Abstract
Shifting the ultra-violet of titania to visible light driven photocatalysis can be realized by coupling with metallic or non-metallic elements. In this study, a highly efficient visible-light-responsive SnO2/CuO/TiO2 heterojunction were synthesized via sol–gel method followed by calcination at different temperatures. The crystalline structures, morphology, constituent contents, chemical state and optical properties of the samples were analyzed in detail. The photodegradation performances of the prepared samples were evaluated by studying the degradation of 2,4-dichlorophenol under UV and visible light irradiation. Among the series of synthesized nanocomposites, the one prepared using Sn(Cu)/Ti molar ratio of 0.1 (SC10) was found to be the most active photocatalyst for the degradation of organic pollutants under investigation. This remarkably improved photocatalytic activity of SC10 could be attributed to the strong absorption of visible light and effective separation of photoinduced e−–h+ pair. The mineralization of 2,4-dichlorophenol was investigated by HPLC/MS and IC analysis. The recycling experiments confirm that SnO2/CuO/TiO2 nanocomposite is essentially stable. The work may set foundation for application of the new photocatalyst of CuO/SnO2/TiO2 and make an important step forward remedying environmental pollution.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photocatalysis has attracted a great deal of attention as a promising candidate for the elimination of organic contaminants in water and air, since the pioneering work by Honda and Fujishima [1]. The photocatalytic reaction is caused by the use of ultraviolet (UV) radiation to stimulate the semiconductor materials [2]. During irradiation of semiconductor photocatalysts with UV, photons are absorbed by the semiconductors and electron (e−)–hole (h+) pairs are generated [3, 4]. However, these photocatalysts show low efficiency under the sun light because UV constitutes only 3% of the total solar energy [5]. From the perspective of both chemistry and practical applications, it is undoubtedly important to develop photocatalytic materials that harvest photons with wider wavelength range [6]. Various strategies have been proposed to efficiently separate the photogenerated electron–hole pairs in semiconductor photocatalysts, for instance by doping, metal loading, and/or introducing heterojunctions. A heterojunction, in general, is defined as the interface between two different semiconductors with unequal band structure, which can result in band alignments. Among the proposed strategies, engineering heterojunctions in photocatalysts has been proved to be one of the most promising ways for the preparation of advanced photocatalysts because of its feasibility and effectiveness for enhancing the optical adsorption of photocatalyst [5, 7]. It also can efficiently reduce the recombination rate of the photogenerated charge carriers in the use of environmental purification and remediation [8].
TiO2 [9], SnO2 [10, 11] and CuO [5], as three well-known semiconductors, have been intensively investigated in the fields of photocatalysis, solar cell and gas sensors due to their special electronic and optical properties. Titanium dioxide (TiO2) has and continues to be one of the most widely studied semiconductors for photocatalytic applications due to its advantages, such as a suitable band gap for redox reactions, long-term stability, low cost, nontoxicity and so on [12]. However, as a single component semiconductor photocatalyst, TiO2 has an inherent drawback that the photogenerated electron/hole (e−/h+) pairs recombine fast (~ 10 ns) and hence only a fraction of e−/h+ pairs are available for the photoreaction [13]. To date, this disadvantage can be overcome by means of semiconductor coupling. With this approach, the assembled semiconductors are selected suitably so that efficient charge transfer occurs between them [14, 15]. This condition spatially separates the photo excited electrons and holes onto the different constituents and reduces the rate of e−/h+ pair recombination.
In multi-heterostructure, the presence of different facets exhibit distinct abilities in modifying the interfaces for better interaction [16]. Several studies on ternary composites such as CdS/TiO2/WO3, TiO2-based V2O5/WO3, Au/TiO2/WO3, CuO/SnO2/TiO2, SnO2/TiO2/WO3, Cu2O/TiO2/g-C3N4, ZnS/ZnO/CNT, Fe3O4/ZnO/CuWO4 and Cu/Cu2O/CuO had shown good photoelectric performance [5, 8, 17,18,19,20,21,22,23,24,25]. The couplings of TiO2 with CuO or SnO2 have been reviewed to greatly enhance the photocatalytic activities in photocatalytic activity by the spatial charge separation efficiency [26,27,28,29,30,31,32,33]. The position of conduction band of SnO2 and CuO that is lower than TiO2 is perfect for the occurrence of charge transfer which reduces the recombination of electron– hole. The presence of CuO with low band gap (1.7 eV) on submonolayer of TiO2 surface not only leads to visible light absorption but also increases the surface acidity for the absorption of more hydroxyl ion and organic reactants [34, 35]. Conversely, SnO2 with wide band gap of 3.9 eV has no absorption of light at wavelength longer than 330 nm. However, when coupled with TiO2, its photocatalytic activity enhance under visible light [36, 37]. It is reported that this system can considerably improve the photocatalytic activity of TiO2, which is attributed to the enhancement of the charge carrier separation due to the different energy band gap position of TiO2 and SnO2, leading to the formation of a cascadal structure [38]. On the basis of the discussion above, the mixed oxides of two semiconductors (e.g. CuO/TiO2 and SnO2/TiO2) as coupling system have attracted extensive interests, and the catalyst samples have been also intensely produced by a variety of synthetic routes [20, 21, 25,26,27,28, 31, 32, 34, 35, 37, 38].
