Abstract
Mg–Ca–Mn–Zn ferrites having general formula Mg0.2−xCaxMn0.3Zn0.5Fe2O4 (x = 0, 0.10, 0.15 and 0.20) were synthesized using solid state reaction method and sintered at 1100 and 1200 °C for 4 h. Structural, microstructural and elemental analyses of synthesized ferrites were performed by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM) and energy dispersive analysis of X-ray. A cubical spinel crystal structure with secondary phases is conformed in synthesized samples by XRD analysis. The lattice parameter decreased initially and then increases at x = 0.20 at 1100 °C. On contrary, the lattice parameter increases with Ca2+ ions concentration at 1200 °C which is attributed due to the different ionic radius of Mg2+ and Ca2+ ions. The FTIR spectra show the presence of high frequency and low frequency band at 559.36–460.14 cm−1 at 1100 °C and 549.71–558.35 cm−1 at 1200 °C corresponding to the tetrahedral and the octahedral sites. The SEM images revealed that the average grain size increases with the increase of Ca2+ ions concentration which may be attributed due to the fact that Ca2+ ions influences the microstructure by forming a liquid phase during sintering process and expedites the grain growth by lowering the rate of cation interdiffusion. Low frequency dielectric dispersion is consistent with the Maxwell–Wagner interfacial polarization. Dielectric constant increases with Ca2+ concentration for both sintering temperatures. The samples x = 0.10 and 0.15 exhibits highest conductivity at 1100 and 1200 °C, respectively because the electron hopping between Fe3+ and Fe2+ ions increases. The conduction process is attributed due to the presence of grain and grain boundary effect as revealed by the impedance study. The significant decrement in permeability with Ca2+ concentration is attributed due to the lower saturation magnetization and increased inner stress or crystal magnetic anisotropy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In due course of time, it is necessary to synthesize of polycrystalline ferrites through different synthetic routes with diverse properties to meet the requirements of today’s technological demands [1, 2]. Among the ferrites, Mg–Mn–Zn ferrites are the important ferrites because they possess moderate initial permeability, high electrical resistivity and relatively easy synthesizing method and due to their wide applications in magnetic cores and deflection yokes in communication, deflection yoke core for high-resolution display monitors, electronic equipment and domestic appliances [3,4,5,6,7]. It is reported that the replacement of nonmagnetic ion of Mg2+ by magnetic ion of Mn2+ (L = 0, S = 5/2, J = 5/2) in Mg–Zn ferrites affect the magnetic properties because the substitution of Mn2+ ions can reduce the magnetic coercivity since the angular momentum is Mn2+ ions is zero (L = 0). In spinel lattice, Mn2+ cations are at its high spin state (S = 5/2). The Mn2+ cations affect the saturation magnetization since it has 5d electron configurations (\({{\text{e}}^2}{\text{t}}_2^3\) and \({\text{t}}_{{\text{2g}}}^3{\text{e}}_{\text{g}}^{\text{2}}\)) at the tetrahedral and octahedral lattice site [8, 9]. Rikukawa et al. have studied low temperature sintering of magnesium–manganese–zinc ferrite [10]. Gorelik et al. have reported the aging mechanism in magnesium–manganese–zinc ferrite [11]. On the other hand, Ebrahimi et al. have investigated the optimum condition for the preparation of pure magnesium–manganese–zinc ferrite [12]. Fan et al. have studied the sintering temperature dependent properties of magnesium–manganese–zinc ferrite [13]. The mechanism of the initial permeability in magnesium–manganese–zinc ferrite has