectively. The diffraction peak at 27.59° belongs to the interlayer stacking of aromatic compounds, which is a characteristic peak specific to graphite structures. The diffraction peak at 13.05° is the characteristic peak of the melon-like substance, which is formed by the 3-s-triazine structure of the in-plane repeating unit [24, 25]. With the ratio of the g-C3N4 increasing, the diffraction peaks of the Zn0.5Cd0.5S (220) and (311) crystal faces gradually decrease, and the (100) crystal plane strength of g-C3N4 gradually increases. Since the diffraction peak of the g-C3N4 (002) crystal plane overlaps with the Zn0.5Cd0.5S(111) crystal plane diffraction peak, the diffraction peaks of g-C3N4 (002) in the CN@ZCS hetero-structure are not detected, which is consistent with previous reports [26, 27].
3.2 Morphology analysis
It can be seen from Fig. 2a, the particles size of Zn0.5Cd0.5S is 75 nm and g-C3N4 is a layer structure with a rough surface and a thickness of about 100–150 nm. Figure 2c, d show that 50-CN@ZCS obviously has a core–shell structure. The Zn0.5Cd0.5S uniformly coats the surface of g-C3N4. The core diameter is 40–80 nm and the thickness of the shell is about 20 nm. The HRTEM picture shows a lattice fringe of 0.327 nm, corresponding to the (111) crystal surface of Zn0.5Cd0.5S. The lattice fringe of g-C3N4 is too weak to be detected. The synthesizing process of the 50-CN@ZCS core–shell heterojunction is shown in Fig. 2e. When the g-C3N4 is mixed with Zn2+ and Cd2+, the Zn2+ and Cd2+ can be adsorbed by the g-C3N4 due to the negative charge of g-C3N4 in the neutral solution [20]. The lamellar g-C3N4 gradually shrinks into a nanoparticle during the nucleating process of Zn0.5Cd0.5S due to the action of the Zn0.5Cd0.5S package [21, 28]. Finally, a 50-CN@ZCS core–shell heterojunction is formed.
Elemental mapping (Fig. 3a–f) and EDS spectrum (Fig. 3g) detected C, N, Zn, Cd and S elements in the 50-CN@ZCS. The elemental mapping results also show that the C, N, Zn, Cd and S elements are uniformly distributed in the 50-CN@ZCS. The solubility product constant of ZnS (2.93 × 10−25) is much higher than that of CdS (8.0 × 10−27). Consequently, the reaction rate between Cd and S during faster than that of Zn and S in the nucleation process, so that the Zn content is lower than that of Cd. In summary, the formation of a 50-CN/ZCS heterojunction photocatalyst can be demonstrated.
3.3 FI-IR analysis
To further verify the functional groups in the 50-CN/ZCS heterojunction, we performed infrared tests on g-C3N4, Zn0.5Cd0.5S and 50-CN/ZCS, and the results are shown in Fig. 4. There are four characteristic absorption peaks in the pure Zn0.5Cd0.5S solid solution. The characteristic peak at 626 cm−1 belongs to the Zn–S bond, and the characteristic peak at 1130 and 1556 cm−1 belongs to the Cd–S bond stretching vibration and bending vibration absorption [17, 19], and the characteristic peak at 933 cm−1 should be attributed to the S-O bond, indicating that Zn0.5Cd0.5S is oxidized to some extent during the hydrothermal process [28]. For g-C3N4, the absorption peak at 809 cm−1 corresponds to the bending vibration characteristic absorption of the triazine cyclic compound, and the absorption peak at 1200 ~ 1650 cm−1 corresponds to the C–N and C=N bond stretching characteristics of the aromatic heterocyclic compounds in g-C3N4 [18, 20]. The absorption peak of 50-CN/ZCS can correspond to the absorption peak of g-C3N4, the absorption peak of Zn0.5Cd0.5S is weak, and the absorption peak at 50-CN/ZCS is not shown, indicating that there is no covalent bond between the g-C3N4 and Zn0.5Cd0.5S nanoparticles. In addition, for all the samples, an absorption peak appeared at 3000–3800 cm−1, which may be related to the stretching vibration of the N–H group in the aromatic group and the O-H stretching vibration of the adsorbed water molecule. In summary, the formation of a 50-CN/ZCS heterojunction photocatalyst can be demonstrated.