On the basis of above discussion, up to date very few studies have been carried out on trimetallic oxide composites. To further make the most of the advantages and hedge the shortcomings of the abovementioned three semiconductors, herein, we designed and synthesized a novel CuO/SnO2/TiO2 ternary heterostructure nanohybrid, displaying an excellent visible-light photocatalytic activity. We presume that mixed oxides of TiO2, CuO and SnO2 with different energy gap would lead to the formation of cascadal structure that would enhance the activity. The photocatalyst were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDX) analysis, transmission electron microscopy (TEM), Raman spectroscopy, ultraviolet–visible diffuse reflectance spectroscopy (UV–vis-DRS), and X-ray photoelectron spectroscopy (XPS). To evaluate the catalytic activity of the synthesized materials, photodegradation experiments were also conducted with 2,4-dichlorophenol (2,4-DCP) under visible light. 2,4-DCP was selected because it is considered a representative compound of an important family of priority pollutants in aquatic environments [39,40,41,42]. Importantly, the initial photocatalytic degradation mechanism of 2,4-DCP was elucidated by means of a gas chromatographymass spectrometry (GC–MS) study of the degradation intermediates. The mechanism of improved photocatalytic activity for SnO2/CuO/TiO2 was probed using photoluminescence spectroscopy. Our findings suggest that incorporation of SnO2 and CuO into TiO2 as a complex system can extend the spectral response to the visible region and that the photocatalytic activity is greatly enhanced due to the promotion of the electron transfer through the interfacial potential gradient in the hybrid structure.
2 Materials and Methods
2.1 Materials
All chemicals were used as received without further purification. Titanium tetraisopropoxide (TTIP, 99.99%) and copper(II) sulfate pentahydrate (CuSO4·5H2O, 99.99% purity) were purchased from Sigma-Aldrich, Tin(IV) chloride pentahydrate (SnCl2·5H2O), Ethanol and 2,4-DCP were from Merck.
2.2 Preparation of CuO/SnO2/TiO2 Composite Photocatalyst
Sol–gel was adopted to synthesize CuO/SnO2/TiO2 composite system in the present work. In brief, 0.02 mol TTIP were added dropwise into 50 ml absolute ethanol to give a solution which was then vigorously stirred for 30 min at room temperature. The desired amounts of SnCl2·5H2O and CuSO4·5H2O (the molar ratio of Sn–Cu was 1:1) were then added in the reaction mixture while stirring continuously for 80 min until the Sn and Cu dopant dissolved. An amount of 2 ml of deionized water was then dropped into the above solution. Afterwards, the resultant solution mixture was maintained at room temperature with continuous stirring for 24 h to form a gel which was then aged for 12 h at room temperature. The samples were dried at 80 °C overnight and then annealed at 450, 550 and 650 °C for 4 h. The resultant composite materials coupled with different Cu and Sn contents by adjusting the molar ratio of Sn(Cu) to Ti from 0.05 to 0.20 were named as SC5, SC10, SC15 and SC20, respectively. Different composites composition were listed in Table 1.
2.3 Material Characterization
X-ray diffraction (XRD) analysis was performed using Bruker AXS D5000 diffractometer utilizing Cu Kα radiation (λ = 1.5406 Ǻ, 40 kV, 30 mA) at 0.050° intervals with 1 s count accumulation per step. The data was collected in the region of 2θ = 20°–80°. Diffuse reflectance UV–vis (DRUV) spectra were recorded using a PerkinElmer Lambda 35 spectrophotometer, with BaSO4 used as reference. Photoluminescence (PL) emission spectra of the samples were measured using FLSP920 Edinburgh with 450 W xenon lamp. The morphology of the samples were analysed using FESEM (SU8020, Hitachi) equipped with an energy dispersive Xray spectrometer (EDX, INCA/350, Oxford). Transmission electron microscopy (TEM) images were obtained with JEOL TEM 2100 high-resolution (HR) TEM (200 kV). The X-ray photoelectron spectroscopy (XPS) measurements were carried out using a PerkinElmer RBD upgraded PHI-5000C ESCA system with monochromatic Mg K excitation and a charge neutralizer. All bonding energies were calibrated to the C 1s peak at 284.8 eV of the surface adventitious carbon.
2.4 Evaluation of Photocatalytic Activity
The photocatalytic activity of all photocatalysts were determined by the photodegradation for 2,4-DCP solution (pH = 5.35) under vis irradiation (k > 400 nm). A sunlamp (Philips HPA 400/30S, Belgium) was used directly for the UV photocatalytic reaction, while for the visible photocatalysis a cutoff filter (k > 400 nm) was employed to remove UV irradiation. The photocatalyst dosages were 30 mg for visible photocatalysis and 10 mg for UV photocatalysis, respectively, which were suspended in 2,4-DCP solution. The reactor was perpendicular to the light beam and located 10 cm away from the light source. The intensity of the UV light and visible light on the samples is evaluated to be 0.2 and 0.1 W/cm2, respectively. An electric fan is applied to cool down the photocatalytic reactor. The suspension was magnetic stirred at (25 ± 2) °C in the dark for 30 min to reach adsorption equilibrium before irradiation, and oxygen gas was continuously bubbled through the solution at a flux of 1 ml min−1. The concentration change of 2,4-DCP was monitored by measuring the UV/vis absorption spectra of the suspension at regular 30 min intervals, using a Shimadzu UV/vis 2550 spectrophotometer. Owing to the very small particle size of CuO/SnO2/TiO2, for each measurement the suspension was filtered three times using a filter syringe to remove the insoluble photocatalyst before UV/vis measurements. GC–MS analysis of the intermediates formed during the mineralisation of 2,4-DCP was obtained using a Shimadzu GC–MS 2010 equipped with a silica capillary column by injecting ~ 0.1 μl of a 500 ppm initial concentration of 2,4-DCP in water. The reproducibility of the photocatalytic degradation was evaluated by repeating experiments at least three times with different batches of photocatalysts prepared by the same procedure.
3 Results and Discussion
3.1 The X-ray Diffraction (XRD)
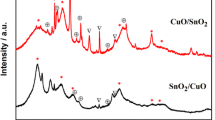
The X-ray diffraction (XRD) patterns for CuO/SnO2/TiO2 calcined at different temperatures are depicted in Fig. 1. The calcinations temperatures were 450, 550 and 650 °C, respectively. It can be seen from Fig. 2a that the composite photocatalyst annealed at 450 °C exhibit pure anatase structure of TiO2 at low Sn and Cu doping level (SC5), no other phases were detected in the SC5 heterojunctions. With the increase of the metal cations doping level, the SC10 mainly has anatase structure, with a minor content of rutile structure. Further increasing the Sn(Cu) doping content, the main (1 1 0) Bragg peak of rutile is observed to become sharper and more intense, indicating that the formation of a large amount of rutile structure in the SnO2/CuO/TiO2 composite catalyst. Moreover, a strong diffraction peak at around 26.6° was observed in the XRD patterns of SC20 sample indicating the presence of a small amount of rutile SnO2 [31].
