Abstract
This study is focused on preparation of electrically conductive nanocomposite adhesive based on epoxy resin as polymeric matrix and nanocarbon black as conductive filler. For this purpose, commercial nanocarbon black was chemically modified by oxidizing acids and then was coated with silver nanoparticles. Nanocarbon black samples with different percentages of conductive filler were synthesized and used in preparation of nanocomposite adhesives. Electrically conductive adhesive with optimum properties was the one containing 30 wt% of conductive filler with bulk resistance of 0.0364 Ω cm and lap shear strength of 6.77 MPa. SEM images showed that good conductive paths are generated in polymer matrix. Also, weight reduction percentage during thermal gravimetric analysis was from 88.71 to 51.31% and degradation temperature was increased from 200 to 340 °C, which showed an improvement in thermal stability with an increment in filler percentage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrically conductive adhesive consists of polymeric matrix filled with conductive fillers. Using electrically conductive adhesives is a technique for internal joint that is harmless for environment. One of the most important challenges in the electric and electronic industries is the topic of joints in parts and microchips. Using lead solder was and still is the most common way of jointing electronic parts to manufacture a device. Due to extreme toxicity of lead for environment and harmful effects of its vapor for human health, Japan and European countries have serious bans for production of such materials.
Electrically conductive adhesives are very popular since their production reaction is not toxic, they are cost effective and their production procedure requires relatively lower temperature to cure. Due to their higher flexibility compared to solder, they are less sensitive to thermo-mechanical tensions. Also, in comparison to solder, these adhesives have higher precision for ultra-small internal joints, which is due to the dimension of particles used in their generation. These materials have high heat conductivity, adequate purity, exceptional jointing strength and uniform production line. While having all these advantages, electrically conductive adhesives have some disadvantages compared to solder. For instance, their conductivity may be lower than solder, their compounds are more sensitive in type and quality and curing time is higher.
Ideal polymeric matrix for an electrically conductive adhesive must have long lasting life, rapid curing, relatively high glass transition temperature, low moisture absorption and proper adherence [1,2,3,4]. Electrically conductive adhesives can be thermoplastic or thermoset. Unlimited usage lifetime in room temperature and reusability are features of electrically conductive adhesives based on thermoplastic polymers. Electrically conductive adhesives based on thermoset polymers are resistant to shape change at very high temperatures. Most of thermosets do not require a solvent.
Most of the commercial electrically conductive adhesives are based on thermoplastic resins [5,6,7,8,9]. Epoxy resins are the most commonly used resins in the formulation of this type of adhesives since they own advantageous features. Silicones, cyanate esters and cyanoacrylates are also used in the formulation of isotropic electrically conductive adhesive [10,11,12,13,14,15]. Electrically conductive adhesives’ formulation consists of resin, conductive filler, curing factor and additives. Polymeric resin provides mechanical and physical properties of electrically conductive adhesives and filler particles provide electrical conductivity properties. Electrically conductive adhesives’ efficiency depends highly on filler loading value, its type and polymeric matrix. Two main categories of electrically conductive adhesives are isotropic and non-isotropic electrically conductive adhesives [16]. Isotropic electrically conductive adhesives are conductive in all directions and filler concentration is higher than percolation threshold in their structure which causes electrons to move in every direction. There are two conductivity paths for Isotropic electrically conductive adhesives. One is natural conductivity which is due to particle to particle contact in polymeric matrix. Second is percolation, which consists of transport of brought electron to the environment by electron’s quantum-mechanical tunneling between particles that are adequately close, so they can cause a possibility of matrix di-electric breakdown. These conduction mechanism schemes are shown in Fig. 1a, b and schematic illustration of the prepared epoxy/CB–Ag nanocomposite adhesive is shown in Fig. 1c. Conductivity of isotropic electrically conductive adhesives depends on uniform distribution of filler particles to shape the conductivity path in polymer matrix [3, 17,18,19,20,21]. Filler fraction in non-isotropic electrically conductive adhesives is 0.5–5% which is much lesser than percolation threshold used in isotropic electrically conductive adhesives. Conductive fillers are separated from each other, so they can move the electrons only in one direction. Curing process of these adhesives is usually done by applying high pressure along with heating. Thus, there is no continuous electrical conductivity path in non-isotropic electrically conductive adhesives before jointing [22,23,24,25].