3.4 XPS analysis
The composition and chemical environment of each element in the sample can be detected by X-ray photoelectron spectroscopy (XPS). Figure 5a shows the xps measurement spectrum of a 50-CN@ZCS photocatalyst. The results show that 50-CN@/ZCS consists of C, N, Cd, Zn and S elements. As shown in Fig. 5b, the peaks of C1s are at 283.7 eV and 287.38 eV, wherein the peak at 283.7 eV is generally considered to be graphitic carbon or sp2 C–N, and the peak at 287.38 eV is the thiazine ring (C3N3) unit or heptazine N–C=N of the (C6N7) unit [29]. The binding energy peak spectrum of N 1 s of 50-CN@ZCS in Fig. 5c can be divided into two peaks, wherein the peak with the binding energy of 397.79 eV is assigned to the sp2 hybridized N atom (C–N=C), and the peak of 403.85 eV belongs to the tertiary N species (N–(C)3) groups. Figure 5d, e are xps spectra of the Cd 3d and Zn 2p orbitals, respectively. Among them, the binding energy of the Cd 3d orbital is located at 403.83 eV and 410.58 eV, and the binding energy of the Zn 2p orbital is located at 1020.6 eV and 1043.74 eV, which is obviously different from the combination of the pure ZnS and CdS at the corresponding orbit. This again confirms that the prepared catalyst is not a mixture of pure ZnS and CdS, but a metal complex sulfide Zn0.5Cd0.5S, and the interaction between metals leads to a difference in the binding energy [30]. Figure 5f shows that the binding energy of the S 2p orbital in 50-CN@ZCS is located at 161.4 eV, indicating the presence of a metal-S bond, and the valence state of sulfur is S2−. In addition to the metal-S bond, sulfur also exhibits another form of existence with a binding energy at 167.54 eV, which is attributed to the C-S bond formed between g-C3N4 and ZnCdS. In summary, there is a strong interaction between g- C3N4 and Zn0.5Cd0.5S.
4 Photocatalytic performance and mechanism
4.1 Photocatalytic performance
The photocatalytic performance of the CN@ZCS catalyst is analyzed without adjusting the pH of the MB solution. The adsorption and photocatalytic decolorization rate curves of MB are shown in Fig. 6a.
In the adsorption stage, the decolorization rates of the g-C3N4, Zn0.5Cd0.5S and 50-CN@ZCS to MB are 6.19%, 16.63% and 34.9%, respectively. The results depend of the specific surface areas of g-C3N4, Zn0.5Cd0.5S and 50 CN@ZCS,which are 4.7477 m2/g, 40.276 m2/g and 55.561 m2/g, respectively. Similarly, the adsorption rates of all the CN@ZCS heterojunction materials also are higher than that of g-C3N4 and Zn0.5Cd0.5S. In a liquid phase photocatalytic reaction, the adsorption to target the contaminants on the surface of the catalyst is a prerequisite for the photocatalytic degradation [31]. The higher adsorption can enrich the pollutants on the surface of the liquid phase [32]. Thus, the active sites in the catalyst have a higher contact ratio with the target pollutants to improve the catalytic efficiency.
In the photocatalytic stage, the degradation rates of all the CN@ZCS heterojunction materials for the MB are greater than 90% and the photocatalytic degradation rate of 50-CN@ZCS for MB reached 99.9%. All of them are higher than Zn0.5Cd0.5S (85.67%) and g-C3N4 (35.5%) alone. This can contribute to the heterojunction structure of the Zn0.5Cd0.5S and g-C3N4. The heterojunction reduces the forbidden band width, enhances the visible light absorption capacity and promotes the effective separation of the photogenerated electrons-holes. The photocatalytic properties of ZnS or CdS directly dispersed on carbon nitride are compared, as shown in Table 1, indicating that this core–shell structure effectively improves its photocatalytic performance.
The photocatalytic activity of CN@ZCS is quantified by the quasi-first-order kinetic model − ln\(\frac{{C}_{t}}{{C}_{0}}\) = k1t [33], as shown in Fig. 6b. C0, Ct and K1 are the original concentration of the dye solution, the concentration at t (min) and the quasi-first-order reaction rate constant (min−1) [34], respectively. It was found that all three curves show a good linear relationship (R2 > 0.97), and the reaction rate constant of 50-CN@ZCS to MB is 0.033 min−1, which is 15.7 times and 3.6 times higher than those of g-C3N4 and Zn0.5Cd0.5S, respectively. The results show that the CN@ZCS heterojunction structure can significantly improve the photocatalytic degradation efficiency.
