Abstract
Long afterglow phosphors of Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ and Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ were successfully synthesized via the sol–gel method. The phase compositions and luminescent properties of the phosphors were analyzed by X-ray diffraction (XRD), fluorescence spectra and decay curves. Compared with the undoped phosphor, the crystallinity of Er3+ co-doping phosphors is decreased. Luminescence spectra show the main peak of excitation was 274, 356 nm and the main emission peak was 467 nm. Furthermore, Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ show excellent luminescent properties, and its initial luminous intensity increased by 1.4 times than Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+. The mechanism of Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ enhancement has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For more than a decade now, Sr2MgSi2O7: Eu2+, Dy3+ has become a concern for long afterglow phosphors, because of its advantages of luminescent properties [1,2,3,4,5,6,7,8]. In 2005, Alvani et al. [9] successfully prepared Sr2MgSi2O7: Eu2+, Dy3+ via high temperature solid phase method, and discussed the performance and structure of the phosphor in detail. Subsequently, Sr2MgSi2O7: Eu2+, Dy3+ phosphors have been research to preparation methods and doping rare earth elements by many researchers [10,11,12,13,14]. There are two important research aspects: doping rare earth elements and preparation methods.
An important factor of the luminescent to long afterglow phosphors is the doping type and doping concentration of rare earth elements. The luminescence properties of the Sr2MgSi2O7 phosphors can be created by doping rare earth ions. The doping type and concentration of rare earth elements are both important factors to phosphors of luminescent properties. Sahu et al. [15] successfully prepared Eu2+ and Ce2+ co-doping Sr2MgSi2O7 phosphors via high temperature solid-state reaction. They focus on the effect of the Eu2+ and Ce3+ ion radius to decay curve. Wu et al. [16] synthesized the Eu2+ and Er3+ co-doping Sr2MgSi2O7 phosphors with high temperature solid-state reaction. The results show that the afterglow time in Sr2MgSi2O7: Eu2+, Er3+ is than Sr2MgSi2O7: Eu2+, Er3+ is 1.2 times stronger than that in Sr2MgSi2O7: Eu2+. In addition to the two rare earth ions co-doping Sr2MgSi2O7 phosphors, Song et al. [17] also attempted that three rare earth ions of Eu2+, Dy3+ and Nd3+ co-doping Sr2MgSi2O7 phosphors.
Although the advantages of high temperature solid-state reaction possesses are less synthesis steps and simple operation, it restricts development to high sintering temperature and unstable luminescent properties. Furusho et al. [18] researched the effect of different sintering temperatures on the luminescent properties. The result indicated the high temperature will influence the structure and phase purity of the Sr2MgSi2O7 phosphors. Then, the researchers tried to reduce the synthetic temperature for improving the crystal structure and luminescent properties of the Sr2MgSi2O7 phosphors by different preparation methods. Pan et al. [19] successfully synthesized Sr2MgSi2O7: Eu2+, Dy3+ phosphors via co-precipitation method. The solid phase reaction can be completed at 1000 °C, indicating the particle size was uniform and the average particle size was about 1 µm. The Sr2MgSi2O7: Eu2+, Dy3+ phosphors [17] was synthesized via combustion method with average particle size of about 20 nm. Zhang et al. [20] successfully prepared Sr2MgSi2O7: Eu2+, Dy3+ phosphors via sol–gel method. This method has obvious advantages for preparing phosphors, such as a lower synthetic temperature and a fine powder.
In this paper, Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ and Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ phosphors were successfully prepared via sol–gel method, and the luminescent properties were improved. Er3+ ions are doped into Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ lattice improve the persistent luminescence due to the formation of exceedingly dense trapping levels situated at appropriate depth. Furthermore, the effects of and Er3+ on the phase compositions and luminescent properties of Sr2MgSi2O7: Eu2+, Dy3+ phosphor were also discussed. The mechanism of Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ enhancement has been discussed.
2 Materials and methods
2.1 Preparation of the Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ (SMSED) and Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ (SMSED) phosphors
The SMSED and SMSEDE samples were prepared via sol–gel method. The rawmaterials Sr(NO3)2, Mg(NO3)2·6H2O and H2BO3 were dissolved in H2O to form solution A ,B and C, and the H3BO3 content was 7.5% of Sr2MgSi2O7. The Dy2O3, Eu2O3 and Er2O3 was dissolved in 3.6M HNO3 to form solution D,E and F. The Si(OC2H5)4 was dissolved in absolute ethylalcohol to form solution G. All solutions were fully dissolved under mechanical stirring, respectively. The solutions of B, C, D, E, F were added to solution A under mechanical stirring, affording a mixture solutions. Then, the solution G was slowly added to the mixture and stirred at 70 °C for 2 h. The mixed solution became wet gel after 2 h and the resulting wet gel was dried in an oven at 70 °C for 72 h to obtain a dry gel. Finally, the dry gel was calcined in a muffle furnace at 1100 °C for 3 h. SMSED was prepared when F was not added.
