Abstract
The electrochemical interaction between graphite and molten salts to produce carbon nanostructures is reviewed. It is demonstrated that, depending on the conditions, it is possible to electrochemically convert graphite in molten salts to either carbon nanoparticles and nanotubes, metal-filled carbon nanoparticles and nanotubes, graphene or nanodiamonds. The application of metal-filled carbon nanotubes as anodes in lithium-ion batteries is reviewed. Surprisingly, this method of preparation is relatively simple and very similar to the mass production of aluminium in molten sodium aluminium fluoride–alumina mixtures, which is performed economically on a tonnage scale, indicating that it may be possible to apply it for the production of novel carbon nanostructures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molten salts and carbon have contrasting properties in that the former are liquids which conduct electricity mainly by the migration of ions whilst the latter is a solid and can either be an electronic conductor (carbon) or an insulator (diamond), depending on the phase. Carbon can exist in molten salts such as CO3 2− or C2 2− ions or combined with another element to form a gas, CO2, CO or a hydrocarbon. It can also exist as an electrode in aqueous or molten salt electrolytes. In the solid state, the electrical conductivity of salts is low due to the lack of defects and the very low diffusion coefficients. On melting, there is usually a significant volume increase (25 %) which indicates significant space in the liquid state and a high diffusion coefficient for the ions leading to a high ionic conductivity coupled with a small electronic conductivity. Application of a voltage to graphite results in a flow of current which is linearly dependent on the applied voltage (Ohm’s law). In comparison, a molten salt will only conduct a significant current above the decomposition potential of the salt which, in turn, leads to its decomposition. At the present time, graphite/carbon is used as electrodes, both anode and cathode, to produce aluminium from alumina dissolved in molten cryolite (Na3AlF6), as the anode in the electrolysis of sodium from molten sodium chloride, magnesium from molten magnesium chloride, lithium from molten lithium chloride and, lastly, the recycling of used nuclear fuel rods using molten lithium chloride [1]. In the Hall–Heroult cell for the production of aluminium, the aluminium ions are discharged on a molten aluminium layer, supported on a graphite cathode, and the oxygen ions are discharged on a carbon anode to form a mixture of carbon dioxide with a small proportion of carbon monoxide [2]. One interesting reaction on the cathode is that sodium is able to codeposit, due to a similar electrode potential to that of aluminium, and can diffuse to the graphite cathode [3]. For used oxide nuclear fuel rods, the fuel rod is made the cathode in a bath of lithium chloride and the cathodic reaction is ionisation of oxygen in the cathode to give oxygen ions which diffuse through the electrolyte to the anode where discharge takes place to form oxygen on an inert anode (platinum) or a mixture of carbon dioxide and carbon monoxide on a carbon anode [4]. In a totally different field, molten alkali carbonates are used at very elevated temperatures and pressures to catalyse the conversion of graphite to diamond [5]. Overall, there is a wealth of information on the interaction of molten salts with carbon which forms the basis of this paper to explore novel and exciting applications.
Interaction of graphite with molten salts
Molten salts may cause various changes on the structure and microstructure of graphite. The exposure of graphite to molten chloride salts [6, 7] will increase the crystallinity of the graphite material. The same effect was reported when molten fluoride salts are used, and the effect was attributed to the improvement of stacking order in graphite exposed to molten salts [8]. If graphite and LiCl are simply heated together to 1250 °C; the graphite tends to break up to form a mixture of exfoliated nanosheets, graphene and nanorods (Fig. 1) [7]. It would be an advantage if the graphite could be treated in such a way to get a specific and well-defined nanomaterial. At present, this is only achieved on an industrial scale in the conversion of carbon to diamond using a molten metal [9] or molten salt catalysts [10].