4.2 Absorption spectrum
In order to further confirm the mechanism of the photocatalytic process, the UV absorption was carried out, as shown in Fig. 7. The peaks at 293 nm and 664 nm indicate the MB absorption. During the adsorption process the two absorption peaks of MB decrease, indicating that the concentration of MB is reduced. After 60 min of photocatalysis, the absorption peak of MB at 664 nm is blue-shifted, the intensity decreased after 240 min, and the absorption peak at 664 nm almost disappeared, indicating that eventually MB decomposes into CO2 and H2O by 50-CN@ZCS.
4.3 UV–Vis DRS analysis
Figure 8a is the UV–Vis spectrum of different ratios of that composite CN@ZCS, which directly reflects the light absorption characteristics of the prepared photocatalyst. The absorption band edges of Zn0.5Cd0.5S and g-C3N4 are about 510 nm and 450 nm, respectively, and the absorption band edges of all the prepared CN@ZCS heterojunctions are located between them. With the composite ratio of g-C3N4 increasing, the absorption band edge shifts to blue, and the color of the CN@ZCS change from yellow to white. Combined with the TEM analysis, it can be concluded that the g-C3N4 and Zn0.5Cd0.5S can form a stable heterojunction structure. According to the Kubelka–Munk function method [35], the band gap of light energy can be calculated by hυ and [F(R)·hυ]0.5, and the result is shown in Fig. 8b. The band gap of Zn0.5Cd0.5S is 2.5 eV. The absorption intensity of the CN@ZCS heterojunction in the visible light range is higher than that of g-C3N4, and the bandgap width increases with increasing the g-C3N4 content. It is concluded that the prepared CN@ZCS heterojunction absorbes more light than g-C3N4, which is beneficial to produce more electron–hole pairs.
4.4 Photoelectric property analysis
The photoactivity of a semiconductor is usually affected by the separation efficiency of the photo-generated carriers. The transient photocurrent response is measured at a specific time interval of the xenon illumination. As shown in Fig. 9a, the increasing transient photocurrent of 50-CN@ZCS is ascribed to the irradiation of visible light, and the generation and separation of electron–hole pairs forming a stable current. The 50-CN@ZCS heterojunction structure increases the separation efficiency of the photogenerated carriers, while the photocurrent response of g-C3N4 is very low.
Electrochemical impedance spectroscopy (EIS) is an effective method to study the interfacial reaction process of materials. Figure 9b shows the electrochemical impedance spectrum of g-C3N4, Zn0.5Cd0.5S and 50-CN@ZCS. Compared to g-C3N4 and Zn0.5Cd0.5S the Nyquist circle radius of the 50-CN@ZCS heterojunction decreases, and the Nyquist circle radius of g-C3N4 is higher. This indicates that the contact resistance and transmission resistance of the 50-CN@ZCS heterojunction surface are reduced. The faster charge transfer on the 50-CN@ZCS heterojunction surface makes the faster photogenerated electron–hole separation [36].
4.5 Photoluminescence spectrum
The semiconductor produces an electron (e−) and hole (h+) separation during light excitation, but the photoelectron and hole easily recombine and produce optical signal. Therefore, the photoluminescence spectroscopy (PL) is used to determine the electron–hole recombination probability of each sample under 325 nm photoexcitation, as shown in Fig. 10. The fluorescence emission peaks of the g-C3N4 and Zn0.5Cd0.5S are around 460 nm and 550 nm. The fluorescence emission peak intensity of the CN@ZCS heterojunction is lower than that of g-C3N4. With the increase in the g-C3N4 content, the position of the emission peak gradually shifted to 460 nm, indicating that g-C3N4 and Zn0.5Cd0.5S formed a heterojunction, and g-C3N4 gradually played a leading role [37]. The lowest intensity of the 30-CN@ZCS and 50-CN@ZCS indicates the lowest photogenerated electron–hole recombination probability.