2.2 Characterization
The X-ray diffraction (XRD) patterns were recorded using an X-ray diffractometer (Rigaku D/Max-2500) with Cu Ka radiation (λ = 0.15406 nm), and diffraction angles ranging from 10° to 80°. The luminescence spectra and decay curves were obtained via a Hitachi F-7000 fluorescence spectrophotometer (Japan) equipped with a 450W Xe lamp as the excitation light-source.
3 Results and discussion
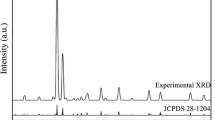
Figure 1 shows the X-ray diffraction (XRD) patterns recorded for SMSED and SESEDE phosphors. It can be seen that all the phosphors are indexed to tetragonal Sr2MgSi2O7 phase with the space group P-421m (No.113) according to JCPDF card no.75-1736. The three peaks (201), (211) and (212) are no offset. Er3+ co-doping has no significant influence on the structure of the phosphors. However, phosphors of Er3+ co-doping reduce the intensity of the peak resulting in a decrease in crystallinity. Eu2+,Dy3+ and Er3+ ions are regarded to occupy the Sr2+ sites in the cell because of the similar covalent radius (RSr = 1.95 Å, REu = 1.98 Å, RDy = 1.92 Å, REr = 1.89 Å) [21, 22].
Figure 2 shows the three-dimensional fluorescence spectra of SMSEDE phosphor. The center of strong emission region appeared at 390 and 467 nm, but the emission region at 390 nm is obviously weaker than 467 nm. This indicates that the best emission wavelength for the Eu2+ in the host crystal was 467 nm. These two strong emission peaks attributed to the 4f65d → 4f7 transitions of Eu2+. Sr2+ at two different positions in the host lattice can be replaced by Eu2+ to form two emission centers Eu1 and Eu2 corresponding to emission at 390 and 467 nm [23]. This because that the RSr = 1.95 Å is close to REu = 1.98 Å. The main excitation peaks are 274 and 356 nm, but due to the impact of instrument peak and peak intensity, the experiment will choose the monitoring wavelengths are 356 and 467 nm for emission spectra and excitation spectra, respectively.
Figures 3 and 4 shows that the excitation spectra and emission spectra of the phosphors. This indicates that emission spectra and excitation spectra show that the fluorescence intensity is significantly increased after Er3+ co-doping. As shown in Fig. 3, under the monitoring wavelength of 467 nm, both of the two phosphors showed two different excitation ranges of 250–330 and 335–450 nm, and the main excitation peak were 274 and 356 nm, indicating that Er3+ had almost no influence to the excitation peak. The reason why the excitation spectra of SMSEDE is enhanced is that Er3+ as a co-doping ion that is Eu2+ ions makes the distribution of the Eu2+ ions becomes random and deep throughout the lattice. This result in enhancement of the two excitation peaks at 274 and 356 nm due to the increase of electrons excited by the two characteristic excitation wavelengths. The excitation peak at 274 nm can be attributed to the charge transition of Eu2+–O2−; the excitation peak at 365 nm can be attributed to the typical 4f–5d emission of Eu2+ in Sr2MgSi2O7 [24]. As shown in Fig. 4 under the 356 nm excitation wavelength, emission spectra show that the fluorescence intensity is significantly increased after Er3+ co-doping. The reason why lattice defects are increased after the introduction of Er3+ and it acts as an trap to capture and release for electrons or holes [25]. The process affects the recombination of electron–hole pairs to make the intensities of the fluorescence spectra stronger. The emission of 467 nm was assigned to the 4f65d1 → 4f7 transition of Eu2+. The emission peak presented broad-featured, which could be attributed to the stronger crystal field strength of the host material. The characteristic emission peaks of Dy3+ and Er3+ are not detected, indicating the Eu2+ acted an activator, but Dy3+ and Er3+ were as co-activators in Sr2MgSi2O7 phosphors. The maximum brightness is achieved when Er3+ and Dy3+ auxiliary luminescent particle are incorporated into the host lattice. The Commission Internationale de l’Eclairage (CIE) chromaticity diagrams [26] of the prepared phosphors are presented in Fig. 5. For all the prepared samples, the CIE chromaticity diagrams fall in the bright blue region.