In this work, epoxy resin and chemically modified nanocarbon black were used as polymeric matrix and for achieving proper conductivity with lower percent of filler, respectively. Nanocarbon black was chemically modified via oxidizing inorganic acids and was coated with silver as a conductive filler. Finally, mechanical properties and heat stability of samples were investigated, and their surface morphology were studied using scanning electron microscopy (SEM) and analyses such as Fourier transform-infrared spectroscopy (FTIR), atomic absorption spectroscopy (AAS), thermal gravimetric analysis (TGA), lap shear strength, electrical resistance measurement were carried out to characterize properties of prepared samples.
2 Experiments
2.1 Materials
Materials used to prepare electrically conductive adhesives are: sodium borohydride 96% (NaBH4), sulfuric acid 98% (H2SO4), nitric acid 65% (HNO3), ammonia solution 25% (NH3) and silver nitrate solution (AgNO3). All materials were purchased from Merck, Germany. Ethanol was supplied by Chem. Lab. company. Commercial nanocarbon black powder (42 nm) was bought from Exir Co., Austria. Resin epoxy, type bisphenol A (DGEBA, RL441), and aminic hardener (Y441) were supplied by Pars Composite company. All reagents were used without further purification.
2.2 Chemical modification of nanocarbon black
In this method, modification of commercial nanocarbon black was carried out using inorganic acids, such as sulfuric acid (H2SO4) and nitric acid (HNO3) by generating acidic functional group (–COOH) on nanocarbon black surface, which was investigated using FTIR spectrum. To study the morphology of nanocarbon black, SEM images were taken [26]. Chemical modification was done using reflux method. 2 g of commercial nanocarbon black was weighed and transferred into a 500 ml, three neck flat bottom flask. 125 ml of nitric acid (20 vol%) and 125 ml of sulfuric acid (20 vol%) were also added. To stabilize the temperature of the mixture at 110 °C it was stirred for 4 h in an oil bath. It should be noted that before the reflux, the mixture was treated by nitrogen gas for 10 min. Modified product was washed by distilled water to eliminate nitrate and sulfate, so the pH of the solution reaches 7. Finally, the solution was put in oven at 80 °C for 24 h [26].
2.3 Silver coating of chemically modified nanocarbon black
To coat chemically modified nanocarbon black with silver, initially, 0.08 g of chemically modified nanocarbon black by acid was transferred into an erlenmeyer flask and 300 ml distilled water was added. Erlenmeyer flask was put in an ultrasonic bath at 30 °C for 15 min, so nanocarbon black particles are completely separated. Erlenmeyer flask was put on a magnetic stirrer, so the mixture is completely mixed. Then, 3.36 ml of 0.1 M silver nitrate titrasol was poured into a flask and was brought up to the volume of 100 ml with distilled water. Silver nitrate solution was added to erlenmeyer flask on the magnetic stirrer and the sample was mixed for 20 min. 1.36 g of sodium borohydride as silver nitrate reduction agent was transferred into another erlenmeyer flask and 12 ml of distilled water was added. Sample was mixed completely so sodium borohydride is dissolved in water and a homogeneous solution is achieved. The erlenmeyer flask was transferred to an ice bath. After 20 min of stirring nanocarbon black with silver nitrate solution, the final solution was transferred to an oil bath and its temperature was kept constant at 70 °C. Sodium borohydride solution was added to this erlenmeyer flask gradually. Afterwards, 5 ml of ammonia solution (25 vol%) was added to the erlenmeyer flask gradually, so the pH of solution reached 11 and it was stirred for 1 h. Then, the solution was filtered and washed with distilled water and ethanol, so pH of the filtered solution reached 7, finally the solid residuals were put in oven at 40 °C for 12 h [27]. It should be noted that all the steps mentioned above were repeated using two times more silver nitrate and sodium borohydride to coat twice the times silver on chemically modified nanocarbon black.