4.6 Capture agent analysis
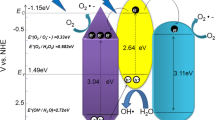
The active substances play a major role in the degradation of MB by the 50-CN@ZCS catalyst. The capture agent tests were performed to study the active substances of catalyst. The operation method is the same as the photocatalytic experiment. The MB concentration is 20 mg/L, 1 mM TBA, EDTA-2Na, and BQ were added to the reaction system, and the degradation results are shown in Fig. 11. With the addition of TBA, the photocatalytic degradation efficiency slightly decreased, indicating that ·OH plays a minor role in the degradation process. When EDTA-2Na and BQ are added, the photocatalytic degradation efficiency significantly decreased demonstrating that O2−· is the active substance. As shown in Fig. 11, the valence band potentials of g-C3N4 (+ 1.4 eV) and Zn0.5Cd0.5S (+ 1.975 eV) are much lower than that of E0(·OH/OH−)(2.38 eV) [38]. However, the conduction band potential of g-C3N4 (− 1.3 eV) and Zn0.5Cd0.5S (− 0.525 eV) are both more negative than those of E0(O2/O2−·) (− 0.28 eV) [39]. Therefore, ·OH cannot be produced, while only O2−· can be produced. The combination of g-C3N4 and Zn0.5Cd0.5S reduces the possibility of photoelectron-hole recombination, allowing a high number of holes and O2−· to react with MB to improve the photocatalytic efficiency.
The valence band level (ECB) of a semiconductor can be calculated from the empirical formula ECB =X-EC-Eg/2 [40] (X is the geometric mean electronegativity of semiconductors; EC is a constant relative to the standard H electrode with a value of 4.5 eV; ECB is the conduction potential of the conduction band; and Eg is the forbidden band width of the semiconductor). By calculation, the VB and CB of g-C3N4 are 1.4 eV and − 1.3 eV, respectively. The VB and CB of the Zn0.5Cd0.5S are 1.975 eV and − 0.525 eV, respectively. Therefore, their energy levels match well. As shown in Fig. 12, when a heterojunction is formed between g-C3N4 and Zn0.5Cd0.5S, the photoelectron energy rapidly transfers from the surface of g-C3N4 to Zn0.5Cd0.5S and the holes aggregate from Zn0.5Cd0.5S to g-C3N4 under the action of potential difference driving force. After the redistribution of electrons and holes, a stable internal electric field will be established at the heterogeneous interface between the two components, which will inhibit the electron–hole recombination process and greatly improve the photocatalytic performance. Photogenerated electrons (e−) are transferred to the surface of Zn0.5Cd0.5S and combine with O2 to form superoxide anions (O2−·), and finally h+ and O2−· degrade the methylene blue dye into CO2 and H2O [41]. The main chemical reactions are as the follows (1–4):
Schematic illustration of electron–hole separation and transport at the g-C3N4/Zn0.5Cd0.5S heterojunction interface. Ec is the contact electric field; Eb is the barrier in the interface depletion layer; and E1 and E2 are the internal electric fields caused by the spatial charge redistribution in g-C3N4 and Zn0.5Cd0.5S, respectively
4.7 Stability analysis of photocatalyst
Recycling of the photocatalyst is a crucial step in the actual photocatalysis process [42]. The degradation rate of MB is shown in Fig. 13a, and the photocatalyst still maintains a high activity (95.7%) after the four successive photocatalytic tests. Figure 13b shows that the chemical structure of 50-CN@ZCS does not change after four photocatalytic cycles. Figure 13c shows the UV–Vis diagram of the 50-CN@ZCS photocatalytic degradation of MB. 50-CN@ZCS does not significantly change in the light absorption band after the photocatalytic degradation. Therefore, it can be seen that the 50-CN@ZCS core–shell heterojunction has an excellent light stability.
5 Conclusion
In this study, a simple co-precipitation-hydrothermal method was used to synthesize a photocatalyst of the g-C3N4@Zn0.5Cd0.5S heterojunction. This core–shell structure enhances the visible light response range, facilitates the separation migration of photogenerated carriers, and improves the activities and stabilities. The photocatalytic performance of the 50-CN@ZCS heterojunction is much higher than those of g-C3N4 and Zn0.5Cd0.5S. The decolorization rate of MB can reach 99.9% in 240 min and the rate constant is 0.033 min−1, which is 3.6 times and 15.7 times higher than those of g-C3N4 and Zn0.5Cd0.5S, respectively. The photocatalytic activity remains at 65% after 4 cycles. The main active substances are h+ and O2−· in the degradation process.
