Abstract
Here novel photocatalysts, SnO2/CuO and CuO/SnO2 nanocomposites were successfully synthesized by chemical method at room temperature. X-ray Diffraction (XRD), transmission electron microscopy (TEM), Fourier transform Infrared (FT-IR), UV–Visible (UV–Vis) and photoluminescence (PL) spectroscopy were utilized for characterization of the nanocomposites. The photocatalytic activity of the nanocomposites was investigated. The hybrid nanocomposites exhibited high photocatalytic activity as evident from the degradation of methylene blue (MB) dye. The result revealed substantial degradation of the MB dye (92 and 69.5% degradation of SnO2/CuO and CuO/SnO2, respectively) under visible light illumination with short period of 30 min. Their large conduction band potential difference and the inner electrostatic field formed in the p–n heterojunction provide a strong driving force for the photogenerated electrons to move from Cu2O to SnO2 under visible light illumination. The excellent photodegradation of methylene blue suggested that the heterostructured SnO2/CuO nanocomposite possessed higher charge separation and photodegradation abilities than CuO/SnO2 nanocomposite under visible light irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanocomposites have been investigated by different research groups in various parts of the world due to the development of new material “architectures” at the nanoscale [1, 2]. In the periodic table, the transition metals are large in number and have number of applications in different field. Some transition metal oxides like ZnO, TiO2, SnO2, CuO, NiO, WO3 CeO2 and Fe3O4 proved as potential candidates for so many applications. Semiconductor materials have been particularly interesting because of their great practical importance in electronic and optoelectronic devices, such as electrochemical cell [3], gas sensors [4], magnetic storage devices [5], field emitters [6], high-Tc superconductors [7], nano fluid [8] and catalysts [9] etc. Even though enormous metal oxides are available, only limited number of them is most useful in science and technology. Physico-chemical properties of special relevance in chemistry are mostly related to the industrial use of oxides as sensors, ceramics, adsorbents and catalysts.
Numerous studies have been reported on the photocatalytic degradation of organic compounds using semiconductor particles as photocatalysts [10,11,12,13,14,15]. Semiconductors as catalysts have acquired great attraction for their potential degradation capacity in the presence of light energy. Among the numerous metal oxides, tin oxide (SnO2) is a wide bandgap (3.6 eV) n-type semiconductor which receives great attention in the field of gas sensors, solar cells, light emission and lithium-ion batteries [15], etc. Substitution of impurities is an effective way to change in physical and chemical properties of the core material. In the same way CuO is also one of the useful metal oxides and which has so many applications in different fields. The unique property of CuO is it acts as a semiconductor. CuO is a distinct p-type metal oxide semiconductor with bandgap of 1.2 eV and SnO2/CuO nanocomposite is synthesized for the many applications including photocatalysis and electrochemical studies [16, 17]. These are known as inexpensive, nontoxic and very effective semiconductor photocatalysts [13,14,15].
Different synthetic techniques can yield SnO2/CuO nanocomposites with different particle sizes and morphologies, which can further affect their properties. Xeu et al. [18] synthesized 1D nanosized core/shell pn-junctions from n-type SnO2 nanorods coated with p-type CuO nanoparticles and they achieved high sensitivity and selectivity against H2S at low temperature. Using electrochemical deposition, Jeun et al. [19] synthesized SnO2/CuO nano-hybrid foams and investigated gas sensing properties. Zhao et al. [20] synthesized a porous pure SnO2 and CuO/SnO2 composite nanofibers via co-dissolution and electrospinning, followed by oxygen plasma treatment and annealing. Compared with pure SnO2 nanofibers, porous CuO/SnO2 composite nanofibers with 5 mol% CuO exhibit enhanced response and selectivity toward low concentration H2S at 200 °C. Accordingly, almost maximum researchers worked on this core/shell material only for sensor applications whereas very few of them applied for photocatalytic applications. Zheng et al. [21] prepared CuO/SnO2 nanocomposites by simple co-precipitation method and the photocatalytic H2 production from acetic acid solution were investigated at room temperature under UV radiations. In this work, both SnO2/CuO and CuO/SnO2 nanocomposites were synthesized by using co-precipitation method and characterized by XRD, TEM, FT-IR, UV–Visible (UV–Vis) and photoluminescence, and the photocatalytic degradations were investigated in detail. The polyvinylpyrrolidone (PVP) was used for synthesis of the nanocomposites. The PVP is low cost and water soluble polymer. The polymer was used as surfactant for reduce particles size. To the best of our knowledge, few studies have reported the construction of heterostructured composed of SnO2 and CuO in organic contaminant photodegradation. However, for the first time, photodegradation study was investigated for both SnO2/CuO and CuO/SnO2 catalyst at simultaneously.