Figure 1b shows the XRD data of CuO/SnO2/TiO2 composite semiconductors annealed at 550 °C. It can be observed that the SC5 sample has anatase as the dominant phase, and the main structure of SC10 sample is still anatase structure, while a weak peak appears at around 27.2°, indicating the formation of a small amount of rutile phase [37]. Compared with SC10, the SC15 sample heated at 550 °C shows a main rutile structure, with some anatase peaks remaining. Whereas, a pure rutile phase is observed in SC20 sample, suggesting that the phase transition from anatase to rutile in the sample occurs. Besides the diffraction peaks for anatase and rutile TiO2, there are two new crystalline structures (rutile SnO2 and CuO, respectively) formed in SC20 sample after annealing, which may be explained by that the tin and copper atomic content have reached saturation in the lattice at higher doping level [43].
The XRD spectra of CuO/SnO2/TiO2 composite semiconductors annealed at 650 °C shows that the significant structure change occurs compared with those of the composite photocatalyst annealed at 450 and 550 °C. As can be seen from Fig. 1c, the SC5 sample shows a mixture of anatase and rutile phase, in addition to the impurity peaks corresponding to CuO that are also present in the composite CuO/SnO2/TiO2 sample. With the increase of tin and copper doping level, the diffraction peaks corresponding to anatase TiO2 disappeared and only rutile TiO2 as well as CuO phase was observed for the SC15 sample, implying the anatase structure is completely transformed into rutile phase. Interestingly, it was also observed the significant diffraction peaks of rutile phase SnO2 in the XRD spectra of the SC20 sample except for the TiO2 and CuO structure. On the basis of the above analysis, it could be found that the Sn and Cu ions doped in TiO2 can promote the phase change from anatase to rutile TiO2 in the range of annealing temperature from 450 to 650 °C. Moreover, higher annealing temperature (650 °C) not only leads to the formation of the impurity such as CuO, but also favors the growth of rutile structure and gives CuO/SnO2/TiO2 catalysts with larger particle size (Table 2). Thus, a fine control of calcination temperature is crucial for obtaining a pure CuO/SnO2/TiO2 heterostructure.
3.2 Raman Spectra
Figure 2 shows the Raman spectra of CuO/SnO2/TiO2 catalysts synthesized by sol–gel methodology, and the obtained samples were calcined at 550 °C. Here, it can be seen that all Raman spectra of composite catalyst samples exhibit rather similar spectra, four well-defined bands at 144, 399, 514 and 639 cm−1 were observed, which are characteristics of anatase structure [44]. Interestingly, as the Sn(Cu) dopant content increases, the intensity of main Raman band at 144 cm−1 assigned to anatase TiO2 gradually reduced, indicating a lower crystallinity and small amount of anatase TiO2 for the composite photocatalysts [45].
3.3 X-ray photoelectron Spectroscopy
Further verification on the nature of surface defects and chemical state of the metal oxides was carried out using XPS that is known for its surface sensitive analysis. Figure 3 shows the typical XPS results of SC15 catalyst annealed at 550 °C. As can be seen from Fig. 3a, the X-ray photoelectron spectroscopy survey spectrum indicates that the SC15 sample contains predominantly O, Ti, Sn, Cu and C elements. It is thought to be the residual carbon from the organic precursor solution and the adventitious carbon from the XPS instrument itself are the main reason to cause the presence of C element .
The XPS spectra of Cu 2p region was shown in Fig. 3b. The figure shows the appearance of the two signals at 938.4 (Cu 2p3/2) and 958.9 eV (Cu 2p1/2); these signals demonstrate the presence of Cu2+ in CuO [46]. The difference in the binding energy gap value between the Cu 2p3/2 and Cu 2p1/2 is 20.5 eV, which confirms the presence of CuO [5, 34, 35, 47]. On the other hand, the shake-up peaks indicate the presence of an unfilled Cu 3d9 shell and reconfirm the presence of Cu2+ in the tested composite [48], which coincide with the Cu2+ position with oxygen. It is known that the radius of Cu2+ is 0.73 Å, which is larger than that of Ti4+ [46]. Thus, the Cu2+ ion is not able to replace the Ti4+ site and occupy its position in theory during the preparation. It is therefore thought that the Cu2+ions possibly resided over the surface of TiO2 particle [46]. In Fig. 3b, the Ti 2p XPS spectra of SC15 has two peaks centered at 458.5 and 463.9 eV, which are attributed to the Ti 2p3/2 and 2p1/2 spin–orbital splitting photoelectrons in Ti4+. These data agree well with previously reported XPS data for TiO2 [46]. Additionally, the band located at the low binding energy (458.5 eV) is broad and asymmetric, demonstrating that there are at least two kinds of Ti chemical states. The peak identified at 457.4 eV suggests the existence of Ti3+ in the composite catalyst [49, 50], and the formation of Ti3+ shows the presence of lots of oxygen vacancies during the doping process. Figure 3b shows the Sn 3d XPS spectra of SC15. The binding energy of 485.6 and 494.6 eV is ascribed to Sn 3d5/2 and Sn 3d3/2, respectively, assigned to Sn in 4 + state species [51]. According to the literature [52], the peak position corresponding to Sn 3d3/2 is centered between those of Sn 3d3/2 (485.0 eV) in metallic Sn and Sn 3d3/2 in SnO2 (486.6 eV), which suggests the Sn4+ ions incorporated into lattice of TiO2 and caused a small structural distortion in SC15 sample. Besides, it is worthy to be noted that the peak at 499.0 eV is due to a plasmon peak (P) in clean Sn metal [53]. The O 1s XPS spectrum is shown in Fig. 3b, too. It can be observed that the broad peak of O 1s is asymmetric, and that can be fitted by two peaks at 528.8 and 530.1 eV, indicating there are two independent chemical states for O within SC15. The peak located at high binding energy for O element can be corresponded to surface hydroxyl groups such as Ti–OH and Ti–O–O [21].