2.4 Preparation of electrically conductive nanocomposite adhesives
To prepare the electrically conductive nanocomposite adhesives, a precise amount of epoxy resin was transferred into a beaker and the exact amount of conductive filler was transferred into another beaker and 20 vol% ethanol was added. The beaker was put in an ultrasonic bath for 20 min. Then mixture was stirred for 20 min. The contents of this beaker were poured in the beaker containing epoxy resin and it was put in an ultrasonic bath and on a magnetic stirrer, for 20 min each, to get a homogenous mixture. After these steps, primary curing is required to eliminate the solvent. To do so, the mixture was put in the oven at 70 °C for 30 min. After primary curing, 10 wt% resin hardener was added to the beaker and the mixture was stirred to get to the gel time, which depends on conductive filler and resin hardener amounts. Adhesive sample was used for molding in silicon frames and for adhering parts. For curing of adhesive, samples were kept in oven for 1 h at 140 °C and afterwards they were kept at room temperature. In this study initially samples having 1, 3, 5, 10, 15, 20, 25, 30, 35 and 40 wt% of modified nanocarbon black were prepared and conductivity tested. Then samples with 10, 15, 20, 25, 30 and 40 wt% of modified nanocarbon black were coated with silver. Finally, the optimum sample was chosen and the further analysis, the mechanical and heat properties, were carried out. Tables 1 and 2 summarize the different types of electrically conductive nanocomposite adhesives in terms of chemically modified nanocarbon black and Ag coated nanocarbon black filler content.
2.5 Characterization methods
2.5.1 Fourier transforms infrared (FT-IR) spectroscopy
The chemical structures of chemically modified nanocarbon black and Ag coated nanocarbon black conductive fillers were investigated by FTIR spectroscopy (Tensor 27, Bruker, Germany). Samples were mixed with KBr powder and pressed to disk. The scanning range of samples by IR spectra was from wave number of 400–4000 cm−1. Each one of measurements were accomplishing at room temperature.
2.5.2 Atomic absorption spectroscopy (AAS)
Atomic absorption spectroscopy (AAS) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state. In this work, flame AAS was used to determine the amount of silver coated on chemically modified nanocarbon black by using the atomic absorption spectroscopy equipment (nov-AA® 400, Germany).
2.5.3 Scanning electron microscopy analysis (SEM)
The surface morphology and particle size of the pristine nanocarbon black, Ag coated nanocarbon black conductive fillers and electrically conductive nanocomposite adhesives were evaluated with using energy dispersive X-ray analysis (EDXA) and field emission scanning electron microscopy associated with them. Nanocomposite adhesive specimen for the SEM observations was prepared by freezing the dry membrane sample in liquid nitrogen up to 10 min and breaking it to produce a fresh cross-section. Fresh cross-sectional cryogenic fractures of the film were vacuum sputtered with a thin layer of gold (Au) using an ion sputtering before observing on the scanning electron microscope (FE-SEM MIRA3 FEG-SEM, Tescan, Czech Republic) with a potential of 300 V to 20 kV. To determine the pristine and Ag coated nanocarbon black fillers’ distribution along the nanocomposite adhesives in cross-section of these sheets, the elemental profiles across the sample thicknesses was obtained by energy dispersive X-ray analysis (EDXA) by Oxford Instruments Microanalysis model 7718 INCA PentaFET.
2.5.4 Measurement of electrical resistivity
Various models and methods have been suggested to measure the electrical resistance. Factors affecting the suitability of various methods and precision attainable include contact resistance and shape of the sample. Among the methods, two-point probe method can be used for higher resistive samples and four-point probe method can be used for the low resistive and single crystals. In this work, we used the two-point probe method for measuring the electrical resistivity of the conductive nanocomposite adhesives. The dimensions of the sample sheets were 25 × 10 × 1 mm (1 mm: the thickness of sample sheet). These rectangular samples were sandwiched between two cooper foils and then the electrical current was conducted between two sides of sample. The electrical resistivity was measured continuously by using a digital multimeter (Afzar Azma Electronic, GDM-8034, Iran) at room temperature. The measured electrical resistivities (R) were converted into specific resistivity (ρ) in terms of Ω cm, by using simple geometric calculation: ρ = R (W × t/L). In this equation, W was the wide, L the length and t the thickness of test sample. Finally, the electrical conductivity (σ) obtained as reciprocal of ρ (σ = 1/ρ) [28].