2 Experimental procedure
2.1 Materials
To synthesize SnO2/CuO and CuO/SnO2 nanocomposites, the following materials were used. The chemical reagents used were analytical reagent grade and used without further purification. Tin chloride (SnCl2, 98%), copper(II) acetate (Cu(CO2CH3)2), sodium hydroxide (NaOH) purchased from Sigma Aldrich company. Polyvinylpyrrolidone (PVP-40,000) was received from Nice chemical company, India. All the glass wares used in this experimental work were acid washed. Ultra pure water was used for dilution and sample preparation.
2.2 Synthesis of SnO2/CuO and CuO/SnO2 nanocomposites
SnO2/CuO and CuO/SnO2 nanocomposites were prepared by a solution proceed single step method. PVP was used as surfactant for control particle size. In a typical procedure, 1.89 g (0.2 M) of SnCl2 in 50 mL of ethanol and an equal molar amount of sodium hydroxide in deionized water were mixed drop by drop. The mixture was stirred magnetically at 80 °C until a homogeneous solution was obtained. After 2 h stirring, 50 mL deionized water of copper(II) acetate solution (0.2 M) was added into the above solution. After 10 min, equal molar concentration of 50 mL of sodium hydroxide solution was added to the above colloidal solution drop by drop with stirred additional 2 h. The precipitate was collected and washed with ethanol and water several times for removal of residuals and then the colloidal was dried in a hot air oven at 120 °C for 2 h.
2.3 Photocatalytic studies
The photocatalytic activity of nanocomposites was investigated by performing degradation of Methyl blue (MB) dye. The dye solution was irradiated in the presence of nanocomposite for 30 min with specific time interval with a 300 W tungsten visible light lamp in the wavelength range of 400–700 nm. The photocatalytic experiments were carried out at room temperature as follows: 50 mg of the as-prepared catalyst was dispersed in 50 mL of Methyl blue dye solution (20 mg L−1) and allowed to magnetically stir for 1 h in dark to reach an adsorption–desorption equilibrium. Afterward, the suspension was continued to stir and undergone visible light irradiation for a specific period. At pre-defined intervals, aliquots were withdrawn and centrifuged to remove the catalyst. The remaining concentration of the dye was determined employing by UV–Vis absorption spectra were recorded in a Hitachi (Model U-4100) UV/Vis NIR spectrophotometer. The kinetics of the photocatalytic degradation processes of the dye was discussed as well. The degradation efficiency (D.E.) was estimated using the following relationship:
where C0 is the initial concentration of the dye at zero time (i.e. before illumination) and Ct is the remaining concentration of the dye after illumination for time t, respectively.
2.4 Characterization
The size and structure of the nanocomposites were determined by X-ray diffraction (XRD) pattern using an X’pert PRO diffractometer with Cu-Kα radiation (λ = 1.54060 Å) at room temperature. The morphology and size of the nanocomposites were obtained from TEM (Technai20G2, FEI). The optical transmission/absorption spectra of the particles in de-ionized water were recorded using a UV-1650PC SHIMADZU Spectrophotometer. Fluorescence measurements have been carried out with a RF-5301PC Spectrophotometer.