3.4 UV–vis Diffuse Reflectance Spectra
The results of UV–vis diffuse reflectance spectra of the catalysts calcined at 550 °C are shown in Fig. 4. The absorption edges for all the samples fall in the visible region (k > 400 nm) which indicates that the entire sample is active at visible light. The optical absorption edges of SnO2/CuO/TiO2 samples locate in the range of 480–500 nm, corresponding to a band gap energy (Eg) of approximately 2.41–2.31 eV. It also can be seen that the band gap decreases monotonically with increasing Sn and Cu concentration, implying that the level of Sn and Cu co-doping has a remarkable effect on the optical absorption of CuO/SnO2/TiO2 composite catalysts.
3.5 Photoluminescence (PL) Spectroscopy
To further address the contribution of charge transfer in the suppression of the recombination of electron hole process which is vital in the photocatalytic reaction [40], photoluminescence (PL) spectroscopy was investigated. Figure 5 shows the photoluminescence spectra attained for all the photocatalysts which were almost identical in shape and position.
The two peaks at around 480 and 520 nm in all samples could be ascribed to the transition from oxygen vacancies with one trapped electron and two trapped electrons to the valence band of TiO2, respectively [2]. Pure TiO2 exhibits a highest PL intensity. The PL intensity is further weakened when CuO and SnO2 were incorporated indicating that the recombination of photoinduced charge carriers is inhibited. This confirms that heterojunction is formed at the interface between TiO2, SnO2 and CuO. Photo-induced electrons would be shifted from the higher CB of TiO2–SnO2 and CuO. Moreover, as the VB potential of CuO is above than SnO2, this might lead to hole transfer in multiple pathways. The holes from the lower valence band of SnO2 would be transferred to TiO2 and CuO. Subsequently, the holes from TiO2 would move to CuO thus promoting the separation of electron–hole pairs. The emission intensity of SC10 that is lower than SC15 and SC20 signifies a lower recombination rate of hetro-generated electrons and holes. This could be correlated to the higher amount of oxygen vacancies that exist in SC10 resulting in a decreased PL intensity. Therefore, SC10 photocatalyst with suppressed electron–hole recombination rate is expected to increase the photocatalytic reaction.
3.6 SEM and TEM
Morphologies of the samples were characterized using SEM and TEM. Figure 6 clearly points out that all the samples (SC5 and SC15) are uniform. Increasing the amount of Sn(Cu) (Fig. 5b) caused the formation of denser particles and the agglomeration between them. These agglomerates were shaped by nanoparticles with an average size at around 30–70 nm. These values were consistent with the calculations made from the corresponding XRD patterns and Scherrer equation. Furthermore, the distribution of Sn(Cu) nanoparticles seemed to be randomly distributed on the TiO2 nanoparticles. The EDX analysis was also employed to detect the chemical compositions of the CuO/SnO2/TiO2 composite catalysts. Table 3 lists the chemical compositions and concentration of CuO/SnO2/TiO2 samples with different Sn(Cu) content. The results of EDX analysis clearly reveal that the SC15 mainly composed of Ti, O, C, Cu and Sn, indicating that the as-prepared composite catalyst have the well-mixed heterojunctions, consistent with XPS and XRD finding. However, Cu element was not observed in the SC5 sample. This is probably due to the Cu content is quite low and dispersed unevenly on CuO/SnO2/TiO2 when the Cu doping level is 0.05.
3.7 Photocatalytic Activity
To determine the efficiency of the photocatalysts, photocatalytic activity of nanocompsite was first investigated by evaluating the degradation of 2,4-DCP under UV light radiation. The catalyst adsorption capacity was tested before irradiation as seen in Fig. 7. Both composites and TiO2 (Commercial) showed very good 2,4-DCP adsorption in the dark (around 10–20%). The adsorption was instantaneous and did not increase with time. After 30 min, the light was turned on starting the photocatalytic process. As can be seen, the highest conversion of 2,4-DCP under UV LEDs was obtained with the TiO2 and SC5 photocatalysts, while that of the other composites was slightly lower. Nonetheless the SC5 photocatalyst presented a quite similar 2,4-DCP conversion than that of TiO2, which could be attributed to a part of 2,4-DCP adsorbed on the composites, especially on the TiO2 surface. This suggests that the only participant in the photocatalytic reaction under UV irradiation was TiO2; while deposited CuO and SnO2 plays a negative role reducing the contact between the TiO2 surface and the photons causing a reduction of 2,4-DCP degradation. This evidences that SnO2 and CuO act like an impurity and the electron–hole pairs generated by nanocomposite are rapidly recombined. When the photocatalytic process is carried on under visible light (470 nm), all of the composites showed a better 2,4-DCP degradation (Fig. 8). The enhanced photocatalytic activity of the composites at visible light could be associated with several synergetic effects as the presence of different facets on the photocatalysts and charge transfer at the interface. Reactive or high-energy facets on photocatalyst contribute significantly to their excellent activities in catalytic reactions. The adsorption of the molecules is instrically determined by surface atomic structures (facets) and the high surface acidity of CuO particles. The divergence in the energy levels drives the electrons and holes to different crystal faces. This suppressed the photoinduced electron–hole pair recombination which is the key to the high efficiency of the photocatalytic reaction [54]. It can also be seen that the co-doping level affects the photocatalytic activity, with the SC10 sample displaying the highest photocatalytic activity under visible light irradiation.
