Abstract
Due to the high agglomerate propensity of Si particles, making aluminum composites with a dispersion of the reinforcement using traditional way is still a challenge. In this study, an innovative approach was utilized to prevent the agglomeration of particles using encapsulating graphene sheets by grinding. Afterwards, the milled mixtures were incorporated with Cr-coated graphite flakes, urea and starch via mechanical mixing. After being mechanically mixed and sintered at 330 °C, the porous preform was fabricated by vacuum gas pressure infiltration. Effects of graphene content (0.5, 1, 3, 5 wt%) on the microstructures and properties of thermal and mechanical were investigated. Thermal conductivity and bending strength of the graphite flakes (Gf)/Si/Al composites increased by 41.4 and 300%, respectively compared to the specimens without graphene. Gf/Si/Al composites with graphene mass fraction of 5% had excellent in-plane thermal conductivity of 346 W/mK and bending strength of 84 Mpa, suggesting the composites can be applied as promising thermal management materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the ever-increasing of high power density of electronic and microelectronic devices, effective thermal management is becoming increasingly important for the reliability and lifetime of electronic components. So many application fields including electronic packaging, aerospace, automotive, battery technologies and space exploration have placed a greater demand on the properties of materials [1,2,3,4,5]. The effective way of improving the efficiency of thermal management is to apply thermal management materials with high thermal conductivity and low coefficient of thermal expansion, which can transport the thermal from the electronic devices [6,7,8,9]. Nowadays, Gf, graphite foams [10], graphite films [11], nanotube [12, 13], carbon fiber [14, 15] and graphene [16,17,18,19,20] have attracted extensive attention due to their excellent thermal conductivity performances. What’s more, those graphite materials can be served as reinforcements for matrixes to synthesis high thermal conductivity materials [9].
Highly oriented Gf have become a kind of promising reinforcement owning to the high thermal conductivity approaching 1000 W/mK, negative coefficient of thermal expansion in-plane thermal expansion, low density, and ease to machinability [21, 22]. In general, the interface of graphite reinforcements plays an important role in the thermal properties of the composites [21]. However, the poor wettability and harmful chemical reaction between molten metals and graphite are two key problems for the fabrication of graphite/Al composites with high performances [23]. Those two problems weaken the reinforcement functions of graphite flakes. On the one hand, Al is incapable of wetting wet the graphite because the contact angle is about 140°. Consequently, it is easy to form pores and voids at the interface of graphite/Al. The existence of the flaws decreased thermal and mechanical properties. On the other hand, it is widely accepted that the harmful interfacial reaction tends to happen during the process of high temperature fabrication, which leads to the formation of carbide [24]. The appearance of carbide has a negative impact on the mechanical and thermal properties of the composites under the same testing conditions [25]. The same problems are found in other carbon materials, such as graphite fibers/Al [26], nanotube/Al [12], graphene/Al [16], SiC/Al and diamond/Al composites [27]. In these carbon/Al composites, an effective way to eliminate the harmful reactions is to coat the carbon materials with metallic coatings, such as Cu, Ni, Gr, Ag, Ti, W and so on [22, 24, 28, 29]. What’s more, the coatings have good contact with graphite, which can effectively eliminate the harmful reaction [30, 31]. Cr coatings show better physical and chemical compatibilities between the coatings and molten aluminum.
In the process of fabricating perform, stacking of Cr-coated Gf leaves almost no space between the graphite layers, which makes the process of infiltration become difficult. Thus, more and more researchers have focused on reinforcing with Gf and nonmetal particles, such as SiC and Si particles [32]. Those particles are doped to the aluminum matrix composites, which can separate the stacked Gf. B4C, Al2O3 and other particles can also form a multiphase hybrid system with Gf to improve the thermal conductivity and mechanical properties [33].