3 Results and discussion
3.1 Structural and morphological studies
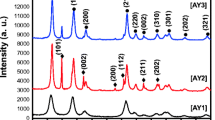
Figure 1 shows the XRD pattern of SnO2/CuO and CuO/SnO2 nanocomposites. The nanocomposites have polycrystalline nature trait to the various diffraction planes of SnO2 and CuO. The XRD profiles exhibit crystalline peaks corresponding to the peaks of SnO2 and CuO. All diffraction peaks can be perfectly indexed as the tetragonal structure for SnO2 (marked as ⊕) and a hexagonal structure for CuO (marked as ∗) which were confirmed from the JCPDS card no. 88-0287 and JCPDS card no. 89-5899, respectively. It exhibits strong diffraction peaks at 2θ values of 26.6°, 33.9°, 37.4°, 51.8°, 54.6°, 61.7° and 64.6°, which coincide well with the corresponding peaks of the tetragonal structure of SnO2. The pure CuO sample displays nine reflection peaks at 2θ values of 32.3°, 35.5°, 38.5°, 48.6° and 53.2°, which can be indexed as a monoclinic crystal structure of CuO. Thus, it is evident that the composites consist of a single phase of either the SnO2 or the CuO phases, not a solid solution phase of SnO2/CuO or CuO/SnO2. The diffraction peaks are broadened, indicating the presence of very fine crystallite size. Most of the observable peaks correspond to SnO2 and few of them for CuO but, intensities of the CuO peaks remain low, suggesting that the concentration of CuO phase in both SnO2/CuO and CuO/SnO2 nanocomposites is small, far smaller than expected from that of initial solution. The possible reason may be due to the dissociation constant value. The dissociation constant value of SnO2 is very much higher compared to that of CuO. The formation of SnO2 is dominant over the CuO for both nanocomposites. Also, some extra peaks in SnO2/CuO and CuO/SnO2 nanocomposites indicated the Cu ions due to higher dissociation constant value. Note that the peaks 2θ values (43.3, 50.4 and 74.1) corresponding planes [(1 1 1), (2 0 0) and (2 2 0)] are due to the copper substrate which is observed only in CuO/SnO2 nanocomposites. No characteristic peaks for impurity, such as SnO, CuSnO3, and Cu2SnO4, were observed [21]. The crystalline size of all the samples was calculated using Scherrer formula [22],
where λ is the wavelength of X-ray radiation, β is the full width at half maximum (FWHM) of the peaks at the diffracting angle θ. The crystallite size was calculated to be approximately 6 and 7 nm for SnO2/CuO and CuO/SnO2 nanocomposites, respectively.
Transmission electron microscopy (TEM) is used to analyze the size and morphology of the product. Powder samples were used for TEM measurements, the samples were prepared by drop casting method on the gold grid for the microscopy study. The different magnifications of TEM images (Fig. 2a, b) reveal that the product consists of spherical particles with a regular morphology and narrow size distribution of SnO2–CuO nanocomposite. Figure 2d, e TEM images of the CuO–SnO2 nanocomposite with uniform and well grain boundaries. Compared to CuO–SnO2, the SnO2–CuO nanocomposite shows very small size. The smaller size is fairly in agreement with optical absorption (significant blue shifting) of the corresponding sample which will be discussed in the optical section in detail. The size of the particles observed in the TEM image is in the range of 5–10 nm, which is in good agreement with that estimated by Debye–Scherrer formula from the XRD pattern. Figure 2c, f shows a histogram of sizes of SnO2/CuO and CuO/SnO2 nanocomposites obtained from microscopy analysis. The particle distribution histogram was obtained counting 120 and 150 particles from the micrograph for SnO2/CuO and CuO/SnO2, respectively. The average particle size of about 6 nm and 7.2 nm for SnO2/CuO and CuO/SnO2, respectively, with a standard deviation σ = 0.22.
TEM images of the different magnifications (70 K for a, d and 145 K for b, e, respectively) of the SnO2/CuO a, b and CuO/SnO2 d, e nanocomposites. For the TEM measurements the powder samples were dispersed in ethanol and then it was drop casted on the gold grid. c, f Particles size histogram of the SnO2/CuO and CuO/SnO2 nanocomposites, respectively
3.2 FT-IR study
Basically FT-IR spectra in the region of 400–4000 cm−1 are executed at room conditions. The FT-IR was performed to understand the reaction process during synthesis. Figure 3 displays the FT-IR spectrum of SnO2/CuO and CuO/SnO2 nanocomposites which shows two broad bands at 3443 and 3354 cm− 1 corresponding to the stretching vibrations of –OH units in the coordinated water and the adsorbed water, respectively. The band appeared at 1400 cm−1 is caused by NH3, the precipitating agent added during the precipitation process. The observed wide vibrational band (at 596 cm−1) could be assigned as a metal–oxygen (Sn–O and Cu–O) stretching vibration [23], which is demonstrated the configuration of doped SnO2/CuO and CuO/SnO2 nanocomposites.