Microstructure of the graphite–LiCl mixture heated at the rate of 80 °C min−1 to 1250 °C. a The dominant microstructure consisted of bent layered grains. b Exfoliated nanosheets which could be found in the microstructure. c TEM image of a graphene sheet. The inset is the selected area electron diffraction pattern taken from edge of the sheet showing the hexagonal structure of the (0001) basal plane. Reproduced from Kamali and Fray [7]
In the 1990s, the group of Kroto found that by electrolysing alkali chlorides using a graphite cathode it was possible to create carbon nanotubes [11]. Subsequent work showed that the process involved intercalation and that lithium atoms produced the highest yield of nanotubes whilst the larger alkali atoms, Na and K, caused the graphite to breakup [12]. It was also found that by using a mixed lithium chloride and tin chloride melt it was possible to obtain carbon nanotubes that were filled with tin and other low-melting point metals, depending on the composition of the second salt [13].
Effects of varying the microstructure of the graphite and the electrochemical parameters
The apparatus for the intercalation of lithium into graphite is shown in Fig. 2 [14]. It was found that the structure and morphology of carbon nanomaterials produced were dependent on those of the graphite material used as the cathode in the molten salt process [15]. It was further found that nanoparticles as well as tubes can be formed by changing the temperature of molten salt [6] and the applied cathode current density [16]. The effect of molten salt temperature on the morphology of the carbon product is illustrated in Figs. 3 and 4 [14]. However, in the earlier experiments, it was observed that the yield of nanotubes and nanoparticles decreased with time and this was interpreted by postulating that the surface of the graphite became saturated with lithium so that instead of the lithium ion discharging and the lithium atoms penetrating the graphite structure, the lithium just formed a layer of lithium metal on the surface of the graphite [17].
Schematic of the experimental set-up used for the small scale preparation of carbon nanostructures in molten LiCl. A graphite rod is used as the cathode and a graphite crucible as the anode. A molybdenum wire is used as the quasi-reference electrode. Reproduced from Schwandt et al. [14]
Scanning electron microscopic image of a carbonaceous product that contains nanotubes as the main constituent. Experimental was conducted at temperature 775 °C. Reproduced from Schwandt et al. [14]
Scanning electron microscopic image of a carbonaceous product that contains nanoparticles as the main constituent. Experimental was conducted at temperature 700 °C. Reproduced from Schwandt et al. [14]
It was found that by switching the current every few minutes the generation of nanoparticles and nanotubes continued indefinitely until all the graphite had been consumed [16]. Under these conditions, the lithium continually moved from one electrode to the other and, overall, this had three distinct advantages—first the LiCl was not consumed, which is important in that lithium chloride is expensive, the production of nanotubes and nanoparticles was continuous and the whole rods were, near enough, totally consumed and chlorine evolution was not a problem.
In 2014 the scalability of the molten salt process was demonstrated using a modified apparatus [16]. The advantages of the molten salt route for the production of these novel materials is that the process is about 1000 times faster than the catalytic route where a hydrocarbon is dissociated into carbon and hydrogen. The space occupied by the equipment for molten salt route is very much smaller than that required for the catalytic route as the reactions take place in condensed phases as opposed to a gaseous environment. However, the product is slightly different in that the catalytic route produces nanotubes whereas the molten salt route produces nanoscrolls. The mechanism for the production of nanoscrolls and nanoparticles, each containing walls consisting of several layers graphene is that the lithium atoms intercalate into the graphite structure causing a strain within the structure which, in turn, encourages the extrusion of graphene sheets. There is a high surface energy between graphite and molten salts so in order to minimise this energy, the sheets roll up to form nanoscrolls and nanoparticles and when tin ions are present in the salt, the tin can deposit as small droplets on the surface of the graphite around which the graphene sheets can enclose.