studied by Kolenbrander et al. [14]. Van der Zaag et al. have studied the effect of intragranular domain walls in magnesium–manganese–zinc [15]. It has been reported in the literature that the substitution of non-magnetic divalent ions in ferrites could enhance the physical, electrical, and magnetic properties as well as improve the quality of material for practical applications [16]. For high frequency applications, it is necessary to study the frequency dependent electrical, dielectric and magnetic properties. It is reported that the physical, electrical and magnetic properties of polycrystalline ferrites are dependent on sintering composition, synthesis method, microstructure, dwell time, heating and cooling rate, nature and amount of dopant, cations distribution and particle size [17, 18]. In the spinel lattice, certain amount of Ca2+ ions can occupy [19]. It has been reported that the maximum 23% of Ca2+ ions can be substituted for Mg2+ ions in magnesium ferrite [20]. Several research works have been performed to evaluate the electrical, magnetic properties and thermal coagulation therapy in ac magnetic field of Ca2+ substituted spinel ferrites such as Ni0.245Zn0.755Fe2O4 [19], Ni0.5Cu0.3Zn0.2Fe2O4 [21], Ni–Zn–Ca [22], Mg1−xCaxNiyFe2−yO4 [23], Mg1−xCaxFe2O4 [20, 24], Cd0.5Mg0.5−xCaxFe2O4 [25], and Mg0.2Ca0.8Fe2O4 [26]. In our previous study, we report the sintering temperature dependent 10% Ca-doped Mn0.6Zn0.4Fe2O4 [27]. Based on the literature survey, it can be assumed that Ca2+ substitution modify the electric and magnetic properties of ferrites considerably. The occupancy of Ca2+ into the spinel lattice would create a lattice distortion due to its larger ionic radius. As results, the modification of material properties to an appreciable extent is expected. There are no studies available in the literature on the influence of Ca2+ ions substitution on the structural, microstructural and electromagnetic properties of Mg0.2Mn0.3Zn0.5Fe2O4 ferrite. It would be interesting to substitute a large cation such as Ca2+ for Mg2+ in Mg0.2Mn0.5Zn0.3Fe2O4 ferrite because the ionic radius of the cations involved are Mg2+ (0.72 Å), Mn3+ (Å), Mn4+ (0.52 Å), Ca (1.14 Å), Zn (0.68 Å) and Fe3+ (0.67 Å) at A-site and B-site are 0.49 and 0.64 Å. The objectives of the present work are to study the effect of Ca2+ ions substitution on structural, microstructural, electrical and magnetic properties of Mg0.2Mn0.5Zn0.3Fe2O4 ferrite. In the present study, the study of dielectric constant, dielectric loss, ac electrical conductivity and complex permeability are made for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 (x = 0.0, 0.10, 0.15, and 0.20) ferrites, which makes better understanding the impact of Ca2+ on the electrical conduction mechanism, dielectric polarization and complex permeability.
2 Experimental
2.1 Synthesis of materials
Mg–Ca–Mn–Zn ferrites having general formula Mg0.2−xCaxMn0.3Zn0.5Fe2O4 (x = 0, 0.10, 0.15 and 0.20) ferrites powder were synthesized by solid state reaction method using MgO (≥99.9%), CaCO3 (≥99.9%), MnCO3 (≥99.9%), ZnO (≥99.9%) and Fe2O3 (≥99.9%) as a raw materials. The raw materials were mixed in stoichiometric proportions and ground for 4 h. Then the mixed powders were calcined at 900 °C for 5 h with the heating and cooling rates at 10 and 5 °C/min, respectively. The calcined powders were ground again with a pestle in an agate mortar for making fine powder. Powders were pressed into pellet-shaped and toroidal samples with a uniaxial pressure of 6400 psi with polyvinyl alcohol (PVA) as binder. Finally, the samples were sintered at 1100 and 1200 °C for 4 h with the same heating and cooling rates as that of calcination process.