The photodegradation process of 2,4-DCP over the SnO2/CuO/TiO2 composites is described as below:
where C0 is the 2,4-DCP concentration before irradiation, C represents the 2,4-DCP concentration at reaction time t, and k is the pseudo-first-order rate constant. Figure 9 shows that the addition of Sn(Cu) to the composite promotes effectively the photocatalytic degradation of 2,4-DCP, and the SC10 composite exhibits highest photocatalytic efficiency (k = 0.0079 min−1.)
Figure 10a shows that the intensity of the maximum absorption band at 352 nm reduce with an increase in irradiation time. As shown in Fig. 10b, the dechlorination rate of 2,4-DCP is 98% after 120 min irradiation, suggesting that the decomposition of 2,4-DCP mainly proceeds a chlorine-released pathway. Furthermore, the TOC removal rate of 2,4-DCP is 70% after 120 min irradiation under visible light (Fig. 10b). The repeated experiments of 2,4-DCP degradation is carried out to evaluate the stability of the SC10 composite (Fig. 11). The photocatalytic activity of the SC10 composite has no obvious variation during three photodegradation cycles, suggesting that the photocatalyst possesses high stability.
An attempt was made to identify the intermediate products formed during the irradiation process through mass spectrometry analysis. The sample was withdrawn from the reaction mixture at the time interval of 30 min and this aqueous sample was extracted into chloroform solvent. It can be seen from the mass spectra in Fig. 12 that the amount of 2,4-DCP decreased gradually as the irradiation time increased. It can be concluded from Fig. 12a–d that 2,4-DCP was decomposed into other new substances whose relative molecular masses were 128.21, 144, 126.2, 110.1, 108.2 and 60 respectively. Figure 12d shows that there were few of 2,4-DCP in the solution after 120 min of treatment. It indicated that most of 2,4-DCP were decomposed into other substances. According to the relative molecular masses of intermediates and principles of organic degradation, the possible molecular structures of the intermediates were listed in Table 4. The intermediate products Chlorophenols, Hydroquinone, benzoquinone, Phenol were identified at 30 min irradiated sample. The Chlorohydroquinones, Hydroxybenzoquinone and Acetic acids were identified at 60 min irradiated sample. In chlorophenols, the attack of hydroxyl radical to the para position leads to the formation of hydroquinone. It undergoes oxidation and reduction to form benzoquinone. The degradation products chlorophenols are obtained by the addition of a hydroxyl radical to the position of the hydroxyl group of chlorophenols. The chlorohydroquinone is converted into hydroxyhydroquinone by oxidation with another hydroxyl radical followed by Cl· abstraction. The hydroxybenzoquinone is formed either by the oxidation followed by reduction of hydroxyhydroquinone and the hydroxyl radical attack of benzoquinone. An attack of hydroxyl radicals on hydroxybenzoquinone and benzoquinone lead to the ring opening to form acetic acid. In addition, the degradation product chlorophenols is obtained by the addition of a hydroxyl radical to the position of the Cl of chlorophenols. The chlorophenols is converted into phenol by oxidation with another hydroxyl radical followed by Cl· abstraction. The aliphatic compound, acetic acid is further oxidized into the smaller molecular weight aliphatic compounds and finally mineralized into CO2 and H2O.
The research of Quanfang Lu showed that part of 2,4-DCP were decomposed into CO2 and H2O by using glow discharge electrolysis [55]. The study of Ying Wang indicated that 2-chlorophenol and 4-chlorophenol may be the intermediate of the degradation of 2,4-DCP [56]. The study of Liu showed that 1,4-dihydroxybenzene was also an intermediate product of the degradation of 2,4-DCP [57]. Xu’s research suggested that there were acetic acid and formic acid generated during the process of Fenton-like degradation of 2,4-DCP when Fe3O4 magnetic nanoparticles as catalyst [58]. The study of Zhang showed that oxalic acid was a kind of degradation products of 2,4-DCP by using pulsed high voltage discharge [59]. Chung et al. showed that 2,4-DCP yielded less-chlorinated products, such as monochlorophenol and monochlorohydroquinone, and the non-chlorinated product, hydroquinone, which might have further reacted by photooxidation [60]. Devi and Nithya reported that the major identified intermediates are hydroquinone and benzoquinone [61]. Rakibuddin and Ananthakrishnan showed that after 5 h irradiation of 2,4-dichlorophenol by graphene encapsulated zno/co3o4 core–shell hybrid under visible light, the final fragments are phenol, pbenzoquinone, malonic acid, butenedioic acid, acetic acid and 25 1,3-butadiene [62].
Combining the studies of other scholars and mass spectrometry analysis, and the molecular structure of the intermediates, one probable degradation pathway of 2,4-DCP could be deduced as shown in Fig. 13.