Figure 6 is shows the decay curve of SMSED and SMSEDE phosphors. Under the 467 nm excitation wavelength and the 356 nm emcitation wavelength, The afterglow decay process usually consists of three sub-decay processes, which can be described by a three-exponential function. The form of the equation is as follow [27]:
where I is phosphorescent intensity, I0, I1, I2, I3 are the constants, t is the time, τ1, τ2 and τ3 are time decay constants, respectively. The fitting results of the parameters of I0, I1, I2, I3, τ1, τ2 and τ3 are listed in Table 1.
The intensity of initial fluorescence (I0) of SMSEDE is 1.4 times stronger than that in SMSED, because Er3+ co-doping could deepen the trap level allowing more free electrons to be trapped. As shown in Fig. 7, the ground state of Eu2+ transitions to the excited state (4f7 → 4f65d1) and then some of the electronic enter into the trap during excitation through the lattice. During the afterglow process, electrons are released from the trap to the excited state through thermal excitation and returned to the ground state of the Eu2+ from the excited state. In other words, the electron transition can be attributed to the 4f65d1 → 4f7. Compared with the undoped phosphor, the trap depth of Er3+ co-doping phosphors is increased, so that the fluorescence intensity and afterglow performance will be enhanced.
4 Conclusions
In this paper, the Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ phosphor were successfully prepared via sol–gel method. The results show that phosphors of Er3+ co-doping reduce the intensity of the peak resulting in a decrease in crystallinity. Moreover the luminescent intensity and afterglow performance of Er3+ co-doping Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+ phosphors is improved and the Sr1.94MgSi2O7: 0.004Eu2+, 0.04Dy3+, 0.016Er3+ phosphor initial luminous intensity increased by 1.4 times than Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ phosphor.
References
H. Furusho, J. Hölsä, T. Laamanen, M. Lastusaari, J. Niittykoski, Y. Okajima, A. Yamamoto, Probing lattice defects in Sr2MgSi2O7: Eu2+, Dy3+. J. Lumin. 128, 881–884 (2008)
W. Pan, G. Ning, X. Zhang, J. Wang, Y. Lin, J. Ye, Enhanced luminescent properties of long-persistent Sr2MgSi2O7: Eu2+, Dy3+ phosphor prepared by the co-precipitation method. J. Lumin. 128, 1975–1979 (2008)
R. Shrivastava, J. Kaur, Characterisation and mechanoluminescence studies of Sr2MgSi2O7: Eu2+, Dy3+. J. Radiat. Res. Appl. Sci. 8, 201–207 (2015)
H. Wu, Y. Hu, X. Wang, Investigation of the trap state of Sr2MgSi2O7: Eu2+, Dy3+ phosphor and decay process. Radiat. Meas. 46, 591–594 (2011)
H. Wu, Y. Hu, Y. Wang, C. Fu, Influence on the luminescence properties of the lattice defects in Sr2MgSi2O7: Eu2+, M (M = Dy3+, La3+, or Na1+). J. Alloy. Compd. 497, 330–335 (2010)
H. Homayoni, L. Ma, J. Zhang, S.K. Sahi, L.H. Rashidi, B. Bui, W. Chen, Synthesis and conjugation of Sr2MgSi2O7: Eu2+, Dy3+ water soluble afterglow nanoparticles for photodynamic activation. Photodiagn. Photodyn. 16, 90–99 (2016)
L. He, B. Jia, L. Che, W. Li, W. Sun, Preparation and optical properties of afterglow Sr2MgSi2O7: Eu2+, Dy3+ electrospun nanofibers. J. Lumin. 172, 317–322 (2016)
P. Dorenbos, Mechanism of Persistent Luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J. Electrochem. Soc. 152, H107–H110 (2005)
A.A.S. Alvani, F. Moztarzadeh, A.A. Sarabi, Preparation and properties of long afterglow in alkaline earth silicate phosphors co-doped by Eu2O3 and Dy2O3. J. Lumin. 115, 147–150 (2005)
S.H.M. Poort, H.M. Reijnhoudt, H.O.T.V.D. Kuip, G. Blasse, Luminescence of Eu2+ in silicate host lattices with alkaline earth ions in a row. J. Alloy. Compd. 241, 75–81 (1996)