2.5.5 Lap shear strength test
The standard test method for apparent shear strength of single-lap-joint adhesively bonded metal specimens by tension loading (metal-to-metal) were carried out by using ASTM D1002. Analysis were carried out using mechanical test’s universal equipment (Santam, STM-20 model, Iran) by rate of 5 mm/min cross-head velocity. Based on this standard, two metal parts with following characteristics were prepared. They were made of aluminum type 209 B and length, width and depth of the parts were 100, 25.4 and 2 mm, respectively. To prepare the parts for joint, initially, the aluminum parts were washed with sulfuric acid (5 wt%). Then, the cross section of two parts were glued with the electrically conductive adhesive prepared and was put in the oven at 140 °C for 1 h to cure. Length and depth of the part covered with the adhesive was 12.5 and 0.8 mm, respectively. To facilitate connection of samples to the pins of mechanical test apparatus, the edges of sample were attached to square pieces, made from same material.
2.5.6 Thermal gravimetric analysis (TGA)
Thermal properties of electrically conductive adhesive samples were determined by thermogravimeteric analysis (TGA). Thermogravimetric analysis (TGA) was investigated using a Linseis Instrument (A 1750) at heating rate of 10°C/min to 600 °C and air atmosphere. The sample weight was 15–20 mg in each run.
3 Results and discussion
3.1 Fourier transforms infrared (FT-IR) spectroscopy
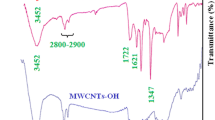
To study the oxidation and modification of commercial nanocarbon black using inorganic acids like sulfuric acid and nitric acid by generating acidic function group (–COOH) on nanocarbon black surface, FT-IR spectrum analysis was carried out on the pristine and modified powder nanocarbon blacks. The analysis was done in the range of 4000–400 cm−1 wavenumbers. The FT-IR measurements of several types of nanocarbon black samples are represented in Fig. 2. Figure 2a shows the FT-IR spectrum of commercial nanocarbon black and Fig. 2b depicts the FT-IR spectrum of modified nanocarbon black with inorganic acids. In graph b, the peak seen in wavenumber range of 3650–3200 cm−1 is related to stretch vibrations of OH groups, in which OH peak appears in wave number of 3333 cm−1. The peak has been shown in the region of 1750–1705 cm−1 is related to stretch vibrations of C=O double bond groups of carboxylic and carbonyl group, and this peak is appeared in the wave number of 1731 cm−1. The peak has been shown in the range of 1570–1550 cm−1 is related to stretch vibrations of strong C=C aromatic bond appeared in the wave number of 1543 cm−1. Whole spectrum found to be in good agreement with the results available in the literature [26]. Rectangular frame drawn in the graph shows the area of carboxylic peak; there is no peak for graph ‘a’ but there is a carboxylic peak for graph ‘b’. This is demonstration of good oxidation and modification of commercial nanocarbon black using mentioned acids.
3.2 Flame atomic absorption spectroscopy (FAAS)
Flame atomic absorption spectroscopy was used to determine the amount of coated silver on the surface of chemically modified nanocarbon black. The FAAS results showed that the amount of coated silver on chemically modified nanocarbon black are 25 and 50 wt% depending on two different amount of silver nitrate solution concentration were used.
3.3 Scanning electron microscopy analysis (SEM)
To investigate the morphology of different types of nanocarbon blacks, SEM micrographs were conducted, and the morphology of these samples is shown in Fig. 3. In this figure, SEM images of commercial nanocarbon black with two different magnifications have been shown. In Fig. 3a, b, the commercial carbon black nanoparticles are in the form of big and spherical agglomerates. While, Fig. 3c, d show the SEM images of nanocarbon black that is chemically modified via acid and is also silver coated with two different magnifications show the modification and coating process has caused agglomerates to become smaller. Modification of nanocarbon black with both oxidizing acids and Ag coating leads to a similar degree of agglomeration in the particles. Acidic function groups are the reason of decreasing agglomeration surface area significantly, which causes better stability, distribution and joint of nanocarbon black in the matrix [26].