Uses of carbon nanoscrolls and nanoparticles
Both conventional carbon nanotubes and nanoscrolls consist of layers of graphene, it is assumed that the mechanical properties are the same and that the nanoscrolls would find similar applications to those of nanotubes. However, at the present time, although the synthesis of nanoscrolls has been proved in the laboratory, there is no industrial production so that the material is not readily available for evaluation. However, there is one electrochemical application where metal-filled carbon nanoscrolls are particularly apposite and that is in lithium-ion batteries. These batteries also use an intercalation process for both anodes and cathodes. Ideally, if a lithium metal anode could be used in the anodic reaction it would be
and the cathode reaction would be
where MeO x is a metal oxide and LiMeO x is an intercalation compound in which Li is mobile and the activity of Li is very low with the difference in activities between the anode and cathode generating the potential to drive the cell [18]. On recharging the cell, the lithium is transferred back to the anode but as it is very difficult to obtain a planar deposit from the electrolyte, the resulting deposit is very dendritic and can grow and contact the cathode, causing a disastrous short circuit leading to fires [19]. In order to overcome this problem, graphite is used as the anode material which can form Li0.167C [20]. The activity of the lithium is reduced in this compound which reduces the overall cell voltage slightly but the main problem is the small amount of lithium contained in the anode [20]. However, it can be increased by using Si or Sn as the anode but the compounds, Li4Sn and Li4Si, suffer from the disadvantage of very large volume changes on the addition and removal of lithium causing the anode to decrepitate to very fine particles with little electrical contact with the neighbouring particles [21]. This problem can be overcome by enclosing the tin or silicon inside a carbon nanotube, where the expansion and contraction can be controlled. Tin and silicon have been inserted into carbon nanotubes using the molten salt route described above. The microstructure of tin-filled carbon nanoscrolls produced in molten LiCl is shown in Fig. 5 [22]. After carrying out charge–discharge cycles, the cell was dismantled and the anode material was studied by means of transmission electron microscopy [23]. It was deduced that on the first insertion the lithium tin compound formed and caused the nanotube to expand but when the anode discharged the alloy shrank but still remained in contact with the highly conducting carbon nanotube. As it is known that lithium cannot pass through graphene, this must mean that the lithium must move between the sheets of the scroll otherwise passing down the centre of the scroll from the ends would be far too slow. It was found that the capacity of the anode has increased by about 50 % which is encouraging bearing in mind that the anode only contained 50 % of the tin-filled carbon nanoscrolls [23]. Using silicon-filled scrolls instead of tin-filled scrolls increased the capacity of the anode considerably [24]. On the other hand, the lack of scalable methods for the preparation of Sn-containing carbon nanostructures is a barrier to the proper evaluation of these nanostructures in an industry where tonnes of material are required. However, the molten salt approach was recently demonstrated to be capable of producing large-scale quantities of Sn-containing carbon nanomaterials [25].
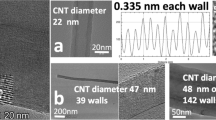
Transmission electron microscopic analysis of tin-filled carbonaceous product prepared using an electrolyte of LiCl with an admixture of SnCl2 of 1 % by mass. Reproduced from Das Gupta et al. [23]
Intercalation of hydrogen atoms into graphite to prepare graphene
It is usually very difficult to get hydrogen molecules to dissolve in graphite or carbon nanotubes. However, it has been shown that it is relatively easy to get lithium to dissolve in graphite and it should be even easier to insert hydrogen atoms into graphite as these atoms are much smaller than the interlayer spacing in graphite. Furthermore, it is known that in comparison to lithium chloride most hydrogen-containing compounds such as H2O, HCl, HBr are far less stable than all lithium compounds and should breakdown under an applied potential before lithium oxide or lithium chloride. Therefore, the presence of water should result in hydrogen atoms intercalating when the graphite, immersed in the molten lithium chloride, is made cathodic. Experiments showed that under electrochemical intercalation using lithium chloride exposed to argon gas containing water, the graphite is exfoliated into graphene sheets which could then be retrieved simply by washing with water and filtration followed by heating in a reducing atmosphere (15 % H2, 85 % N2) at around 1300o C which is above the boiling points of LiCl and Li2CO3 [26]. Figure 6 shows the starting and the final graphite rod, showing very even erosion. Figures 7 show a TEM micrograph of the graphene product [26]. The rate of production was about 5 g/cm2 of electrode/day which is an order of magnitude higher than other graphene producing methods, such as CVD and low temperature exfoliation.
