Abstract
We report on scalable, time saving, and cost effective method to synthesize core–shell based nanodielectrics for energy storage applications. Nanodielectric films were fabricated by embedding highly ferroelectric β-phase polyvinilydine fluoride (PVDF) polymer with high electrical conductivity alumina coated aluminum nanoparticles. Al-Al2O3 core–shell nanoparticles were successfully synthesized through a simple, cost effective, and scalable blending procedure in PVDF to yield high performance energy storage capacitors. The results on electrical permittivity values, structural morphology and phases change with thermal annealing, thermal and frequency response of capacitance, dielectric strength, and dielectric loss of the produced films with different loadings of core–shell nanoparticles are presented. The dielectric permittivity K of the nanocomposite is found to increase from 12 for pure PVDF polymer to a value of 23.6 when PVDF was embedded with a 20% volume loading of Al-Al2O3 core–shell nanoparticles. Electrical characterization showed reproducible and stable capacitance values of 10–25 nF/in2 over frequencies of up to 10 MHz as well as an increase in capacitance with temperature below 70 °C, and a decrease thereafter. Breakdown voltage for the Al-Al2O3/PVDF composite films reached 70 V/µm at a loading of 10% and decreased to 63 V/µm for 20% loading films. Results show that proper loadings (10–20%) of engineered oxidized aluminum (Al-Al2O3) in PVDF provide high permittivity low loss nanodielectrics on par with commercially leading dielectrics manufacturer (3M C-Ply) along with the added structural flexibility and cost-saving to the end user.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Every generation of capacitors are primarily credited to the innovation and engineering of new dielectric materials. Dielectric materials are categorized into organic polymers, inorganic ceramics, filled and unfilled polymeric thin films, ceramic films and nanodielectric composites. Nanodielectric composites belong to a new type of materials engineered for improved functions, performance for dielectrics, and electrical insulation.
High electrical permittivity (k) materials have received a tremendous interest recently due to their potential applications in high capacitive gate dielectrics, field effect transistors, actuators, and photonic devices [1,2,3,4,5]. Extensive interest is being invested into the research of polymer based nanocomposites films [6,7,8,9,10,11,12,13,14,15]. Such a material provides a more practical energy storage solution primarily for flexible electronics [16]. The amount of stored energy depends on the nature of materials and polarizability of elaborated nanocomposites. It can be increased substantially by addition of nanoparticles to the host polymer which can result in better electrical, mechanical, and thermal properties of the nanocomposites.
Achieving permittivity values K > 100 is difficult even with the mature ceramic-filled polymer technology. Experimentally, relative K values of 70 are considered excellent for polymer/ferroelectric composites. The dielectric performance of the nanocomposites can only be improved through highly dispersion of the nanoparticles. Conventional mixing techniques cannot break-up nanoparticles due their easy agglomeration as a result of high surface energy. Therefore, a key issue to achieving higher performance nanodielectrics is by making well dispersed nanoparticles in the host polymer.
The commonly used metal particles are silver and aluminum with usual size in the order of 1 µm. Epon 58034 (resin) and bispheno-A epoxy (DER 661) (polymer) are commercially available from the Shell company. However, the use of micro-size fillers impacts negatively the dielectric strength of polymeric materials by creating local charging, known as Maxwell–Wagner interfacial polarization effects at the micro-clusters sites [17]. Nanosize fillers are predicted to enhance the dielectric strength as opposed to micro-fillers. Experimental results suggest that when the particle size becomes comparable to the size of polymer chain, the particles act cooperatively with the host matrix and mitigate the internal charge creation [18]. The interface changes both the magnitude and distribution of the internal charge and gives rise to an interaction zone called diffuse layer, which affects the interfacial polarization through the formation of local conductivity.
This research paper reports on synthesis and characterization of high permittivity core–shell metal/ferroelectric polymers for use in energy storage capacitors. Polyvinilydine fluoride or Polyvinilydine difluoride (PVDF) is being considered as dielectric in energy storage capacitors. PVDF is highly non-reactive and resistant to solvents, acids, bases, thermal heat, and has low smoke generation during a fire event. It also has a low density (1.78) and costs less compared to other fluoropolymers. Strong piezoelectricity was observed in PVDF, with the piezoelectric coefficient of poled (placed under a strong electric field to induce a net dipole moment) thin films as large as 6–7 pC/N: 10 times larger than that observed in any other polymer. In this paper, we used PVDF as a polymer matrix and Al/Al2O3 core–shell nanoparticles for producing high dielectric composite materials.