The cross-sectional morphology of prepared conductive nanocomposite adhesives and quality of Ag coated nanocarbon black dispersion into epoxy resin matric were conducted by SEM–EDXA measurements and the morphology of these adhesives is shown in Fig. 4. In this figure, the SEM images of cured epoxy resin, conductive nanocomposite adhesive with 20, 30 and 40 wt% of modified nanocarbon black that is coated with silver are given (samples with numbers of 7-2, 9-1 and 11-1 respectively in Table 2). It can be seen from Fig. 4a; the cross-section of the cured epoxy resin reveals the smooth and homogenous structure of adhesive with no conductive filler. Whereas, in the microstructure of conductive nanocomposite adhesive with 20 wt% of modified nanocarbon black that is coated with silver (CB 10% + Ag 10%) in Fig. 4b, the formation of conductive paths is not performed because of the particles in the matrix of epoxy resin are not enough. Density of nanocarbon black dispersion into epoxy resin matrix is low which leads to higher electrical resistance. In Fig. 4c agglomeration of conductive particles are completely visible and distribution density of particles is lower compared to previous samples. In this sample, it was able to create conductive paths and electrical resistance is decreased very well. Finally, Fig. 4d depicts the electrically conductive adhesive with 40 wt% of modified nanocarbon black that is coated with silver (CB 20% + Ag 20%). In this case, conductive paths are not adequate which is due to generation of agglomerates and distribution density of particles is not uniform which leads to higher electrical resistance compared to previous sample.
SEM images of a cured epoxy resin, b conductive nanocomposite adhesive with 20 wt% of modified nanocarbon black that is coated with silver (CB 10% + Ag 10%), c conductive nanocomposite adhesive with 30 wt% of modified nanocarbon black that is coated with silver (CB 15% + Ag 15%) and d conductive nanocomposite adhesive with 40 wt% of modified nanocarbon black that is coated with silver (CB 20% + Ag 20%)
Also, the dot-mapping of SEM images for two conductive nanocomposite adhesives with 30 and 40 wt% of Ag coated nanocarbon blacks performed and the distribution of different elements into polymer matrices is shown in Fig. 5. The elemental analysis of silver (Ag) and carbon (C) on the cross-section of the adhesive samples can facilitate better estimation of distribution of Ag coated nanocarbon black particles in the nanocomposite adhesive matrix. Figure 5a shows that the silver and carbon element have a very uniform distribution along the cross section of the conductive nanocomposite adhesive with 30 wt% of Ag coated nanocarbon black (CB 15% + Ag 15%) which indicates a good distribution of these particles in this nanocomposite epoxy resin matrix. This implies that Ag coated nanocarbon black particles are not recrystallized into large particles after incorporating with epoxy. But in the Fig. 5b the agglomeration formation of Ag coated nanocarbon blacks are seen in the epoxy resin matrix and then the lower resistivity of nanocomposite adhesive is resulted.
3.4 Measurement of electrical resistivity
Although electrical conductivity in conductive polymeric systems is measured usually by the four-probe method, however, we measured electrical conductivity of the conductive nanocomposites by using a different method. We used test samples described in preparation of the reference materials section for the electrical measurements. The method is usually used for the electrical measurements of the polymeric materials filled with conductive fillers. The possibility of using common electrical measurement tools such as an ohm-meter, ease of preparation of the test samples, and facile investigation of the conductivity–temperature behavior is among the advantages of the method. Table 3 summarizes the room temperature electrical and specific resistivity (R and ρ) measured for different conductive nanocomposite adhesives. The measured electrical properties in Table 3 for each sample four replications were performed, and the mean value of each sample is reported in Table 3.
As it can be seen in the Table 3, initially critical concentration and percolation threshold of chemically modified nanocarbon black are investigated to find the optimum value for silver because of the economical restrictions of silver. As conductivity is function of modified nanocarbon black, the data in Table 3 show that adhesive matrix is non-conductive when having low concentration of nanocarbon black. But it shows lower conductivity in critical concentration of carbon black (5 wt%). Conductive elements generate the conductive paths by physical contact or electron transport (tunneling). Percolation threshold was observed in the range of 15–20 wt%, where a little increment in nanocarbon black concentration leads to a high effect in conductivity. An increase in nanocarbon black content to more than percolation threshold (25 wt%) leads matrix to its conductivity paths and generation of conductivity network and decrease in resistance. After this range, nanocarbon black increment did not cause any high increase in conductivity. So, a decision was made to use silver in the percolation threshold range of nanocarbon black to increase conductivity [29]. Table 4 summarizes the room temperature electrical and specific resistivity (R and ρ) measured for different conductive nanocomposite adhesives filled with Ag coated nanocarbon blacks. The measured electrical properties in Table 4 for each sample four replications were performed, and the mean value of each sample is reported in Table 4.