3.3 Optical study
The optical absorption spectra of nanocomposites are measured by UV–Vis spectrophotometer in the visible range of 200–800 nm as shown in Fig. 4a, b as insert. In UV–Vis absorption method, the outer-electrons of atoms or molecules are absorbed by incident radiation sources, which undergo electron transition from lower to higher energy levels [24, 25]. According to this phenomenon, the spectrum is obtained due to the optical absorption, which can be used to analyze the band-gap energy of SnO2/CuO and CuO/SnO2 nanocomposites. The optical absorption measurement was carried out at ambient conditions. No extra peaks associated with impurities and structural defects were observed in the spectrums, which proved that the synthesized nanocomposites control crystallinity of SnO2/CuO and CuO/SnO2 nanocomposites. Band-gap energy (Eg) is calculated on the basis of the maximum absorption band of nanocomposites according to following equation,
where h is the Planck’s constant, ‘c’ is the velocity of light and ‘λ’ is the absorbed wavelength.
The room temperature PL spectra of SnO2/CuO and CuO/SnO2 nanocomposites are shown in Fig. 4a, b and corresponding UV–Vis absorption spectrum is inserted. The spectrum of SnO2/CuO nanocomposite showed only a broad band with strong orange emission which centered at about 600 nm. This strong emission might originate from the interaction between oxygen vacancies and interfacial tin vacancies [26]. In the case of CuO/SnO2, a strong broad emission peak was observed around 400 nm (violet emission). The emissions may be attributed to the different luminescent centers such as defect energy levels arising due to tin interstitials and oxygen vacancies as well as dangling bonds into nanocrystals. The similar PL result reported for CuO contains binary nanocomposite and pure CuO [27, 28]. The enhancement of PL intensity of CuO/SnO2 nanocomposite is mainly caused by the absorption and desorption of oxygen on the surface of sensing materials.
SnO2 shows p-type (Stable state) and also n-type semiconductor where as CuO shows only p-type. Due to this SnO2/CuO and CuO/SnO2 nanocomposites appeared as p–n homojunction.
Compared with the spectrum of SnO2/CuO nanocomposite, the emission band showed blue shift in the spectrum of CuO/SnO2 nanocomposite. The luminescence peak at 677 nm was also referred in the Cu-doped SnO2 [29], which were believed to originating with Cu ions. However, in the SnO2/CuO nanocomposite, the SnO2 and CuO were two independent phases (it was not found any trace of Cu related diffraction peak in Fig. 1). In addition, the peak at 678 nm was not found in the PL spectrum of CuO [30]. So it was concluded that the interface between SnO2 and CuO was the origin of the red shoulder.
Band gap of the nanocomposite was determined from the UV absorption data by Tauc plot. Figure 4c shows the exact value of bandgap which is determined by extrapolating the straight line portion of (αhν)2 versus energy (hν). The bandgaps are found to be around 2.35 and 3.0 eV for SnO2/CuO and CuO/SnO2 nanocomposites, respectively, which is red shifted from bulk SnO2 (Eg = 3.6 eV). Generally, we expect a blue shift in the nano regime but, here it is red shifted from bulk. The red shift confirms that the carrier concentrations of the CuO composited SnO2 nanoparticles increased compared to the pure SnO2 nanoparticles. The new electronic states created below the conduction band of the material may reduce the band gap of the CuO/SnO2 nanocomposite. Vijyalakshmi et al. reported the different mol% of the Zn doped SnO2 decreased the band gap value [31]. This shift toward lower energy in the composite is due to the contribution of CuO, the band gap of which is 1.2 eV. The red shifting PL emission shows the nanocomposite can be used in optoelectronic application.
Figure 5a, b shows the photocatalytic degradation activity of SnO2/CuO and CuO/SnO2 composites, respectively, were evaluated by MB dye under visible light irradiation time up to 30 min. The irradiation of visible light and the rate of degradation of the MB dye solution with the SnO2/CuO and CuO/SnO2 nanocomposites for different time intervals were presented in Fig. 5c, d. The major absorbance peak of MB was observed at 663 nm and also no other peaks were observed. As well, no shift was observed during the photocatalytic procedure, which implied that only the pure photochemical reaction was generated during the decoloration process for MB [32]. For the SnO2/CuO nanocomposites, the photodegradation was almost completed after 30 min, whereas CuO/SnO2 nanocomposites had more than 25% residual of MB. The results reveal that compared to CuO/SnO2 composite, SnO2/CuO composite solution degrades faster and easier with visible light irradiated MB dye. This degradation result may be due to suitable bandgap of SnO2/CuO composite (2.35 eV). In general, generation of more photo electron–hole pairs and less recombination for electron–hole pairs could come about prior to participation in oxidation–reduction reaction. More effective degradation reaction would take place to enhance photocatalytic efficiency. The factors involved in the increase in photocatalytic efficiency are the formation of p–n type heterojunction and as absorbing visible light, n-type could help p-type absorb more incident photons.