References
J. Chen, B.B. Ding, T.Y. Wang, F. Li, Y. Zhang, Y.L. Zhao, H.S. Qian, J. Mater. Sci. 25, 4103–4109 (2014)
J. Yan, K. Wang, H. Xu, J. Qian, W. Liu, X. Yang, H. Li, Chin. J. Catal. 34, 1876–1882 (2013)
L. Li, S. Xue, P. Xie, H. Feng, X. Hou, Z. Liu, R. Zou, Electron. Mater. Lett. 14, 739–748 (2018)
D. Zewde, O.P. Yadav, A.M. Taddesse, Curr. Phys. Chem. 7, 172–180 (2017)
C. Zhang, H. Liu, W. Wang, H. Qian, S. Cheng, Y. Wang, Y. Hu, Appl. Catal. B 239, 309–316 (2018)
A.G. Kyazym-zade, M.A. Jafarov, E.F. Nasirov, C.A. Jahangirova, R.S. Jafarli, Semiconductors 51, 454–457 (2017)
H.F. Ye, R. Shi, X. Yang, W.F. Fu, Y. Chen, Appl. Catal. B 233, 70–79 (2018)
M. Askari, N. Soltani, E. Saion, W.M.M. Yunus, H.M. Erfani, M. Dorostkar, Superlattices Microstruct. 81, 193–201 (2015)
W. Wang, W. Zhu, H. Xu, J. Phys. Chem. C 112, 16754–16758 (2008)
J. Zhang, W.N. Wang, M.L. Zhao, C.Y. Zhang, C.X. Huang, S. Cheng, H.S. Qian, Langmuir. 34, 9264–9271 (2018)
W.J. Ong, L.L. Tan, Y.H. Ng, S.T. Yong, S.P. Chai, Chem. Rev. 116(12), 7159–7329 (2016)
L. Yang, J. Huang, L. Shi, L. Cao, Q. Yu, Y. Jie, J. Ye, Appl. Catal. B 204, 335–345 (2017)
F. Wang, P. Chen, Y. Feng, Z. Xie, Y. Liu, Y. Su, G. Liu, Appl. Catal. B 207, 103–113 (2017)
Z. Li, Q. Zhang, X. He, M. Chen, Appl. Clay. Sci. 151, 201–210 (2018)
H. Wu, Y. Yao, W. Li, L. Zhu, N. Ni, X. Zhang, Mol. Microbiol. Biotechnol. 13, 2225–2234 (2011)
H. Xu, L. Wu, L. Jin, K. Wu, J. Mater. Sci. Technol. 33, 30–38 (2017)
Q. Wang, Y. Shi, Z. Du, J. He, J. Zhong, L. Zhao, B. Su, Eur. J. Inorg. Chem. 2015, 4108–4115 (2015)
D. Li, Z. Wu, C. Xing, D. Jiang, M. Chen, W. Shi, S. Yuan, J. Mol. Catal. A 395, 261–268 (2014)
Y.X. Dong, J.T. Cao, B. Wang, S.H. Ma, Y.M. Liu, ACS Appl. Mater. Interface 10, 3723–3731 (2018)
L.C. Wu, H.Y. Xu, H. Zhao, Appl. Mech. Mater. 618, 215–219 (2014)
J.Y. Zhang, Y.H. Wang, J. Jin, J. Zhang, Z. Lin, F. Huang, J. Yu, ACS Appl. Mater. Interface 5, 10317–11032 (2013)
U. Mizutani, MRS Bull. 37, 169–169 (2012)
R.G. Chaudhuri, A. Chaturvedi, E. Iype, Mater. Res. Express. 5, 036202 (2018)
Y. Deng, L. Tang, G. Zeng, Z. Zhu, M. Yan, Y. Zhou, J. Wang, Appl. Catal. B 203, 343–354 (2017)
J. Wen, J. Xie, X. Chen, X. Li, Appl. Surf. Sci. 391, 72–123 (2017)
W.K. Jo, N.C.S. Selvam, Chem. Eng. J. 317, 913–924 (2017)
Z. Qian, H. Shaozheng, L. Fayun, F. Zhiping, W. Qiong, W. Fei, L. Daosheng, Chem. J. Chin. U. 37, 521–528 (2016)
B. Xue, H.Y. Jiang, T. Sun, F. Mao, Catal. Lett. 146, 2185–2192 (2016)