3.8 Photocatalytic Mechanism
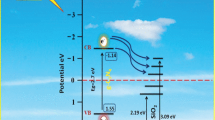
On the basis of the above experimental results, a possible photocatalytic mechanism is proposed to explain the enhanced photocatalytic performance, as shown in Fig. 14. The intergrowth and coexistence effects of the mixed-phase TiO2–SnO2–CuO heterojunctions play a vital role in the enhancement of its photocatalytic activity. The mechanism for the band configuration at the contacted interface of mixed-phase TiO2–SnO2–CuO material is proposed. Figure 14 shows the schematic diagram of the energy band gap and the charge transfer in the mixed-phase heterojunction system. As we have known, the conduction band (CB) edges of TiO2 (R), TiO2 (A), SnO2 and CuO are − 0.43, − 0.21, + 0.07 and 0.27 eV with respect to normal hydrogen electrode (NHE) while the valence band (VB) edges of TiO2 (R), TiO2 (A), SnO2 and CuO are + 2.60, 2.99, 3.67 and 1.6 eV respectively, suggesting that the system has different electron affinity and band configuration [34, 63, 64]. TiO2(A) has the conduction band (CB) edge that is below that of TiO2 (R) and above that of SnO2 and CuO [65]. Thus, a ‘‘staggered’’ type II heterojunction at the interface of the mixed-phases is formed. Under light irradiation, electrons in the valence band (VB) of TiO2 (R) are excited to its CB, while the hole remains in VB, and due to the Fermi energy level of TiO2 (R) is higher than that of TiO2(A), the electrons in CB are driven by the potential energy to transfer to the CB of TiO2(A) and then to SnO2 and CuO. Conversely, the photogenerated hole can move from VB of SnO2 to that of TiO2 and CuO [41,42,43]. It can be seen that in this process the electrons and holes are separated efficiently at the TiO2–SnO2–CuO interfaces, resulting in a large number of charges could participate in reaction and enhance quantum efficiency. The charge transfer from TiO2 causes reduction of Ti3+ (Ti3+ − e− → Ti4+) and the formation of Cu2+ (Cu2+ + e− → Cu+).The excess electrons accumulate in CuO, leading to negative shift in Fermi level of CuO. As a result, the Cu2+ translates into Cu+ or Cu, which is consistent with the XPS results. Then Cu+ or Cu gives the excited electrons to oxygen adsorbed on the surface of catalyst. The hole locate in the VB of TiO2 (R) then reacts with hydroxyl group to produce the hydroxyl radical (·OH), which are responsible for photoreaction. On the basis of the above analysis, we tentatively conclude that combining three semiconductors and two phases with different energy levels to form a ternary hybrid catalyst can facilitate the transfer of the electrons, reduce the recombination probability and increase the charge carrier lifetime, as a consequence of the enhancement of the photocatalytic activity for TiO2–SnO2–CuO catalysts.
4 Conclusion
In summary, a new type of heterostructured photocatalyst by compositing TiO2 with SnO2 and CuO is achieved via sol–gel method. Incorporating SnO2 and CuO into TiO2 nanoparticles was not improved its photocatalytic performance in UV-light region. On the other hand, under visible light, CuO and SnO2 addition evidenced the injection of charge-carriers and surely explains the enhanced activity of the composites. Based on the results, it can be concluded that combining three semiconductors with different energy levels to form a ternary hybrid catalyst can facilitate the transfer of the electrons, reduce the recombination probability and increase the charge carrier lifetime, as a consequence of the enhancement of the photocatalytic activity for SnO2/CuO/TiO2 samples. Indeed, enhanced activity was observed for the synthesized SC-0.1 heterojunction, indicating that the recombination of charge carrier is inhibited greatly among all the SnO2/CuO/TiO2 samples.
References
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Al-Hetlani E, Amin MO, Madkour M (2017) Detachable photocatalysts of anatase TiO2 nanoparticles: annulling surface charge for immediate photocatalyst separation. Appl Surf Sci 411:355–362
Gu L, Jin C, Wang W, He Y, Yang G, Li G (2017) Transition-metal-free, visible-light induced cyclization of arylsulfonyl chlorides with o-azidoarylalkynes: a regiospecific route to unsymmetrical 2,3-disubstituted indoles. Chem Commun 53:4203–4206
Park J-H, Ramasamy P, Kim S, Kim YK, Ahilan V, Shanmugam S, Lee J-S (2017) Hybrid metal-Cu2S nanostructures as efficient co-catalysts for photocatalytic hydrogen generation. Chem Commun 53:3277–3280
Wu X, Zhao J, Wang L, Han M, Zhang M, Wang H, Huang H, Liu Y, Kang Z (2017) Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl Catal B 206:501–509
Hirose Y, Itadani A, Ohkubo T, Hashimoto H, Takad J, Kittaka S, Kuroda Y (2017) Tubular nitrogen-doped TiO2 samples with efficient photocatalytic properties based on long-lived charge separation under visible-light irradiation: synthesis, characterization and reactivity. Dalton Trans 46:4435–4451
Hu JYR, Meng W, Du Y (2016) A novel p-LaFeO3/n-Ag3PO4 heterojunction photocatalyst for phenol degradation under visible light irradiation. Chem Commun 52:2620–2623
Okeil S, Krausmann J, Dönges I, Pfleger S, Engstler J, Schneider JJ (2017) ZnS/ZnO@CNT and ZnS@CNT nanocomposites by gas phase conversion of ZnO@CNT. A systematic study of their photocatalytic properties. Dalton Trans 46:5189–5201
Zhang H, Zhang W, Zhao M, Yang P, Zhu Z (2017) A site-holding effect of TiO2 surface hydroxyl in the photocatalytic direct synthesis of 1,1-diethoxyethane from ethanol. Chem Commun 53:1518–1521