Recently, graphite films, such as artificial graphite films synthesized from polyimide films [11], graphene [33,34,35,36,37] and reduced graphene oxide, have drawn attracted much attention in thermal management due to their excellent thermal performances. Some researchers have demonstrated almost a decade ago that graphene is one atomic layer of carbon. Nowadays, graphene is considered to be one of the main reinforcing fillers for Gf/Al composites. Aluminum matrixes reinforced with graphene demonstrate promising improvement in their thermal and mechanical properties [31, 34, 36, 38, 39]. In addition, graphite also exhibits self-lubricating properties when added to metal matrix for tribological applications. Besides, graphene has a function of enhancing the mechanical properties of metal–graphene composites. Despite lots of advantages of adding reinforcements in composites for thermal applications, there are still many challenges waiting for being graphene [40]. Some researchers pay attention to dope higher content of graphene to improve the mechanical properties, but the excess addition leads to the agglomeration and improper space of Gf. Actually, graphene is used as reinforcement to fabricate Gf/Al composites and the fabrication processes of the composites are well designed and controlled, thus high thermal conductivity and mechanical properties can be expected [41]. Furthermore, in the case of utilizing graphene and Si particles as raw materials to prepare Gf/Si/Al composites, we can easily control the distribution by grinding, which is effective to enhance mechanical properties. This way not only simplifies the fabrication process but also attains the high thermal conductivity composites [19, 42].
In this study, the aim is to investigate the influence of adding graphene up to 5 wt% into aluminum alloy on the thermal and mechanical properties of the composites. Finally, we successfully fabricated Gf/Si/Al composites with expected properties by vacuum gas pressure process [33]. The effects of appearance and mass fraction of graphene in the Al matrix on microstructures and properties of the composites are investigated. The microstructures of the composites are characterized at in-plane and out-of-plane to investigate the distribution of Gf and Si particles [43]. In addition, the coatings also are characterized compared to the raw Gf. Moreover, the influence of graphene content on the thermal and mechanical properties is evaluated [19, 35].
2 Materials and experimental procedure
2.1 Raw materials
The Gf with average diameter of 270 μm and thickness of around 25 μm, purchased from Shangdong Graphite Corporation. The in-plane and out-of-plane TC values of Gf were measured to be 1000 and 38 W/mk. Si particles with average diameter of 200 meshes, purchased from Aladdin, Shanghai. Urea and starch were acquired from PaiNi, China. The chromium powders (mesh 200, purity 99.8%), purchased from SINOPHARM, China, were applied as coating materials. Meanwhile, ZAlSi7Mg was chosen for infiltration instead of pure Al due to its good fluidity and lower melting temperature. Furthermore, graphene nanosheets with 6–10 nm thick × 15 microns wide were used as fillers, which bought from Molbase.
2.2 Preparation of graphene coating process
Graphene nanosheets were processed with drastic oxidation methods [44]. Then the graphene oxide is reduced in tube furnace at 600 °C for 4 h.
Meanwhile, the Gf wereetched in strong alkali solution consisting of NaOH and Na2CO3 and Na3PO4 under 120 °C. The solution can effectively remove the oil and impurities on the surface of Gf. After being degreased, the Gf mentioned in last step are washed with distilled water to neutral and vacuumed for next step. Then Gf were etched for 10 min in sulfuric acid to increase the specific surface area, which can enhance the sensitization-activation on the surface of the graphite layers. After coarsening, the Gf were rinsed with distilled water to neutral and dried under vacuum at 60 °C for 10 h.
The pretreated Gf and salt mixture were blended in mixer for 15 min. The final mixture was transferred to ceramic crucible and sintered in the tube furnace under inert gas at 1000 °C for 2 h. And the heating and cooling speed in the process of coating was controlled at 3 °C/min. In the process of coating, salt mixture consisting of KCl and NaCl (1.2:1 wt%) was heated above melting points to serve as electrolyte. Then the sinter is immersed in distilled water for 1 h and the coated Gf were collected by the method of pumping filtration. The final Cr-coated Gf were dried in vacuum oven at 50 °C for 24 h. Detailed parameters are shown in Table 1.
2.3 Fabrication of preform
Si powders were encapsulated by graphene via milling in mortar for 15 min. The encapsulated Si powders, urea, starch and Cr-coated Gf were blended in mixer for 15 min. The final mixture mentioned in last step was processed into a preform in steel mould under certain pressure. Then the preform was sintered in furnace at the speed of 3 °C/min to 330 °C according to the thermogravimetric curve of urea, which obtained the porous graphite preform. During the process of heating, the urea had decomposed into NH3 and HCNO which can produce pores structure. Meanwhile, the starch transformed dextrinization as binders.