As it can be seen in Table 4, by increasing conductive nanofiller content into epoxy resin matrix, the electrical conductivity of adhesive samples slightly increased but not satisfied and not the value of our expectation. The adhesive sample containing 30 wt% conductive nanofiller (CB 15% + Ag 15%) resulted in desired electrical conductivity, because the well distribution of filler and generation of conductivity bridges between non-binding particles are the reason of this decrease in resistance, which decreases tunneling resistance in electrically conductive adhesive matrix. Also, more increment of conductive filler percentage in the adhesive sample with 40 wt% (CB 20% + Ag 20%) resulted in more agglomeration of filler particles and the mechanical and electrical properties of nanocomposite adhesive decreases. Also, by increasing the filler percent, preparation of electrically conductive adhesive encounters some problems like rapid drying, not a uniform distribution and difficult molding process [29]. Figure 6 shows the effect of silver coating on chemically modified nanocarbon black surface on electrical conductivity of conductive nanocomposite adhesives in comparison with the adhesives with no coating. As it can be seen in Fig. 6, the positive effect of silver coating on increasing electrical conductivity and decreasing resistivity is proved.
3.5 Lap shear strength analysis of electrically conductive adhesive
Lap shear strength of electrically conductive adhesive was measured after preparation of samples using universal mechanical test equipment. Samples used in this test contained cured resin epoxy, electrically conductive adhesive with 15 wt% of modified nanocarbon black and electrically conductive adhesive with 30 wt% of modified nanocarbon black, coated with silver (CB 15% + Ag 15%). The results of lap shear strength analysis are reported in Fig. 7. Based on data, lap shear strength of pure resin epoxy is 12.19 MPa. Lap shear strength decreases to 6.51 MPa as result of increase in conductive filler and weakening of epoxy matrix due to weak interaction of filler and epoxy resin, are inevitable. Increment of conductive filler along with decrease of resin wt% and agglomeration, shear strength was decreased to 6.77 MPa which is in good agreement with the results available in the literature [30]. The high concentration of filler causes a decrease of the mechanical properties in terms of toughness, causing a rigid failure in the joint. The values were comparable to the lap shear strength values of commercial adhesives.
3.6 Thermal gravimetric analysis (TGA)
To study the effect of nanocarbon black and Ag coated modified nanocarbon black addition into epoxy resin in conductive nanocomposite adhesives, thermal gravimetric analysis of these samples was carried out. The TGA thermograms of neat epoxy resin, conductive nanocomposite adhesive with 15 wt% of chemically modified nanocarbon black and conductive nanocomposite adhesive with 30 wt% of Ag coated modified nanocarbon black (CB 15% + Ag 15%) as shown in Fig. 8. It was observed that epoxy resin starts to decompose at 200 °C and it completely degrades at 570 °C. Weight loss percentage for resin epoxy was 88.71%. Addition of chemically modified nanocarbon black to epoxy resin (15 wt% CB) causes a thermal decomposition which occurs at temperature of 340 °C and weight loss percentage for this sample is 71.2%. Electrically conductive adhesive containing 30 wt% of Ag coated modified nanocarbon black (CB 15% + Ag 15%) starts to thermally decompose at temperature of 340 °C. Weight loss percentage for this sample is 51.31%. The TGA thermograms show that better thermal stability of nanocomposite adhesive compared to neat epoxy resin adhesive due to the introduction of nanofiller into polymer matrix. It is obvious from the graphs that the degradation of epoxy resin adhesive has a sharp slope compared to the nanocomposite adhesives which implies that the thermal stability of nanocomposite adhesives have improved than neat epoxy resin adhesive [31].