PVDF exists in four different crystalline phases: α, β, γ and δ. It displays such diverse crystalline phases depending on crystallization conditions. The non-polar α phase is the common phase, which is obtained by melt crystallization at temperatures above 120 °C. The β-phase is the polar desirable phase due to its ferroelectric characteristics, and hence it is important to maximize β-phase in PVDF films used as dielectrics. The γ and δ are polar phases, but the magnitude of their dipole moment is much smaller than that of β-phase. Thus, the PVDF polymer containing β-phase has garnered the most interest for electronic applications as it can provide the best ferroelectric properties. It is also possible to extract β-phase from other phases by varying crystallization temperature and structural deformation [19].
The concept of putting metal nanoparticles instead of ceramics comes up because metals can, in principle, be thought of as the limiting case of high permittivity particles [17, 20,21,22,23]. We have shown during a modeling and simulation study that k of the nanocomposite increase considerably with increasing metal nanoparticle loading below the percolation threshold of 16% [20]. In order to increase the energy storage capability without compromising the inter-particle spacing, we make core–shell capacitors uniformly distributed in the host polymeric films. These improved core–shell embedded devices should allow for storage of a large amount of charge per unit volume that can be released rapidly on demand. The present work shows that nanocomposites made of low loading of metal nanoparticles (~ 60 nm) fillers into polymers such as PVDF is a forceful and influential concept for producing high electric permittivity films with a minimum dielectric loss possible. We tested the elaborated nanocomposite into standard macroscale capacitors for energy storage devices.
2 Experimental section
2.1 Materials and sample preparation
It has been reported that PVDF films derived using N,N-Dimethylformamide (DMF) polar solvent have a polar β-phase [19]. Such β-phase PVDF films were used here in the experimental procedure to prepare polymer and nanoparticle solutions. Post-processing is one of the key stages in fabrication as it plays an important role in deciding the material characteristics of the dielectric. Post-processing a PVDF dielectric increases its breakdown strength and decreases the dielectric loss. This post-processing involves heating the spin-coated dielectric to a temperature that allows PVDF to crosslink. As part of this work PVDF samples were cured at curing temperatures over a range of 30–120 °C for 10 min.
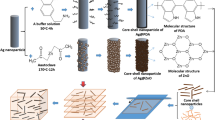
The blending procedure for making Al-Al2O3 core–shell-PVDF nanodielectric is shown in Fig. 1. Aluminum nanoparticles used in the experiments are manufactured by American Elements Inc [24]. These nanoparticles were thermally oxidized to form an Al2O3 capping shell on the metallic nanoparticle (Al-Al2O3) for electrical insulation as shown on Fig. 2. This eliminates the need of a functionalization procedure to form an insulating shell around the metal aluminum core. The solid oxide shell around the Al core prevents agglomeration of Al nanoparticles. In the event where two core-shells interact with each other, our simulation results has shown a drop in the net polarization at the interface region [20]. Furthermore, the shell-isolated structure enhances the thermal and chemical stability of the nanoparticles, improves solubility and reactivity as well as allows conjugation of other molecules to these particles. The important step in determining the characteristics of the nanodielectric is the mixing of the PVDF and Al-Al2O3 nanoparticles in DMF (Dimethylformamide) solutions in proper proportions as the capacitance of the film coated using Al-Al2O3 nanoparticles and PVDF depends on the concentration of nanoparticles (also termed as loading) in the dielectric. A solution of Al-Al2O3 nanoparticles in DMF solvent was stirred and sonicated to completely disperse the nanoparticles. A second solution of PVDF polymer in DMF solvent was made by stirring and heating for few hours. Next, these two solutions were slowly mixed together and stirred for a few hours on a magnetic stirrer. Finally, the mixed solution was sonicated for 2–5 min by a high power Misonix sonicator to break-up any agglomerates. A small amount of mixture, approximately 0.2 ml, was used to make thin Al-Al2O3/PVDF films on a quarter section of a 2-inch silicon wafer (Si) using a Laurell Technologies WS-400 spinner followed by thermal curing on a hotplate. Prior to casting and curing the nanocomposite on silicon substrates, we first applied a thin layer of aps (3-aminopropyl triethoxysilane) solution to enhance the interfacial adhesion of PVDF [25].