The photographs of a the graphite rod which was used as cathode in the molten salt process conducted under moist gas flow, b the graphite cathode after the molten salt process, and c the graphene product stored in a jar. Reproduced from Kamali and Fray [25]
TEM micrograph of the graphene nanosheets produced as well a typical electron diffraction pattern recorded at relatively flat edge of a graphene sheet, with the peaks labelled by Miller–Bravais indices. Reproduced from Kamali and Fray [25]
Graphene has some remarkable properties including high ballistic electron mobility, high thermal and electrical conductivity which may allow the material to be used in electrochemical capacitors [27–29] and anodes and cathodes in lithium-ion batteries [30], electrodes for fuel cells [31] and many other applications [32]. Existing methods for the production of graphene are based upon chemical oxidation of graphite [33], exfoliation of graphite in organic solvents [34], chemical vapour deposition [35–37] and molten metals [38]. Compared to the molten salt intercalation route, most of the other processes operate at very low production rates.
Synthesis of nanodiamonds
It has been shown by theoretical analysis that sp 3 diamond nucleation from sp 2 carbon is possible inside carbon nanotubes or nanoparticles due to surface tension effects [39] and there have been many attempts to convert carbon nanotubes to diamonds using plasma techniques, laser irradiation, shock waves and plasma sintering [40–42] but all these techniques require very high pressures and temperatures as well as metallic catalysts. Non-metallic catalysts, such as alkali carbonates, have also been used although even higher temperatures and pressures are required as well as longer reaction times [43].
When electrolysing LiCl containing lithium oxide, the reaction at the anode is likely to be reaction of the oxygen ions with the carbon to give carbon dioxide which would be soluble in the melt and can react with the lithium oxide to form lithium carbonate [44]. Examination of the nanocarbon particles and nanoscrolls showed lithium carbonate nanoparticles incorporated into the structure between the graphene layers (Fig. 8) [44]. As mentioned above, lithium carbonate is a known catalyst for the conversion of graphite to diamond, albeit at very high temperatures and pressures and, even then, it takes several hours for the diamonds to form.
TEM micrograph of the as-synthesised electrolytic carbon material. The right panel shows a number of CNTs, and the inset is a typical selected area diffraction pattern confirming the presence of graphitic carbon and Li2CO3 single-crystals. The left panel exhibits two high-resolution TEM image. The upper image shows a wall of a CNT demonstrating that Li2CO3 nanocrystals, pointed by arrows, embedded into the graphitic structure of the wall. The down image presents a carbon nanoparticle in which Li2CO3 nanocrystals are encapsulated in carbon shells. Reproduced from Kamali and Fray [40]
The oxidation of the carbon nanostructures produced in molten LiCl proceeds at considerably lower temperatures than those typically needed for the oxidation of carbon nanomaterials, due to the catalytic effect of lithium carbonate crystals encapsulated in graphitic nanostructures [45]. It was found that when the carbon nanoparticles, containing lithium carbonate were heated in air at about 500 °C, the nanoparticles were converted into octahedral nanodiamonds (5 nm–1 µm) as shown in Fig. 9 [44]. Although the particles were ignited in air at 500 °C, it is likely that the true temperature is very much higher and probably closer to 4500 °C. The formation of nanodiamonds was confirmed by the diffraction pattern of the [111] plane of diamonds with additional evidence given by the Raman Spectra of the material which confirmed the existence of diamond. After the heat treatment it was found that the density increased from 2.2 to 3.0 gcm−3 which is in accordance with the transformation of diamonds form graphite.