4 Conclusions
In this work, nanocomposite adhesive based on the epoxy resin filled with nanocarbon black filler, chemically modified and Ag coated nanocarbon blacks were prepared and their thermal and electrical properties were studied. FT-IR analysis showed good oxidation and modification of commercial nanocarbon black using acids and this lead to better stability of nanocarbon black in epoxy resin matrix. SEM–EDXA analysis shows that the silver and carbon element have a very uniform distribution along the cross section of the conductive nanocomposite adhesive matrix. Flame atomic absorption spectroscopy showed that the amount of 25 and 50 wt% metallic silver has been coated on the surface of chemically modified nanocarbon black. Conductivity measurements depicted that the proper concentration of conductive filler is 30 wt% of Ag coated chemically modified nanocarbon black (CB 15% + Ag 15%) has a best specific resistivity of 0.0364 Ω cm. The results of lap shear strength test showed that the increase of conductive filler cause a reduction in of lap shear strength adhesive. Lap shear strength of adhesive with 30 wt% of Ag coated chemically modified nanocarbon black (CB 15% + Ag 15%) is 6.77 MPa, which is acceptable compared to commercially adhesives. The results of thermogravimetric analysis show an increment of thermal stability in nanocomposite samples. Weight loss and degradation start temperature of electrically conductive adhesive were reduced and increased significantly.
References
Z. Czech, A. Kowalczyk, R. Pełech, R. Wróbel, L. Shao, Y. Bai et al., Using of carbon nanotubes and nano carbon black for electrical conductivity adjustment of pressure-sensitive adhesives. Int. J. Adhes. Adhes. 36, 20–24 (2012)
H. Ma, M. Ma, J. Zeng, X. Guo, Y. Ma, Hydrothermal synthesis of graphene nanosheets and its application in electrically conductive adhesives. Mater. Lett. 178, 181–184 (2016)
X. Nana, L. Zhiling, X. Hui, Z. Yuzhen, W. Yuehui, L. Jingze, Influence of curing procedures on the electrical properties of epoxy-based isotropic conductive adhesives. Rare Met. Mater. Eng. 45, 2524–2528 (2016)
J.C. Jagt, Reliability of electrically conductive adhesive joints for surface mount applications: a summary of the state of the art. IEEE Trans. Compon. Packag. Manuf. Technol. A 21, 215–225 (1998)
S. Vons, Q.K. Tong, R. Kuder, D. Shenfield, Surface mount conductive adhesives with superior impact resistance,” in Advanced Packaging Materials, 1998. Proceedings. 1998 4th International Symposium on, 1998, pp. 261–267
M. Zwolinski, J. Hickman, H. Rubin, Y. Zaks, S. McCarthy, T. Hanlon et al., Electrically conductive adhesives for surface mount solder replacement. IEEE Trans. Compon. Packag. Manuf. Technol. C 19, 241–250 (1996)
J. Liu, K. Gustafsson, Z. Lai, C. Li, Surface characteristics, reliability, and failure mechanisms of tin/lead, copper, and gold metallizations. IEEE Trans. Compon. Packag. Manuf. Technol. A 20, 21–30 (1997)
J. Luo, Z. Cheng, C. Li, L. Wang, C. Yu, Y. Zhao et al., Electrically conductive adhesives based on thermoplastic polyurethane filled with silver flakes and carbon nanotubes. Compos. Sci. Technol. 129, 191–197 (2016)
S.K. Bhudolia, P. Perrotey, S.C. Joshi, Enhanced vibration damping and dynamic mechanical characteristics of composites with novel pseudo-thermoset matrix system. Compos. Struct. 179, 502–513 (2017)
M. Lutz, R. Cole, High performance electrically conductive silicone adhesives. Microelectron. Int. 7, 27–30 (1990)
J. Pujol, C. Prud’homme, M. Quenneson, R. Cassat, Electroconductive adhesives: comparison of three different polymer matrices. Epoxy, polyimide and silicone. J. Adhes. 27, 213–229 (1989)