2.4 Infiltration of the preform
The porous preform was infiltrated with molten aluminum alloy (ZALlSiMg) at 700 °C under a vacuum of 3 Mpa. Then, the cuboid Gf/Si/Al composites were fabricated into the size of 51 mm × 37 mm × 5 mm. Figure 1 demonstrates the detailed process of fabricating the Gf/Si/Al composites.
2.5 Characterization
The microstructures of Gf/Si/Al composites on the fractured surface were analyzed by field-emission scanning electron microscope (FE-SEM, Quanta 600FEG). X-ray photoelectron spectroscopy (XPS, Thermal Scientific K Alpha) was applied to examine the interface composition of graphite and aluminum. The thermal diffusivity of Gf/Si/Al composites (parallel to the graphite layers) was measured by a laser flash instrument via a NETZSCH LFA447 thermal analyzer. The density and porosity of Gf/Si/Al composites were characterized by electron density meter DE-120M (DahoMeter, Shenzheng, China). The heat distribution was obtained by Fluke Tis20 9 Hz Thermal Imager (American Fluke). The crystal phase structures of the samples were characterized by X-ray diffraction instrument (XRD, Rigaku, Cu Kα).
3 Results and discussion
3.1 Characterization of raw materials and Cr-coatings
Figure 2a demonstrates the Gf platelets. The smooth interface weak the wettability between Gf and molten aluminum. Figure 2b displays the image of Si particles which have irregular forms. The high dispersion of reduced graphene is shown in Fig. 2c. Figure 2d shows the surface morphology and thin cracks of the Cr-coated Gf by salt bath plating, and the result indicates that the tight coated layers and well-distributed coatings on the interface of the Gf. Thin cracks have formed due to different thermal expansion under different thermal stress. In other words, the existence of implies displays that the tight combination of molten Cr and Gf. Compared with uncoated Gf, the surface of Cr-coated Gf becomes smooth, which can bind with molten aluminum tightly.
Figure 3a–e reveal that Gf are uniformly distributed and oriented perpendicularly to the pressing direction due to the uniaxial pressure. The coatings are used to eliminate the harmful interfacial reaction and increase the bonding force between Gf and aluminum alloy. The Cr-coated Gf can be observed in Fig. 3a–e, which shows a tightly adhered interface between Gf and Cr. As shown in Fig. 3f, the existence of silvery coating is investigated to testify the homogeneity of the coating.
As shown in Fig. 4a–d, the interfaces between Gf, Cr and Al are closely attached, which have an positive impact on the thermal and mechanical properties. It is apparent that no obvious voids can be observed in the composites, thus the coatings are able to enhance the bonding strength greatly. At the same time, Si particles are observed in the boundary of coatings and Al, which shows a good combination of Si phase and Al phase. In Fig. 4b, some Si particles are observed to agglomerate together, it may resulting in cracks along the boundary in the process of infiltration. The agglomeration isn’t found in other samples with the increasing of content of graphene content, it indicating that graphene does improve the distribution of Si particles.
The images of sections are observed in Fig. 5a–e, and the space of the layers increases gradually with the higher graphene content. Uniform distribution is verified by those pictures which is the key step to fabricate Gf/Si/Al composites. What’s more, the graphite layers are all paralleled to the direction of TC, which is the promising direction of the composites. It can be regarded as an evidence to testify the exits of coating according to the existence of the composites in Fig. 5f. Meanwhile, the corresponding EDS images reveal that the uniform content of aluminum and Si which can effect density, thermal and mechanical properties of the composites.
Figure 6 shows the metallographic micrographs of Gf/Si/Al composites with different content of graphene. The darker flake areas represent Gf and the brighter phase is Si/Al matrix. it can be seen in Fig. 6a–e that, with the addition of Si particles, Gf are seperated by Si particles which can apparently enhance the TC property. Si particles play a role in separating the Gf, which can produce suitable distance for a uniform infiltration. Moreover, Si particles are apparently observed in the Al matrix in Fig. 6f–j. Some particles are agglomerates without encapsulating graphene in Fig. 6f, so the addition of graphene promotes the dispersion of Si particles. At the same time, graphene leads to the expansion of perform in the process of heating. Some reashers have found that adding a small amount of Si particles to Al matrix can reduce the solubility of carbon in the aluminum, which effectively prevents the formation of Al4C3.