2.2 Fabrication and characterization
The steps in making a capacitor device using synthesized nanodielectrics are shown in Fig. 3. Prior to spin coating the nanocomposite, the Si wafer substrate is cleaned to remove grease and surface dust. Trichloroethylene, acetone and methanol are used in similar volumes to clean the wafer. 10 ml of each of these solvents are put into a beaker with the Si wafer. This is sonicated in an ultrasound sonicator bath for 1 min with the substrate then dried on a hotplate at 120 °C for 1 min. Finally, the wafer is rinsed with deionized water and blown with dry nitrogen gas. The bottom aluminum (Al) electrode of the planar capacitor was deposited by using a thermal evaporator. Al top electrodes were deposited through a mechanical mask with 3 mm × 3 mm openings which correspond to the actual size of the tested capacitors. We fabricated one capacitor array using unloaded PVDF as a reference sample and two capacitors arrays with 10 and 20% volume loadings of Al-Al2O3 fillers in PVDF host polymer. The three types of devices were then characterized for their capacitance characteristics using an HP-4275A high frequency LCR meter.
The LCR meter was attached to the probe station which has two positioners connected to the top and bottom electrodes, respectively. Current–voltage measurements were obtained using a Keithley 6487 Pico ammeter/voltage source controlled by LabVIEW software to supply the input voltage and to read the output current. The leakage current and corresponding applied voltage till film breakdown were recorded and plotted. The thickness of the polymer was determined from the nanocomposite cross section using an FEI XL-30FEG scanning electron microscope (SEM).
3 Results and discussion
The high resolution SEM micrograph in Fig. 4 shows a high dispersion of 10% volume loading of Al core–shell nanoparticles in PVDF matrix. The size of Al nanoparticles varies between 60 and 100 nm and the Al2O3 shell is about 15 nm thick as depicted by the inset picture. X-ray diffraction analysis was carried out on nanocomposite samples in order to identify the phase of PVDF polymer and to study the aluminum and alumina materials structure. The XRD peaks in Fig. 5 and which are positioned at diffraction angles (2θ) of 20.1° and 20.3° confirm the presence of γ (110), and β (200,110) phases of PVDF [17]. The other XRD peaks at 38.52°, 44.67°, 65.13°, 78.27°, 28.56°, 26.64°, 34.67° identify the presence of Al (111), Al (200), Al (220), Al (211) [26], Si (111) [27], α-Al2O3 (012), and α-Al2O3 (104) [28], respectively. Based on these results, it was determined that cured samples at 120 °C for 10 min showed β and γ phases in the composite. The β-phase has the highest share and it is of great interest due to its ferroelectric nature as stated above. Therefore, identical curing process was performed on all samples associated with this work. Regarding the crystallinity of the shell, it was found to be α-phase Al2O3 (Fig. 5), a well-known material with high dimensional stability. Figure 6 shows the surface morphology of Al-Al2O3/PVDF composite films which were spin coated at 2000 RPM and thermally treated in the range of 30–120 °C.
Table 1 reports the specifications of fabricated capacitors as well as the average value parameters of tested capacitors at a frequency of 10 kHz. The dielectric loss is reduced as Al nanoparticles loading is increased in the nanocomposite. This is most likely due to enhancement of electrical conductivity right at the interface between both top and bottom aluminum electrodes and the nanocomposite. The permittivity of the bare PVDF is about 12 and it doubles in value for a volume loading of 20%. Figure 7 shows the frequency behavior of typical capacitors having 3 mm × 3 mm Al electrodes. Each reading of the capacitance represents the average value of measurements taken on an array of five capacitor devices. For comparison purpose, the capacitance values are normalized to the same film thickness of 6300 nm. Figure 7 indicates a very small variation (0.3–1.1%) of capacitance over 10 kHz–10 MHz frequency range.
Table 2 shows the embedded dielectric characteristics of fabricated capacitors in comparison with existing commercial ones. The leading commercial dielectric under 3M trade mark “C-Ply” is made of an epoxy/BaTiO3 composite. Such polymer-ceramic composites combine the processability of the polymer with the high electrical permittivity of the ceramics. However, if the dielectric constant of the polymer-ceramic composite has to be maintained high then it has to be heavily doped with ceramic fillers (about 80%) which unfortunately is detrimental to the composite’s mechanical performance and sometimes to its electromechanical reliability [29, 30]. For Al-Al2O3/PVDF composites, results show that proper loading (10–20%) of engineered oxidized aluminum nanoparticles in PVDF provide high permittivity, low loss nanodielectrics on par with commercially available capacitor devices along with the added structural flexibility and cost-saving to the end user.