SEM micrographs of the ECM after heat treatment in air showing nano- and micron-sized diamonds. The left hand panel shows diamond crystal which are growing on a carbon substrate. Kamali and Fray [40]
Nanodiamonds are usually made by taking diamonds that have been made at very high temperatures and pressures followed by grinding to create nanodiamonds. However, the shapes of the nanodiamonds are no way as perfect as those found by the molten salt precursor route. The properties of these novel perfect diamonds, shown in Fig. 8, prepared by this electrochemical molten salt route are, at present, being determined.
Overview of the molten salt synthesis of nanocarbons
As described earlier, molten salts have been used for decades for the industrial production of aluminium, magnesium, sodium and lithium. By far the largest production is for aluminium at around 50 million tonnes/year. For each tonne of aluminium that is produced, about 0.4 tonne of carbon is consumed. The Hall-Heroult cells for aluminium operate at about 4 V with a current density of around 1 A/cm2. The temperature of the cells is around 950 °C and, somewhat surprisingly, the aluminium product is sold for about $3/kg which can be compared with nanoparticles and nanodiamonds which sell for $3/g. The technology described in this paper where the temperature is around 800 °C, the voltages are of the order of 4 V and the current density is about 1 A/cm2 which is very similar to that of the Hall-Heroult cell. As the rate of erosion of the graphite rods is similar to the erosion of the anodes in the Hall-Heroult cell, this indicates that carbon nanoproducts could be made for an order of magnitude smaller cost than at present! This applies to nanotubes, graphene and nanodiamonds. Furthermore, it can be envisaged that planar electrodes could be used, perhaps of 1 m2 dimensions so a unit, with about 20 graphite plates, about 1 m3 in size could produce about 600 kg/day or 4 tonnes/week or 200 tonnes/year which is much higher production rate than other methods for nanoparticles. In addition, it is also very interesting to note that in the western world there are plenty of redundant Hall-Heroult cells as much of the aluminium industry has moved from Europe to regions of the world where energy costs are significantly lower [46].
Conclusions
Carbon is basically a very cheap commodity and is used industrially on an enormous scale. However, as the size of the carbon product goes from cm scale to nanoscale, the cost increases very significantly. This paper demonstrates that by using molten salts, electrochemistry and technology that is readily available from the metallurgical industry, it should be possible to reduce the cost of carbon nanoproducts such as nanoscrolls, nanoparticles, nanodiamonds and graphene considerably. Although the basic electrochemistry has been proven but there is still much to do to convert a successful laboratory experiment into an industrial process.
References
Habashi F (ed) (1977) Handbook of extractive metallurgy. Wiley-VCH, Weinheim
Thonstad J, Fellner P, Haarberg GM, Hives J, Kvande H, Sterten A (2001) Aluminium electrolysis; fundamentals of the Hall-Heroult process. Aluminium-Verlag, Dusseldorf
Mikhalev Y, Oye HA (1996) Absorption of metallic sodium in cathode carbon materials. Carbon 34:37–41
Joseph TB, Sanil N, Mohandas KS, Nagarajan K (2015) Study of graphite as anode in the electro-deoxidation of solid UO2 in LiCl-Li2O melt. J Electrochem Soc 162:E51–E58
Pal’yanov YN, Sokol AG, Borzdov YN, Khokhryakov AF, Sobolev NV (1999) Diamond formation from mantle carbonate fluids. Nature 400:417–418