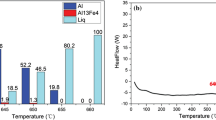
In order to determine the phase composition of the composites, the XRD is performed and presented in Fig. 7. Cr7C3 and Cr3C2 peaks are observed in the samples with the addition of Cr. The molten salts promoted the interface reactions of Cr and Gf. In the process of reacting, Cr react with Cr3C2 to form Cr7C3 gradually. So the coating on the surface of Gf is composed of two types of carbides. In the infiltration of Gf/Si/Al composites, the immediate contact of Gf and molten Al promotes the reaction of Al4C3. The existence of Al4C3 limits the thermal and mechanical properties of the composites. As shown in Fig. 5, the four different Gf/Si/Al composites with different graphene mass fraction show different space of layers. In the process of liquid infiltration, the molten aluminum reacts with graphene leading to the formation Al4C3. With the increasing of graphene, the content of Al4C3 became slight. Furthermore, it can be concluded that Al4C3 only exits in the specimens containing graphene because there is no peaks in the curve of RGO 0%.
In order to confirm whether there exists Al4C3, the surface element components of Gf/Si/Al composites are detected by XPS. As shown in Fig. 6a, the composites consist of Al, C, Si, Cr and O elements. Compared with the samples without graphene, there is no peak at 73.6 eV in Fig. 8b, which further confirms the existence of Al4C3 formed in the reaction of graphene and molten aluminum. With the increasing of graphene, the content of Al increases gradually according to Fig. 8c–f. But the content of Al4C3 is controlled into a permissible range, so the existence of carbide doesn’t have a significant impacts on thermal performances. Furthermore, the presence of a few nanosized Al4C3 is necessary for enhancing interfacial bonding and minimizing interfacial thermal resistance.
3.2 Thermal properties of Gf/Si/Al composites
In order to evaluate the thermal conductivity of Gf/Si/Al composites, the equations are applied proved to be accurate for thermal conductivity predictions of anisotropic composites with Si and graphene reinforcements. It could be described as follows [19]:
where α, λ, C p, ρ are the thermal diffusivity, TC, specific heat capacity and average density of Gf/Si/Al composites, respectively. The specific heat capacity of Gf/Si/Al composites is calculated by the linear rule of mixtures using the specific heat capacity values of the components (Gf, Si particles, graphene, and Al alloy). Detailed properties of the composites are listed in Table 2.
With the increasing of graphene, the thermal conductivity of Gf/Si/Al composites increases gradually. However, the limit addition of graphene cannot improve the thermal conductivity obviously. Based on the microstructures of Gf/Si/Al composites, Si particles encapsulated graphene are distributed into aluminum matrix uniformly. High thermal conductivity reinforcements have been introduced to the composites to reinforce the thermal conductivity properties. Furthermore, it is an effective way to prevent agglomeration by grinding. The thermal conductivity of the composites obviously obtains improvement with the graphene mass fraction of 5%.
Thermal images photograph of the preforms monitored from 1 to 11 min are shown in Fig. 10a. The images of the preform without graphene are displayed in Fig. 10b. The thermal transfer performances of a, b and c specimens are compared by putting those three specimens in the same temperature area of hot plate, and then measured the temperature distribution. The addition of graphene greatly improves the thermal conductivity greatly with the excellent performance by the uniform temperature distribution of Fig. 10c. Consistent with the result of highest thermal conductivity with RGO 5% as shown in Fig. 9.
3.3 Bending strength properties
Figure 11 shows the bending strength of the specimens as a comparison of graphene content. The addition of 5% to preform has improved the bending strength for about three times compared to the original specimen. A spot of graphene doesn’t enhance the bending strength apparently and distribute to composition uniformly.
Thus, the in-plane TC and mechanical performance of composites are all improved owning to the introduction of graphene. Cr coatings can decrease the interfacial defects and improve the interfacial bonding of the Al/graphite. Gf/Si/Al composites possess high thermal conductivities and favorable mechanical properties, which makes them a kind of promising materials for effective thermal management.