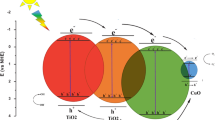
The schematic diagram showing the possible mechanism for the photocatalytic degradation of dye through SnO2–CuO nanocomposites is depicted in Fig. 6. The mechanism of photocatalysis can be understood as follows. When sun light is incident on SnO2–CuO nano-heterojunctions, electrons from the valance band are excited into the conduction band, leaving behind holes. The photogenerated electrons generated in the conduction band of CuO jump to the conduction band of SnO2, while the holes from the valance band of SnO2 transfer to the valance band of CuO [33]. This helps to inhibit the recombination of photogenerated electrons and holes and improves the charge separation efficiency. Then the photo-generated electrons reacted with the adsorbed oxygen (or dissolved in the solution) to produce active ˙O2− radicals. Therefore, efficient separation of electron–hole pairs in the as-achieved SnO2–CuO nanocomposite facilitates enhanced stability and photocatalytic activity towards photodegradation of malachite green under UV light. The concurrent active species of h+, and other radicals produced in the solution, such as ˙OH, can directly oxidize dye molecules in the system.
4 Conclusion
SnO2/CuO and CuO/SnO2 nanocomposites have been successfully synthesized by the simple chemical precipitation method and characterized by the XRD, TEM, FT-IR, UV–Vis and PL analysis. XRD results showed that tetragonal structure for SnO2 and a hexagonal structure for CuO. TEM images revealed that the product consists of spherical particles with a regular morphology and narrow size distribution. Size of the particles observed in the TEM image is in the range of 5–10 nm, which is in good agreement with data of the XRD analysis. Wide vibrational band at 596 cm−1 observed from FT-IR could be assigned as a metal–oxygen (Sn–O and Cu–O) stretching vibration. From the optical study, emission band showed significant blue shift in CuO/SnO2 compared with SnO2/CuO nanocomposite. The band gaps are found to be around 2.35 and 3.0 eV for SnO2/CuO and CuO/SnO2 nanocomposites, respectively. The obtained results indicate that SnO2/CuO nanocomposite having both the advantages of photodegradation–adsorption process which considered a promising new photocatalysts that involve in the abatement of various organic pollutants and also it can apply in sensor application due to it good electrochemical response.
References
F. Ansari, A. Sobhani, M. Salavati-Niasari, J. Colloid Interface Sci. 514, 723–732 (2018)
M. Mahdiani, F. Soofivand, F. Ansari, M. Salavati-Niasari, J. Clean. Prod. 176, 1185–1197 (2018)
P. Poizot, S. Laruelle, S. Grugeon, L. Dupontl, J.M. Tarascon, Nature 407, 496 (2000)
V.R. Katti, A.K. Debnath, K.P. Muthe, M. Kaur, A.K. Dusa, S.C. Gadkari, Sens. Actuators B Chem. 96, 245–252 (2003)
H.M. Fan, L.T. Yang, W.S. Hua, X.F. Wu, Z.Y. Wu, S.S. Xie, Nanotechnology 15, 37 (2004)
C.T. Hsieh, J.M. Chen, H.H. Lin, H.C. Shih, Appl. Phys. Lett. 83, 3383 (2003)
M.K. Wu, J.R. Ashburn, C.J. Torng, P.H. Hor, R.L. Meng, L. Gao, Phys. Rev. Lett. 58, 908 (1987)
M.H. Chang, H.S. Liu, C.T. Tai, Powder Technol. 207, 378 (2011)