Lamb R, Umar A, Mehta SK, Kansal SK (2015) ZnO doped SnO2 nanoparticles heterojunction photo-catalyst for environmental remediation. J Alloy Compd 653:327–333
Reddy CV, Ravikumar RVSSN., Srinivasc G, Shim J, Cho M (2017) Structural, optical, and improved photocatalytic properties of CdS/SnO2 hybrid photocatalyst nanostructure. Mater Sci Eng B 221:63–72
Pitre SP, Yoon TP, Scaiano JC (2017) Titanium dioxide visible light photocatalysis: surface association enables photocatalysis with visible light irradiation. Chem Commun 53:4335–4338
Lee S-Y, Park S-J (2013) Review: TiO2 photocatalyst for water treatment applications. J Ind Eng Chem 19(6):1761–1769
Klaysri R, Tubchareon T, Praserthdam P (2017) One-step synthesis of amine-functionalized TiO2 surface for photocatalytic decolorization under visible light irradiation. J Ind Eng Chem 45:229–236
Batalović K, Bundaleski N, Radaković J, Abazović N, Mitrić M, Silva RA, Savić M, Belošević-Čavor J, Rakočević Z (2017) Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)—a combined XPS and DFT study. Phys Chem Chem Phys 19:7062–7071
Liu G, Yang HG, Pan J, Yang YQ, Lu GQ, Cheng HM (2014) Titanium dioxide crystals with tailored facets. Chem Rev 114:9559–9612
Kim H-i, Kim J, Kim W, Choi W (2011) Enhanced photocatalytic and photoelectrochemical activity in the ternary hybrid of CdS/TiO2/WO3 through the Cascadal electron transfer. J Phys Chem 115(19):9797–9805
Albonetti S, Blasioli S, Bonelli R, Mengou JE, Scirè S, Trifirò F (2008) The role of acidity in the decomposition of 1,2-dichlorobenzene over TiO2-based V2O5/WO3 catalysts. Appl Catal A 341(1–2):18–25
Kovács G, Baia L, Vulpoi A, Radu T, Karácsonyi É (2014) TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, part I: aeroxide P25 based composites. Appl Catal B 147:508–517
Yang G, Yan Z, Xiao T (2012) Preparation and characterization of SnO2/ZnO/TiO2 composite semiconductor with enhanced photocatalytic activity. Appl Surf Sci 258:8704–8712
Nadarajan R, Bakar WAWA., Ali R, Ismail R (2016) Effect of structural defects towards the performance of TiO2/SnO2/WO3 photocatalyst in the degradation of 1,2-dichlorobenzene. J Taiwan Inst Chem Eng 64:106–115
Min Z, Wang X, Li Y, Jiang J, Li J, Qian D, Li J (2017) A highly efficient visible-light-responding Cu2O–TiO2/g-C3N4 photocatalyst for instantaneous discolorations of organic dyes. Mater Lett 193:18–21
Shekofteh-Gohari M, Habibi-Yangjeh A (2016) Fabrication of novel magnetically separable visible-light-driven photocatalysts through photosensitization of Fe3O4/ZnO with CuWO4. J Ind Eng Chem 44:174–184
Liu L, Deng J, Ni T, Zheng G, Zhang P, Jin Y, Jiao Z, Sun X (2017) One-step synthesis of Ag/AgCl/GO composite: a photocatalyst of extraordinary photoactivity and stability. J Colloid Interface Sci 493:281–287
Guerrero-Araque D, Acevedo-Peña P, Ramírez-Ortega D, Calderon HA, Gómez R (2017) Charge transfer processes involved in photocatalytic hydrogen production over CuO/ZrO2-TiO2 materials. Int J Hydrogen Energy 42:9744–9953
Sheng P, Lia W, Du P, Cao K, Cai Q (2016) Multi-functional CuO nanowire/TiO2 nanotube arrays photoelectrode synthesis, characterization, photocatalysis and SERS applications. Talanta 160:537–546
Luna AL, Valenzuela MA, Colbeau-Justin C, Vázquez P, Rodriguez JL (2016) Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl Catal A 521:140–148
Zhu L, Hong M, Ho GW (2015) Fabrication of wheat grain textured TiO2/CuO composite nanofibers for enhanced solar H2 generation and degradation performance. Nano Energy 11:28–37
Ajmal A, Majeed I, Malik RN, Iqbal M, Nadeem MA, Hussainc I, Yousafa S, Zeshan G (2016) Photocatalytic degradation of textile dyes on Cu2O–CuO/TiO2 anatase powders. J Environ Chem Eng 4(2):2138–2146
Wu J, Li C, Chen X, Zhang J, Zhao L, Huang T (2017) Photocatalytic oxidation of gas-phase Hg0 by carbon spheres supported visible-light-driven CuO–TiO2. J Ind Eng Chem 46:416–425
Du F, Zuo X, Yang Q, Yang B, Li G, Ding Z, Wu M, Ma Y, Jin S, Zhu K (2016) Facile assembly of TiO2 nanospheres/SnO2 quantum dots composites with excellent photocatalyst activity for the degradation of methyl orange. Ceram Int 42(11):12778–12782
Yuan J, Zhang X, Li H, Wang K, Gao S, Yin Z, Yu H, Zhu X, Xiong Z, Xiea Y (2015) TiO2/SnO2 double-shelled hollow spheres-highly efficient photocatalyst for the degradation of rhodamine B. Catal Commun 60:129–133
Huang M, Yu S, Li B, Lihui D, Zhang F, Fan M, Wang L, Yu J, Deng C (2014) Influence of preparation methods on the structure and catalytic performance of SnO2-doped TiO2 photocatalysts. Ceram Int 40(8):13305–13312
Bandara J, Udawatta CPK, Rajapakse CSK (2005) Highly stable CuO incorporated TiO2 catalyst for photocatalytic hydrogen production from H2O Photochem. Photobiol Sci 4:857–861
Moniz SJA, Tang J (2015) Charge transfer and photocatalytic activity in CuO/TiO2 nanoparticle heterojunctions synthesised through a rapid, One-Pot, Microwave Solvothermal Route. ChemCatChem 7(11):1659–1667
Réti B, Péter N, Dombi A, Hernadi K (2013) Preparation of SnO2-TiO2/MWCNT nanocomposite photocatalysts with different synthesis parameters. Phys Status solidi 250(12):2549–2553
Zhou B, Yang S, Wu W, Sun L, Lei M, Pan J, Xiong X (2014) Self-assemble SnO2@TiO2 porous nanowire-nanosheet heterostructures for enhanced photocatalytic property. CrystEngComm 16:10863–10869
Li Y, Hu Z, Liu S, Duan X, Wang B (2014) Comparison of the preparation methods for a highly efficient CuO/TiO2 photocatalyst for hydrogen generation from water Reaction Kinetics. Mech Catal 112(2):559–572