4 Conclusions
This study proposes an innovative fabrication method, making use of encapsulating and coating, for manufacturing Al-based composites reinforced with Si particles, graphite flakes, and graphene. Cr-coatings are successfully coated on the surface of Gf by salt bath plating to weaken the formation of carbide effectively. Cr-coatings with the ratio of Cr to Gf (2.5:10 wt%) on the Gf by salt bath plating process are low density, uniform and integrated. Moreover, the optimized fabrication parameters of the preform are programmed with 3 °C/min from room temperature to 330 °C. The uniform preforms are manufactured by the decomposition of urea and starch (10:1 wt%), which can be infiltrated more easily and uniformly with lower infiltration pressure. Furthermore, Gf/Si/Al composites are processed by vacuum gas pressure infiltration. The composites with graphene mass fraction of 5% have excellent in-plane thermal conductivity of 346 W/mK. The thermal conductivity of in-plane directions and the bending strength have been enhanced to 41.4 and 300% of the composites. In this study, the effective way can be applied to fabricate homogeneous Gf/Si/Al composites.
References
Y. Huang, Q. Ouyang, Q. Guo, X. Guo, G. Zhang, D. Zhang, Mater. Des. 90(59), 508 (2016)
K. Raza, F.A. Khalid, J. Alloys Compd. 615(8), 111 (2014)
Y. Huang, Q.B. Ouyang, D. Zhang, J. Zhu, R.X. Li, H. Yu, Acta Metall. Sin. 27(5), 775 (2014)
M. Rajashekhar, A.A. Khan, Int. J. Innov. Res. Sci. Eng. Technol. 2(11), 6150 (2013)
K. Komuro, M. Suwa, K. Soeno, M. Ohsawa, Carbon 22(1), ii (1984)
M.K. Aghajanian, D.K. Creber, A.W. Urquhart, D.R. White, Met. Matrix Comp. 21(1), 98 (1990)
D.D.L. Chung, Appl. Therm. Eng. 21(16), 1607 (2001)
G. Xin, H. Sun, T. Hu, H.R. Fard, X. Sun, N. Koratkar, T. Borca-Tasciuc, J. Lian, Adv. Mater. 26(26), 4521 (2014)
C. Zhou, G. Ji, Z. Chen, M.L. Wang, A. Addad, D. Schryvers, H.W. Wang, Mater. Des. 63, 719 (2014)
N.C. Gallego, J.W. Klett, Carbon 41(7), 1461 (2003)
Y. Huang, Y. Su, X. Guo, Q. Guo, Q. Ouyang, G. Zhang, D. Zhang, J. Alloys Compd. 711, 22 (2017)
J.F. Xiang, L.J. Xie, S.A. Meguid, S.Q. Pang, J. Yi, Y. Zhang, R. Liang, Comput. Mater. Sci. 128, 359 (2017)
B.S. Guo, M. Song, J.H. Yi, S. Ni, T. Shen, Y. Du, Mater. Des. 120, 56 (2017)
T.T. Liu, X.B. He, Q. Liu, L. Zhang, L. Wang, Q.P. Kang, X.H. Qu, J. Mater. Eng. Perform. 22(6), 1649 (2013)
B.B. Singh, M. Balasubramanian, J. Mater. Process. Technol. 209(4), 2104 (2009)
J. Wozniak, A. Jastrzębska, T. Cygan, A. Olszyna, J. Eur. Ceram. Soc. 37(4), 1587 (2017)
B.F. Xu, Z.D. Lin, C.M. Du, H.B. Lin, K.Y. Liang, W.P. Qiu, G.L. Yang, Mater. Res. Innov. 19, S1-388 (2015)
V.G. Konakov, I.A. Ovid’ko, N.V. Borisova, E.N. Solovyeva, S.N. Golubev, O.Y. Kurapova, N.N. Novik, I.Y. Archakov, Rev. Adv. Mater. Sci. 39(1), 41 (2014)
Y. Yang, Y. Huang, H. Wu, H. Fu, M. Zong, J. Mater. Res. 31(12), 1723 (2016)