Figure 8 shows the variation in capacitance with loading over a temperature range up to 170 °C for the same characterized capacitors in Fig. 7. The capacitance value showed steady response with temperature below 70 °C, and diminished thereafter, for different loadings of Al-Al2O3 core–shell nanoparticles. However, the capacitor with the high loading (20%) showed a drastic fall in capacitance when temperature goes beyond 70 °C. This phenomenon was found to be reversible i.e., capacitors retrieved their original capacitance values when temperature was brought down to room temperature. This could be related to an increase in the electrical contact resistance at the interface between both top and bottom aluminum electrodes and the nanocomposite. The change of resistance at 20% loading may be attributed to high number of core–shell nanoparticles in the diffuse layer, which affects the interfacial polarization through the formation of local conductivity. In this case, we believe as the temperature increases above 70 °C, there might be an air gap that separated the capacitor electrodes providing areas of isolation and which results in poor electrical contacts. Figure 9 shows the breakdown voltage (BDV) measurements of Al-Al2O3/PVDF capacitor structures at different loadings. The breakdown voltage of the reference host polymer was about 50 V/µm at a standard leakage current of 1 µA. The dielectric strength reached 70 V/µm at a loading of 10% and decreases to 63 V/µm as the loading increases to 20%.
Dependence of capacitance on temperature for the capacitors of Fig. 7
4 Conclusions
Embedding metallic nanoparticles into polymers is emerging as an effective approach for producing high permittivity films. Al-Al2O3 core–shell nanoparticles were successfully synthesized through a simple, cost effective, and scalable blending procedure in a PVDF polymer matrix to yield high performance energy storage capacitors. Analysis revealed a uniform nanodielectric film thickness which can be controlled by varying the concentration of the PVDF polymer solution and also showed a principally ferroelectric β-phase for the PVDF matrix. Different loadings of Al-Al2O3 nanoparticles were embedded into PVDF polymer, the dielectric permittivity K of the nanocomposite increased from 12 for pure PVDF polymer to a value of 23.6 when PVDF was embedded with a 20% volume loading of Al-Al2O3 nanoparticles. Electrical characterization showed reproducible and stable capacitance values of 10–25 nF/in2 over frequencies of up to 10 MHz as well as an increase in capacitance with temperature below 70 °C, and a decrease thereafter. Breakdown voltage for the Al-Al2O3/PVDF composite films reached 70 V/µm at a loading of 10% and decreased to 63 V/µm for the 20% loading films. We conclude that entraining metallic nanoparticles into polymers to make nanodielectric films is a viable concept for producing high permittivity material at low metal nanoparticles loading. Results show that proper loadings (10–20%) of engineered oxidized aluminum (Al-Al2O3) in PVDF provide high permittivity low loss nanodielectrics on par with commercially leading dielectrics films along with the added structural flexibility and cost-saving to the end user. Due to the low loading of nanoparticles, capacitors fabricated with this technique retain the advantages of polymers while displaying an increased capacitance density.
References
A. Mahadevegowda, N.P. Young, P.S. Grant, Engineering the nanostructure of a polymernanocomposite film containing Ti-based core-shell particles to enhance dielectric response. Nanoscale. 7, 15727 (2015)
Y.J. Li, X. Xiong, C.L. Zou, X.F. Ren, Y.S. Zhao, One-dimensional dielectric/metallic hybrid materials for photonic applications. Small. 11, 3728 (2015)
S.K. Saha, Nanodielectrics with giant permittivity. Bull. Mater. Sci. 31, 473 (2008)
J.Y. Li, L. Zhong, S. Duchame, Appl. Phys. Lett. 90, 132901 (2007)
J. Lu, C.P. Wong, Recent advances in high-k nanocomposite materials for embedded capacitor applications. IEEE Trans. Dielectr. Electr. Insul. 15, 1322 (2008)
C. Tang, G. Long, X. Hu, K.W. Wong, W.M. Lau, M. Fan, J. Mei, T. Xu, B. Wang, D. Hui, Conductive polymer nanocomposites with hierarchical multi-scale structures via self-assembly of carbon- nanotubes on graphene on polymer-microspheres. Nanoscale. 6, 7877 (2014)
G. Keledi, J. Hári, B. Pukánszky, Polymer nanocomposites: structure, interaction, and functionality. Nanoscale. 4, 1919 (2012)