O. Hai, H. Jiang, D. Xu, M. Li, The effect of grain surface on the long afterglow properties of Sr2MgSi2O7: Eu2+, Dy3+. Mater. Res. Bull. 76, 358–364 (2016)
Y. Lin, Z. Tang, Z. Zhang, X. Wang, J. Zhang, Preparation of a new long afterglow blue-emitting Sr2MgSi2O7-based photoluminescent phosphor. J. Mater. Sci. 20, 1505–1506 (2001)
H. Wu, Y. Hu, Y. Wang, B. Zeng, Z. Mou, L, Deng Influence on luminescent properties of the Sr2MgSi2O7: Eu2+, Dy3+, Nd3+ co-doping. J. Alloy. Compd. 486, 549–553 (2009)
L. Xiao, J. Zhou, G. .Liu, L. Wang, Luminescent properties of R+ doped Sr2MgSi2O7: Eu2+, Dy3+, (R+ = Li+, Ag+) phosphors. J. Alloy. Compd. 712, 24–29 (2017)
I.P. Sahu, D.P. Bisen, N. Brahme, R. Sharma, Luminescence properties of Eu2+, Dy3+-doped Sr2MgSi2O7 and Ca2MgSi2O7 Phosphors by solid state reaction Method. Res. Chem. Intermediat. 41, 6649–6664 (2015)
H. Wu, Y. Hu, L. Chen, X. Wang, Enhancement on the afterglow properties of Sr2MgSi2O7: Eu2+ by Er3+ codoping. Mater. Lett. 65, 2676–2679 (2011)
F. Song, D. Chen, Y. Yuan, Synthesis of Sr2MgSi2O7: Eu, Dy and Sr2MgSi2O7: Eu, Dy, Nd by a modified solid-state reaction and their luminescent properties. J. Alloy. Compd. 458, 564–568 (2008)
H. Furusho, J. Hölsä, T. Laamanen, M. Lastusaari, J. Niittykoski, Y. Okajima, Probing lattice defects in Sr2MgSi2O7: Eu2+, Dy3+. J. Lumin. 128, 881–884 (2008)
W. Pan, G. Ning, J. Wang, Y. Lin, J. Ye, Erratum to “Enhanced luminescent properties of long-persistent Sr2MgSi2O7: Eu2+, Dy3+ phosphor prepared by the co-precipitation method”. J. Lumin 129(2009), 584 (2008)
F. Ye, S. Dong, Z. Tian, S. Zhou, S. Wang, Fabrication and study of properties of the PLA/ Sr2MgSi2O7: Eu2+, Dy3+ long-persistent luminescence composite thin films. Opt. Mater. 40, 130–135 (2013)
C.R. Kesavulu, V.B. Sreedhar, C.K. Jayasankar, K. Jang, D.S. Shin, S.S. Yi et al., Structural, thermal and spectroscopic properties of highly Er3+-doped novel oxyfluoride glasses for photonic application. Mater. Res. Bull. 51, 336–344 (2014)
D. Singh, V. Tanwar, A.P. Samantilleke, B. Mari, S. Bhagwan, K.C. Singh, P.S. Kadyan, I. Singh, Synthesis of Sr(1–x–y)Al4O7: Eux 2+, Lny 3+ (Ln = Dy, Y, Pr) nanophosphors using rapid gel combustion process and their down conversion characteristics. Electron. Mater. Lett. 13, 222 (2017)
X. Wei, Y. Shen, G. Zuo, L. Hou, Y. Meng, F. Li, Preparation of porous Sr2MgSi2O7: Eu2+, Dy3+ energy storage carriers via sol-hydrothermal synthesis. Ceram. Int. 41, 13872–13877 (2015)
W. Tian, K. Song, F. Zhang, P. Zheng, J. Deng, J. Jiang, J. Xu, H. Qin, Optical spectrum adjustment of yellow–green Sr1.99SiO4–3x/2Nx: 0.01Eu2+ phosphor powders for near ultraviolet–visible light application. J. Alloy. Compd. 638, 249–253 (2015)
F. Li, Z. Li, X. Wang, M. Zhang, Y. Shen, P. Cai, Crystal structure and luminescent property of flaky-shaped Sr4Al14O25: Eu2+, Dy3+ phosphor doped with Er3+ ions. J. Alloy. Compd. 692, 10–21 (2017)
D. Singh, V. Tanwar, A.P. Simantilleke, B. Mari, P.S. Kadyan, I. Singh, Rapid synthesis and enhancement in down conversion emission properties of BaAl2O4: Eu2+, RE3+ (RE3+=Y, Pr) nanophosphors. J. Mater. Sci.: Mater. Electron. 27, 2260–2266 (2016)
I. Chen, T. Chen, Sol-gel synthesis and the effect of boron addition on the phosphorescent properties of SrAl2O4: Eu2+, Dy3+ phosphors. J. Mater. Res. 16, 644–651 (2001)
Acknowledgements
This work is supported by the National Science Foundation Project of China (Grant Nos. 51572069 and 51772099).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Y., Li, F., Chang, S. et al. Er3+ co-doped Sr1.956MgSi2O7: 0.004Eu2+, 0.04Dy3+ phosphors and enhancement of luminescent properties. J Mater Sci: Mater Electron 29, 9239–9244 (2018). https://doi.org/10.1007/s10854-018-8952-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8952-0