Kamali AR, Divitini G, Schwandt C, Fray DJ (2012) Correlation between microstructure and thermokinetic characteristics of electrolytic carbon nanomaterials. Corros Sci 64:90–97
Kamali AR, Fray DJ (2013) Molten salt corrosion of graphite as a possible way to make carbon nanostructures. Carbon 56:121–136
He Z, Gao L, Wang X, Zhang B, Qi W, Song J et al (2014) Improvement of stacking order in graphite by molten fluoride salt infiltration. Carbon 72:304–311
Pal’yanov NV, Kupriyanov IN, Khokhryakov AF, Ralchenko VG (2001) Crystal growth of diamond, handbook of crystal growth: bulk crystal growth, 2nd edn. Elsevier, Amsterdam, pp 671–713
Tomlinson E, Jones A, Milledge J (2004) High-pressure experimental growth of diamond using C–K2CO3–KCl as an analogue for Cl-bearing carbonate fluid. Lithos 77:287–294
Hsu WK, Terrones M, Hare JP, Terrones H, Kroto HW, Walton DRM (1996) Electrolytic formation of carbon structures. Chem Phys Lett 262:161–166
Chen GZ, Fan X, Luget A, Shaffer MSP, Fray DJ, Windle AH (1998) Electrolytic conversion of graphite to carbon nanotubes in molten salts. J Electroanal Chem 446(1–2):1–6
Hsu WK, Li J, Terrones H, Terrones M, Grobert N, Zhu YQ, Trasobares S, Hare JP, Pickett CJ, Kroto HW, Walton DRM (1999) Electrochemical production of low melting metal nanowires. Chem Phys Lett 301:159–166
Schwandt C, Dimitrov AT, Fray DJ (2010) The preparation of nano-structured carbon materials by electrolysis of molten lithium chloride at graphite electrodes. J Electroanal Chem 647:150–158
Kamali AR, Schwandt C, Fray DJ (2011) Effect of the graphite electrode material on the characteristics of molten salt electrolytically produced carbon nanomaterials. Mater Charact 62:987–994
Kamali AR, Fray DJ (2014) Towards large scale preparation of carbon nanostructures in molten LiCl. Carbon 77:835–845
Kinloch IA, Chen GZ, Howes J, Boothroyd C, Singh C, Fray DJ, Windle AH (2003) Electrolytic, TEM and Raman studies on the production of carbon nanotubes in molten NaCl. Carbon 41:1127–1141
Thackeray MM (1995) Structural considerations of layered and spinel lithiated oxides for lithium ion batteries. J Electrochem Soc 142:2558–2563
Zavalis TG, Behm M, Lindbergh G (2012) Investigation of short circuit scenarios in lithium ion battery cell. J Electrochem Soc 159:A848–A859
Tarascon JM, Guyomard D (1991) Lithium metal free rechargeable batteries based upon Li1+xMn2O4 cathodes and carbon anodes. J Electrochem Soc 38:2864–2868
Winter M, Besenhard JO (1999) Electrochemical lithiation of tin and tin-based intermetallics and compounds. Electrochem Acta 145:31–50
Das Gupta R, Schwandt C, Fray DJ (2014) Preparation of tin filled carbon nanotubes and nanoparticles by molten salt electrolysis. Carbon 70:142–148
Das Gupta R (2009) The electrochemical production of tin filled carbon nanotubes and their use as anode materials in lithium-ion batteries. Ph.D. Thesis, University of Cambridge
Kamali AR, Fray DJ, Unpublished results
Kamali AR, Fray DJ (2015) A possible scalable method for the synthesis of Sn-containing carbon nanostructures. Mater Today Commun 2:38–48
Kamali AR, Fray DJ (2015) Large-scale preparation of graphene by high temperature insertion of hydrogen in graphite. Nanoscale 7:11310–11320
Hou L, Lian L, Li D, Pang G, Li J, Zhang X, Xiong S, Yuan C (2013) Mesoporous N-containing carbon nanosheets towards high performance electrochemical capacitors. Carbon 64:141–149