Lv X, Wang Y, Lin Z (2016) Study on photocatalytic degradation of 2,4-dichlorophenol by ZnS microsphere. J Nanosci Nanotechnol 16(1):1060–1066
Kargi F, Eker S (2005) Removal of 2,4-dichlorophenol and toxicity from synthetic wastewater in a rotating perforated tube biofilm reactor. Process Biochem 40(6):2105–2111
Sinirtas E, Gulin M, Soylu SP (2016) Photocatalytic degradation of 2,4-dichlorophenol with V2O5–TiO2 catalysts: effect of catalyst support and surfactant additives. Chin J Catal 37(4):607–615
Jian Z, Huang S, Zhang Y (2013) Photocatalytic degradation of 2,4-dichlorophenol using nanosized Na2Ti6O13/TiO2 heterostructure particles. Int J Photoenergy. https://doi.org/10.1155/2013/606291
Rangel-Vázqueza I, Angel GD, Bertina V, González F, Vázquez-Zavalaa A, Arrieta A, Padilla JM, Barrerad A (2015) Synthesis and characterization of Sn doped TiO2 photocatalysts: effect of Sn concentration on the textural properties and on the photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. J Alloy Compd 643:144–149
Ana H-R, Parka SY, Huh JY, Kim H, Leed Y-C, Lee YB (2017) Nanoporous hydrogenated TiO2 photocatalysts generated by underwater discharge plasma treatment for solar photocatalytic applications. Appl Catal B 15:126–136
Yang G, Jiang Z, Shi H, Jones MO, Xiao T (2010) Study on the photocatalysis of F–S co-doped TiO2 prepared using solvothermal method. Appl Catal B 96(3–4):458–465
Nguyen DCT, Cho K-Y, Oh W-C (2017) Synthesis of frost-like CuO combined graphene-TiO2 by self-assembly method and its high photocatalytic performance. Appl Surf Sci 412:252–261
Renuka L, Anantharaju KS, Vidyad YS, Nagaswarupa HP, Prashantha SC, Sharma SC, Nagabhushana H (2017) A simple combustion method for the synthesis of multi-functional ZrO2/CuO nanocomposites: excellent performance as Sunlight photocatalysts and enhanced latent fingerprint detection. Appl Catal B 210:97–115
Chen J, Liu X, Zhang H, Liu P, Li G, An T, Zhao H (2016) Soft-template assisted synthesis of mesoporous CuO/Cu2O composite hollow microspheres as efficient visible-light photocatalyst. Mater Lett 182:47–51
El-Sheikh SM, Khedr TM, Hakki A, Ismail AA, Badawy WA, Bahnemann DW (2017) Visible light activated carbon and nitrogen co-doped mesoporous TiO2 as efficient photocatalyst for degradation of ibuprofen. Sep Purif Technol 173:258–268
Seifvand N, Kowsari E (2017) TiO2/in-situ reduced GO/functionalized with an IL-Cr complex as a ternary photocatalyst composite for efficient carbon monoxide deterioration from air. Appl Catal B 206:184–193
Santhi K, Rani C, Karuppuchamy S (2016) Synthesis and characterization of a novel SnO/SnO2 hybrid photocatalyst. J Alloy Compd 662:102–107
Hu L, Chen F, Hu P, Zou L, Hu X (2016) Hydrothermal synthesis of SnO2/ZnS nanocomposite as a photocatalyst for degradation of Rhodamine B under simulated and natural sunlight. J Mol Catal A: Chem 411:203–213
Ji X, Liu Y, Wu X, Zhang Z (2014) A low temperature situ precipitation route to designing Zn-doped SnO2 photocatalyst with enhanced photocatalytic performance. Appl Surf Sci 311:609–613
Kuang Q, Wang X, Jiang Z, Z ZX, and Zheng L (2014) High-energy-surface engineered metal oxide micro- and nanocrystallites and their applications. Acc Chem Res 47(2):308–318
Lu Q, Yu J, Gao J (2006) Degradation of 2,4-dichlorophenol by using glow discharge electrolysis. J Hazard Mater 136(3):526–531
Wang Y, Shen Z, Li Y, Niu J (2010) Electrochemical properties of the. erbium–chitosan–fluorine-modified PbO2 electrode for the degradation of. 2,4-dichlorophenol in aqueous solution. Chemosphere 79:987–996
Liu L, Chen F, Yang F (2009) Stable photocatalytic activity of immobilized Fe0/TiO2/ACF on composite membrane in degradation of 2,4-dichlorophenol. Sep Purif Technol 70(2):173–178
Xu LJ, Wang JL (2012) Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl Catal B 123–124:117–126
Zhang J, Liu D, Bian W, Chen X (2012) Degradation of 2,4-dichorophenol by pulsed high voltage discharge in water. Desalination 304:49–56
Chung S-G, Chang Y-S, Choi J-W, Baek K-Y, Hong S-W, Yun S-T, Lee S-H (2013) Photocatalytic degradation of chlorophenols using star block copolymers: removal efficiency, by-products and toxicity of catalyst. Chem Eng J 215–216:921–928
Devi LG, Nithya PM (2018) Photocatalytic activity of Hemin (Fe(III) porphyrin) anchored BaTiO3 under the illumination of visible light: synergetic effects of photosensitization, photo-Fenton & photocatalysis processes. Inorg Chem Front 5:127–138
Rakibuddina M, Ananthakrishnan R (2016) Effective photocatalytic dechlorination of 2,4-dichlorophenol by a novel graphene encapsulated ZnO/Co3O4 core-shell hybrid under visible light. Photochem Photobiol Sci 15:86–98
Marschall R (2014) Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv Funct Mater 24:2421–2440
Scanlon D, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CR, Powell MJ, Palgrave RG (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12(9):798–801
Kumar A, Rout L, Achary LSK, Mohanty A, Marpally J, Chand PK, Dash P, (2016) Design of binary SnO2–CuO nanocomposite for efficient photocatalytic degradation of malachite green dye. In: AIP Conference Proceedings 1724
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golestanbagh, M., Parvini, M. & Pendashteh, A. Preparation, Characterization and Photocatalytic Properties of Visible-Light-Driven CuO/SnO2/TiO2 Photocatalyst. Catal Lett 148, 2162–2178 (2018). https://doi.org/10.1007/s10562-018-2385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2385-5