J. Feng, T. Chen, S. Liu, Q. Zhou, Y. Ren, Y. Lv, Z.J. Fan, J. Colloid Interface Sci. 479, 1–6 (2016)
Y.G. Li, X.L. Wei, H.J. Li, R.R. Wang, J. Feng, H. Yun, A.N. Zhou, RSC Adv. 5(1), 14074–14080 (2015)
H. Li, J. Li, C. Xu, P. Yang, D.H. Ng, P. Song, M. Zuo, J. Alloys Compd. 698, 852–862 (2017)
X. Liu, W. Yang, C. Yu, H. Zhang, J. Environ. Chem. Eng. 6(4), 4899–4907 (2018)
X. Liang, P. Wang, M. Li, Q. Zhang, Z. Wang, Y. Dai, B. Huang, Appl. Catal. B 220, 356–361 (2018)
X. Liu, P. Lv, G. Yao, C. Ma, Y. Tang, Y. Wu, Y. Yan, Colloids Surf. A 441, 420–426 (2014)
W. Wang, S. Li, C. Pan, S. Liu, T. Luo, G. Dai, J. Chin. Chem. Soc. 65, 252–258 (2018)
Y. Bai, L. Ye, T. Chen, L. Wang, X. Shi, X. Zhang, D. Chen, ACS Appl. Mater. Interface 8, 27661–27668 (2016)
Q. Nie, Q. Yuan, Q. Wang, Z. Xu, J. Mater. Sci. Lett. 39, 5611–5612 (2004)
Q. Liu, C. Fan, H. Tang, X. Sun, J. Yang, X. Cheng, Appl. Surf. Sci. 358, 188–195 (2015)
X.H. Yang, H.T. Fu, X.Z. An, X.C. Jiang, A.B. Yu, RSC Adv. 6, 34103–34109 (2016)
J. Cao, B. Luo, H. Lin, B. Xu, S. Chen, J. Hazard. Mater. 217, 107–115 (2012)
C. Zhang, Y. Lu, Q. Jiang, J. Hu, Nanotechnology. 27, 355402 (2016)
M. Sun, T. Yan, Q. Yan, H. Liu, L. Yan, Y. Zhang, B. Du, RSC Adv. 4, 19980–19986 (2014)
F. Jiang, T. Yan, H. Chen, A. Sun, C. Xu, X. Wang, Appl. Surf. Sci. 295, 164–172 (2014)
Y. Xu, W.D. Zhang, Eur. J. Inorg. Chem. 2015, 1744–1751 (2015)
Y. Sun, J. Jiang, Y. Cao, Y. Liu, S. Wu, J. Zou, Mater. Lett. 212, 288–291 (2018)
L. Zhang, F. Huang, C. Liang, L. Zhou, X. Zhang, Q. Pang, J. Taiwan Inst. Chem. Eng. 60, 643–650 (2016)
D. Peng, H. Wang, K. Yu, Y. Chang, X. Ma, S. Dong, RSC Adv. 6, 77760–77767 (2016)
D. Wang, Z. Xu, Q. Luo, X. Li, J. An, R. Yin, C. Bao, J. Mater. Sci. Lett. 51, 893–902 (2016)
X. Hao, Z. Jin, S. Min, G. Lu, RSC Adv. 6, 23709–23717 (2016)
Z.L. Fang, H.F. Rong, L.Y. Zhou, P. Qi, J. Mater. Sci. 50, 3057–3064 (2015)
L. Shi, F. Wang, J. Zhang, J. Sun, Ceram. Int. 42, 18116–18123 (2016)
L. Shi, J. Ma, L. Yao, L.S. Cui, W. Qi, J Colloid Interface Sci. 519, 1–10 (2018)
Acknowledgements
The project was supported by National Youth Fund Project of China (Grant No. 51404083).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, S., Wang, D., Zhao, Y. et al. Core–shell g-C3N4@Zn0.5Cd0.5S heterojunction photocatalysts with high photocatalytic activity for the degradation of organic dyes. J Mater Sci: Mater Electron 30, 5284–5296 (2019). https://doi.org/10.1007/s10854-019-00828-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00828-w