C.L. Carnes, K.J. Kalbunde, J. Mol. Catal. A Chem. 194, 227 (2003)
W. Zhao, W.H. Ma, C.C. Chen, J.C. Zhao, Z.G. Shuai, J. Am. Chem. Soc. 126, 4782 (2004)
M. Ghanbari, F. Ansari, M. Salavati-Niasari, Inorg. Chim. Acta 455, 88–97 (2017)
G. Murugadoss, R. Thangamuthu, R. Jayavel, M. Rajesh Kumar, J. Lumin. 165, 30–39 (2015)
O. Amiria, N. Mir, F. Ansarid, M. Salavati-Niasari, Electrochim. Acta 252, 315–321 (2017)
F. Ansari, P. Nazari, M. Payandeh, F.M. Asl, B. Abdollahi-Nejand, V. Ahmadi, J. Taghiloo, M. Salavati-Niasari, Nanotechnology 29 075404 (2018)
P. Nazari, F. Ansari, B.A. Nejand, V. Ahmadi, M. Payandeh, M. Salavati-Niasari, J. Phys. Chem. C 121, 21935–21944 (2017)
J.G. Lu, P. Chang, Z. Fan, Mater. Sci. Eng. R 52, 49–91 (2006)
X.J. Zheng, Y.J. Wei, L.F. Wei, B. Xie, M.B. Wei, Int. J. Hydrogen Energy 35, 11709–11718 (2010)
X. Xue, L. Xing, Y. Chen, S. Shi, Y. Wang, T. Wang, J. Phys. Chem. C 112, 12157–12160 (2008)
J.-H. Jeun, D.-H. Kim, S.-H. Hong, Mater. Lett. 105, 58–61 (2013)
Y. Zhao, X. He, J. Li, X. Gao, J. Jia, Sens. Actuators B 165, 82–87 (2012)
S. Fuentes, R.A. Zárate, P. Muñoz, D.E. Díaz-Droguett, J. Chil. Chem. Soc. 55, 147–149 (2010)
A.L. Patterson, Phys. Rev. Online Arch. 56, 978–982 (1939)
D. Shaposhnik, R. Pavelko, E. Llobet, F. Gispert-Guirado, X. Vilanova, Int. J. Hydrogen Energy 34, 3621–3630 (2009)
M. Sauer, J. Hofkens, J. Enderlein, Copyright © 2011 Wiley, Weinheim
G. Murugadoss, M.Rajesh Kumar, Appl. Nanosci. 4, 67–75 (2014)
J.H. He, T.H. Wu, C.L. Hsin, K.M. Li, L.J. Chen, Y.L. Chueh, L.J. Chou, Z.L. Wang, Small 2, 116–120 (2006)
K. Wang, W. Zhang, F. Lou, T. Wei, Z. Qian, W. Guo, J. Solid State Electrochem. (2018). doi.https://doi.org/10.1007/s10008-018-3936-9
R. Sasikala, S.K. Rani, D. Easwaramoorthy, K. Karthikeyan, RSC Adv. 5, 56507–56517 (2015)
L.J. Li, K. Yu, H.B. Mao, Z.Q. Zhu, Appl. Phys. A 99, 865–869 (2010)
C. Jin, K. Baek, S. Park, H.W. Kim, W.I. Lee, C. Lee, Solid State Commun. 150, 1812–1817 (2010)
S. Vijayalakshmi, S. Venkataraj, M. Subramanian, R. Jayavel, J. Phys. D: Appl. Phys. 41, 035505 (2008)
R. Shao, L. Sun, L. Tang, Z. Chen, Chem. Eng. J. 217, 185–191 (2013)
D. Li, J. Hu, R. Wu, J.G. Lu, Nanotechnology 21, 485502 (2010)
Acknowledgements
The authors would like grateful thanks to the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), India for awarding Young Scientist Project, Grant Agreement No. SB/FTB/PS/-158/2013 (GAP 06/15). One of the author (Manavalan Rajesh Kumar) thanks to the contract no. 40/is2. This research was supported in part by the State contract (No. 3.6121.2017/8.9) between Ural Federal University and the Ministry of Education and Science of Russian Federation as well as was supported in part by grant of state assignment of FASO of Russia (theme “Flux” No. AAAA-A18-118020190112-8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajesh Kumar, M., Murugadoss, G., Pirogov, A.N. et al. A facile one step synthesis of SnO2/CuO and CuO/SnO2 nanocomposites: photocatalytic application. J Mater Sci: Mater Electron 29, 13508–13515 (2018). https://doi.org/10.1007/s10854-018-9476-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9476-3