Chen JC, Liu YQ, Li N, Wu C, Xu L, Yang H (2015) Nanostructured polystyrene/polyaniline/graphene hybrid materials for electrochemical supercapacitor and Na-ion battery applications. J Mater Sci 50:5466–5474. doi:10.1007/s10853-015-9092-z
Xian HY, PengTJ Sun HJ, Wang JD (2015) Preparation of graphene nanosheets from microcrystalline graphite by low-temperature exfoliated method and their supercapacitive behavior. J Mater Sci 50:4025–4033. doi:10.1007/s10853-015-8959-3
Wang G, Shen X, Yao J, Park J (2009) Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 47:2049–2053
Wang Z, Shoji M, Ogata H (2012) Synthesis and characterisation of platinum nanoparticles on carbon nanosheets with enhanced electrocatalytic activity toward methanol oxidation. App Surf Sci 259:219–224
Li X, Wang X, Zhang L, Lee S, Dai H (2008) Chemically derived ultrasmooth graphene nanoribbon semiconductors. Science 319:1229–1232
Li D, Müller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3:101–105
Nicolosi V, Chhowalla M, Kanatzidis MG, Strano MS, Coleman JN (2013) Liquid exfoliation of layered materials. Science 340:1420
Malesevic A, Vizireanu S, Kemps R, Vanhulsel A, Haesendonck CV, Dinescu G (2007) Combined growth of carbon nanotubes and carbon nanowalls by plasma-enhanced chemical vapour deposition. Carbon 45:2932–2937
Tanaike O, Kitada N, Yoshimura H, Hatori H, Kojima K, Tachibana M (2009) Lithium insertion behaviour of carbon nanowalls by dc plasma CVD and its heat: treatment effect. Solid State Ionics 180:381–385
Mori S, Veno T, Suzuki M (2011) Synthesis of carbon nanowalls by plasma-enhanced vapour deposition in a CO/H-2 microwave discharge system. Diam Relat Mater 20:1129–113215
Shaahin A, Kalaantari H, Garey J, Balandin AA, Abbaschian R (2011) Growth of graphene and graphite nanocrystals from a molten phase. J Mater Sci 46:6255–6263. doi:10.1007/s10853-011-5432-9
Liu QX, Wang CX, Li SW, Zhang JX, Yang GW (2004) Nucleation stability of diamond nanowires inside carbon nanotubes: a thermodynamic approach. Carbon 42:629–633
Zhu YQ, Sekine T, Kobayashi T, Takazawa E, Terrones M, Terrones H (1998) Collapsing carbon nanotubes and diamond formation under shockwaves. Chem Phys Lett 287:689–693
Zhang F, Ahmed F, Holzhüter G, Burkel E (2012) Growth of diamond from fullerene C60 by spark plasma sintering. J Cryst Growth 340:1–5
Yusa H (2002) Nanocrystalline diamond directly transformed from carbon nanotubes under high pressure. Diam Relat Mater 11:87–91
Pal’yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF, Shatsky AF, Sobolev NV (1999) The diamond growth from Li2CO3, Na2CO3, K2CO3 and Cs2CO3 solvent at P = 7 GPa and T = 1700–1750 °C. Diam Relat Mater 8:1118–1124
Kamali AR, Fray DJ (2015) Preparation of nanodiamonds from carbon nanoparticles at atmospheric pressure. Chem Commun 51:5594–5597
Kamali AR, Schwandt C, Fray DJ (2012) On the oxidation of electrolytic carbon nanomaterials. Corros Sci 54:307–313
House of Commons Environmental Audit Committee, Fourth Report of Session 2009–2010 The role of carbon materials in preventing dangerous climate change. The Stationary Office 2010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The University of Cambridge has licensed the technology to two companies, one dealing with the production of nanodiamonds and the other dealing with the production of graphene.
Rights and permissions
About this article
Cite this article
Kamali, A.R., Fray, D. Electrochemical interaction between graphite and molten salts to produce nanotubes, nanoparticles, graphene and nanodiamonds. J Mater Sci 51, 569–576 (2016). https://doi.org/10.1007/s10853-015-9340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9340-2