L. Zhu, Q. Wang, Novel ferroelectric polymers for high energy density and low loss dielectrics. Macromolecules. 45, 2937 (2012)
J. Ho, R. Jow, Characterization of high temperature polymer thin films for power conditioning capacitors, DTIC Document (2009)
Q. Chen, Y. Shen, S. Zhang, Q. Zhang, Polymer-based dielectrics with high energy storage density. Annu. Rev. Mater. Res. 45, 433–458 (2015)
S. Wu, W. Li, M. Lin, Q. Burlingame, Q. Chen, A. Payzant, K. Xiao, Q. Zhang, Aromatic polythiourea dielectrics with ultrahigh breakdown field strength, low dielectric loss, and high electric energy density. Adv. Mater. 25, 1734 (2013)
M. Sindu Shree, H. Schulz-Senft, N.H. Alsleben, Y.K. Mishra, A. Staubitz, R. Adelung, Light, force, and heat: a multi-stimuli composite that reveals its violent past. ACS Appl. Mater. Interfaces. 9, 38000 (2017)
Y.K. Mishra, R. Adelung, ZnO tetrapod materials for functional applications. Mater. Today. (2018). https://doi.org/10.1016/j.mattod.2017.11.003
J.N. Ansari, S. Khasim, A. Parveen, O.A. Al-Hartomy, Z. Khattari, N. Badi, Synthesis, characterization, dielectric and rectification properties of PANI/Nd2O3: Al2O3 nanocomposites. Polym. Adv. Technol. 27, 1064–1071 (2016)
Z.M. Dang, M.S. Zheng, J.W. Zha, (2016) 1D/2D carbon nanomaterial-polymer dielectric composites with high permittivity for power energy storage applications. Small 12, 1688
J.K. Nelson, J.C. Fothergill, Internal charge behavior of nanocomposites. Nanotechnology. 15, 586–595 (2004)
T.J. Lewis, Interfaces: nanometric dielectrics. J. Phys. D. 38, 202 (2005)
S. Sataparthy, S. Pawar, P.K. Gupta, K.B.R. Varma, Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. Bull. Mater. Sci. 34, 727 (2010)
P. Barber, S. Balasubramanian, Y. Anhuchamy, S. Gong, A. Wibowo, H. Gao, H.J. Plochn, H.Z. Loye, Polymercomposite and nanocomposites dielectric, materials for pulse power energy storage, Materials. 2, 1697 (2009)
R. Mekala, N. Badi, Modeling and simulation of high permittivity core-shell ferroelectric polymers for energy storage solutions. COMSOL Conference, Boston, 9–11 October 2013
R. Bikky, N. Badi, A. Bensaoula, Effective medium theory of nanodielectrics for embedded energy storage capacitors. COMSOL Conference, Boston. 7–9 October 2010
D.M. Ekanath, N. Badi, A. Bensaoula, Modeling and simulation of artificial core-shell based nanodielectrics for electrostatic capacitors applications. COMSOL Conference, Boston. 13–15 October 2011
N. Badi, A. Benqaoula, A.V. Simakin, G.A. Shafeev, Laser engineered core–shell nanodielectrics with giant electrical permittivity. Mater. Lett. 108, 225–227 (2013)
W.T. Whang, W.H. Cheng, A Study on Interfacial Adhesion of Poly(vinylidene fluoride) with Substrates in a Multilayer Structure (Society of Plastic Engineers Inc., Bethel, 2002)
(1998) http://www.omicsonline.org/scientific-reports/srep385.php
M. Zervos, D. Tsokkou, M. Pervolaraki, A. Othonos, Low temperature growth of In2O3 and InN nanocrystals on Si(111) via chemical vapour deposition based on the sublimation of NH4Cl in In (2001)
A. Bourbia, S. Boulkhessaim, H. Bedboudi, M. Draissia, Phase transformation in rapidly solidified Al-Al2O3 alloys by high-frequency melting. Phys. Scripta. 85, 055601 (2002)
C. Yang, Y. Lin, C.W. Nan, Modified carbon nanotube composites with high dielectric constant, low dielectric loss and large energy density. Carbon. 47, 1096 (2009)
H. Liu, Y. Shen, Y. Song, C.W. Nan, Y. Lin, X. Yang, Carbon nanotube array/polymer core/shell structured composites with high dielectric permittivity, low dielectric loss, and large energy density. Adv. Mater. 23, 5104 (2011)
Acknowledgements
The authors would like to acknowledge financial support for this work, from the Deanship of Scientific research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under Grant No. S-0214/1438.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. NB planned this study and carried out all electrical characterizations, data analysis, and plotting. RM prepared all figures. SK wrote the manuscript. ASR prepared the SEM samples and performed analysis. AI read and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Badi, N., Mekala, R., Khasim, S. et al. Enhanced dielectric performance in PVDF/Al-Al2O3 core–shell nanocomposites. J Mater Sci: Mater Electron 29, 10593–10599 (2018). https://doi.org/10.1007/s10854-018-9123-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9123-z