B.M. Amoli, J. Trinidad, A. Hu, Y.N. Zhou, B. Zhao, J. Mater. Sci. Mater. Electron. 26(1), 590 (2015)
H. Kurita, T. Miyazaki, A. Kawasaki, Y. Lu, J.F. Silvain, Compos. A 73, 125 (2015)
A. Rodriguez-Guerrero, S.A. Sanchez, J. Narciso, E. Louis, F. Rodriguez-Reinoso, Acta Mater. 54(7), 1821 (2006)
W. Li, Y. Liu, G. Wu, Carbon. 95, 545 (2015)
S.W. Ip, R. Sridhar, J.M. Toguri, T.F. Stephenson, A.E.M. Warner, Mater. Sci. Eng. A 244(1), 31 (1998)
R. M, N. A, A. Khan, Int. J. Innov. Res. Sci. Eng. Technol. 2(5), 1383 (2013)
T.T. Liu, X.B. He, Q. Liu, S.B. Ren, Q.P. Kang, L. Zhang, X.H. Qu, J. Mater. Sci. 49(19), 24784 (2014)
Z. Tan, Z. Li, G. Fan, Q. Guo, X. Kai, G. Ji, L. Zhang, D. Zhang, Mater. Des. 47(9), 160 (2013)
S.G. Warrier, C.A. Blue, R.Y. Lin, J. Mater. Sci. 28(3), 760 (1993)
J. Pelleg, D. Ashkenazi, M. Ganor, Mater. Sci. Eng. A 281(1–2), 239 (2000)
A.R. Kamali, D. Fray, J. Mater. Sci. 51(1), 569 (2016)
A. Saboori, C. Novara, M. Pavese, C. Badini, F. Giorgis, P. Fino, J. Mater. Eng. Perform. 26(3), 993 (2017)
M. Murakami, N. Nishiki, K. Nakamura, J. Ehara, H. Okada, T. Kouzaki, K. Watanabe, T. Hoshi, S. Yoshimura, Carbon 30(2), 255 (1992)
S.W. Feng, Q. Guo, Z. Li, G.L. Fan, Z.Q. Li, D.B. Xiong, Y.S. Su, Z.Q. Tan, J. Zhang, D. Zhang, Acta Mater. 125, 98 (2017)
G. Li, B.W. Xiong, J. Alloys Compd. 697, 31 (2017)
A. El-Ghazaly, G. Anis, H.G. Salem, Compos. A 95, 325 (2017)
X.M. Du, R.Q. Chen, F.G. Liu, Dig. J. Nanomater. Biostruct. 12(1), 37 (2017)
L.A. Yolshina, R.V. Muradymov, I.V. Korsun, G.A. Yakovlev, S.V. Smirnov, J. Alloys Compd. 663, 449 (2016)
H. Kwon, J. Mondal, K.A. AlOgab, V. Sammelselg, M. Takamichi, A. Kawaski, M. Leparoux, J. Alloys Compd. 698, 807 (2017)
C.-C. Hsieh, W.-R. Liu, Carbon. 118, 1 (2017)
S.E. Shin, Y.J. Ko, D.H. Bae, Compos. B 106, 66 (2016)
Y. Huang, Q. Ouyang, Q. Guo, X. Guo, G. Zhang, D. Zhang, Mater. Des. 90, 508 (2016)
R. Pérez-Bustamante, F. Pérez-Bustamante, I. Estrada-Guel, L. Licea-Jiménez, M. Miki-Yoshida, R. Martínez-Sánchez, Mater. Charact. 75(1), 13 (2013)
X. Chen, Y. Huang, K. Zhang, W. Zhang, J Colloid Interface Sci. 506, 291 (2017)
W.S. Hummers Jr, R.E. Offeman, J. Am. Chem. Soc. 80(6), 1339 (1958)
Acknowledgements
This work was financially supported by the ShenZhen institute of Technology, Northwestern Polytechnical University; School of Natural and Applied Science, Northwestern Polytechnical University, and the fundamental Research Funds for the Central Universities (No. JCJY20170306153232969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Huang, Y., Zhou, S. et al. Effects of graphene content on thermal and mechanical properties of chromium-coated graphite flakes/Si/Al composites. J Mater Sci: Mater Electron 29, 4179–4189 (2018). https://doi.org/10.1007/s10854-017-8363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8363-7