J. Gonzales, M. Mena, Moisture and thermal degradation of cyanate-ester-based die attach material, in Electronic Components and Technology Conference, 1997. Proceedings., 47th, 1997, pp. 525–535
I.Y. Chien, M. Nguyen, Low stress polymer die attach adhesive for plastic packages, in Electronic Components and Technology Conference, 1994. Proceedings., 44th, 1994, pp. 580–584
D.P. Galloway, M. Grosse, M.N. Nguyen, A. Burkhart, Reliability of novel die attach adhesive for snap curing, in Electronics Manufacturing Technology Symposium, 1995.’Manufacturing Technologies-Present and Future’, Seventeenth IEEE/CPMT International, 1995, pp. 141–147
X. Kong, Z. Xu, L. Guan, M. Di, Study on polyblending epoxy resin adhesive with lignin I-curing temperature. Int. J. Adhes. Adhes. 48, 75–79 (2014)
K. Gilleo, Assembly with conductive adhesives. Solder. Surf. Mt. Technol. 7, 12–17 (1995)
P.B. Jana, S. Chaudhuri, A. Pal, S. De, Electrical conductivity of short carbon fiber-reinforced polychloroprene rubber and mechanism of conduction. Polym. Eng. Sci. 32, 448–456 (1992)
A. Malliaris, D. Turner, Influence of particle size on the electrical resistivity of compacted mixtures of polymeric and metallic powders. J. Appl. Phys. 42, 614–618 (1971)
G. Ruschau, S. Yoshikawa, R. Newnham, Resistivities of conductive composites. J. Appl. Phys. 72, 953–959 (1992)
G. Xiao, E. Liu, T. Jin, X. Shu, Z. Wang, G. Yuan et al., Mechanical properties of cured isotropic conductive adhesive (ICA) under hygrothermal aging investigated by micro-indentation. Int. J. Solids Struct. 122, 81–90 (2017)
M. Erinc, M. Van Dijk, V. Kouznetsova, Multiscale modeling of residual stresses in isotropic conductive adhesives with nano-particles. Comput. Mater. Sci. 66, 50–64 (2013)
Y. Li, K. Moon, C. Wong, Electronics without lead. Science 308, 1419–1420 (2005)
Y. Li, C. Wong, Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: materials, processing, reliability and applications. Mater. Sci. Eng. R: Rep. 51, 1–35 (2006)
J. Lau, C. Wong, N.C. Lee, S.R. Lee, Electronics Manufacturing with Lead-Free Halogen-Free, and Conductive-Adhesive Materials (McGraw Hill, New York, NY, 2002)
J. de Vries, J. Caers, Anisotropic conductive adhesives in electronics. Adv. Adhes. Electron.: Mater. Prop. Appl. 53, (2011)
D. Borah, S. Satokawa, S. Kato, T. Kojima, Characterization of chemically modified carbon black for sorption application. Appl. Surf. Sci. 254, 3049–3056 (2008)
X. Peng, F. Tan, W. Wang, X. Qiu, F. Sun, X. Qiao et al., Conductivity improvement of silver flakes filled electrical conductive adhesives via introducing silver–graphene nanocomposites. J. Mater. Sci.: Mater. Electron. 25, 1149–1155 (2014)
S.J. Peighambardoust, B. Pourabbas, Preparation and chracterization of nylon-6/PPy/MMT composite of nanocomposites. J. Appl. Polym. Sci. 106, 697–705 (2007)
W. Zhang, R.S. Blackburn, A. Dehghani-Sanij, Electrical conductivity of epoxy resin–carbon black–silica nanocomposites: effect of silica concentration and analysis of polymer curing reaction by FTIR. Scr. Mater. 57, 949–952 (2007)
A. Zandiatashbar, R. Picu, N. Koratkar, Mechanical behavior of epoxy-graphene platelets nanocomposites. J. Eng. Mater. Technol. 134, 031011 (2012)
M. Atif, Surface modification and characterization of carbon black; UV cured colored epoxy composites, Politecnico di Torino, 2014
Acknowledgements
The authors are grateful for the financial support of “Iran Nanotechnology Initiative Council”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masaebi, N., Peighambardoust, S.J. & Ahadzadeh, I. Electrically conductive nanocomposite adhesives based on epoxy resin filled with silver coated nanocarbon black. J Mater Sci: Mater Electron 29, 11840–11851 (2018). https://doi.org/10.1007/s10854-018-9284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9284-9