2.2 Characterization
Crystal structure study was performed using Rigaku Ultima IV (Japan) using X-ray powder diffractometer (RIGAKU Ultima IV, Japan). The infrared absorption spectra were carried out by an infrared spectrophotometer (Shimadzu FTIR 8400S). The crystallite size was calculated by the Debye–Scherrer’s formula [28]. The lattice constant (a) was calculated by using \({d_{hkl}}\) spacing and \(hkl\) parameters by using: \(a=\frac{{\lambda {{\left[ {{h^2}+{k^2}+{l^2}} \right]}^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}}}{{2\sin \theta }}\), where \(hkl\) are the Miller indices of the crystal plane [29]. Hopping length of octahedral site (\({L_B}\)) and tetrahedral (\({L_A}\)) were determined using: \({L_A}=\frac{{a\sqrt 3 }}{4}\) and \({L_B}=\frac{{a\sqrt 2 }}{4}\), respectively [30]. The value of the tetrahedral radii (\({R_{tetra}}\)) and the octahedral radii (\({R_{octa}}\)) is calculated by \({R_{tetra}}=a\sqrt 3 \left( {u - 0.25} \right) - {R_0}\) and \({R_{octa}}=a\left( {\frac{5}{8} - u} \right) - {R_0}\) respectively, where \(a\) is lattice parameter, \(u\) is oxygen positional parameter, \({R_0}\) is the radius of oxygen ion (1.32 Å) [31]. The value of oxygen parameter for an ideal FCC crystal is 0.375 [31]. The tetrahedral bond length \({d_{AO}}\left( {{d_{A - {O^{2 - }}}}} \right)\) and the octahedral bond length \({d_{BO}}\left( {{d_{B - {O^{2 - }}}}} \right)\) can be calculated using the relations: \({d_{AO}}=a\sqrt 3 \left( {u - 0.25} \right)\) and \({d_{BO}}=a{\left( {3{u^2} - \frac{{11}}{4}u+\frac{{43}}{{64}}} \right)^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\) [32]. The tetrahedral edge \({d_{AE}}\) and the octahedral edge \({d_{BE}}\) and unshared edge \({d_{BEU}}\) can be determined using the relations: \({d_{AE}}=a\sqrt 2 \left( {2u - 0.5} \right)\), \({d_{BE}}=a\sqrt 2 \left( {1 - 2u} \right)\) and \({d_{BEU}}=a{\left( {4{u^2} - 3u+\frac{{11}}{{16}}} \right)^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\) [32]. The morphology and elemental analysis were characterized by scanning electron microscopy (SEM) (Model: JEOL-JSM-6490LA) equipped with energy dispersive X-ray spectrophotometer (EDAX). The average grain size was calculated according to Rahaman et al. [27]. X-ray density (\({\rho _x}\)), bulk density (\({\rho _B}\)) and porosity were obtained based on the available literatures [33]. Electrical and magnetic properties were carried out by Wynne Kerr impedance analyzer (Model: 6500). The real part (\(Z'\)) and the imaginary part (\(\varepsilon ''\)) of complex impedance were determined using: \(Z'=\frac{G}{{{G^2}+{\omega ^2}C_{p}^{2}}}\) and \(Z''=\frac{{\omega {C_P}}}{{{G^2}+{\omega ^2}C_{P}^{2}}}\) relations [34]. The initial permeability of the samples was calculated according to Rahaman et al. [27].
3 Results and discussion
3.1 X-ray diffraction analysis
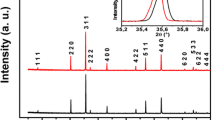
Figure 1a, b show the X-ray diffraction spectra of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 (x = 0.0, 0.10, 0.15 and 0.20) ferrites at 1100 and 1200 °C, respectively. The XRD patterns confirm the formation of a cubic spinel structure. It is observed that secondary phases appeared in the XRD patterns of Mg0.2Mn0.5Zn0.3Fe2O4 at 1100 °C which is attributed due to the hematite (α-Fe2O3). The possible reason for the appearance of secondary phase in Mg0.2Mn0.5Zn0.3Fe2O4 sintered powder is that the solid state reaction was not completed under the sintering process at 1100 °C. Hematite (α-Fe2O3) phase is completely disappeared in Mg0.2Mn0.5Zn0.3Fe2O4 ferrite at 1200 °C which means that the Mg0.2Mn0.5Zn0.3Fe2O4 was considered to be pure single-phase spinel structure. The impurity phases appeared in the Ca2+ substituted samples at 1100 and 1200 °C are attributed due to Ca2Fe2O5 or CaFe2O4 [19, 21, 24]. In Table 1, the lattice parameters for the samples sintered at 1100 and 1200 °C are presented. At 1100 °C, the lattice parameter increases up to x = 0.15 and then decreases for x = 0.20. On the other hand, the lattice parameter increases with Ca2+ concentration at 1200 °C. The little increase in the lattice parameter in the Ca2+ substituted samples is attributed to the differences of the ionic radii of Ca2+ and Mg2+. Ca2+ has larger ionic radius (1.14 Å) as compared to Mg2+ (0.66 Å). The substitution of Ca2+ leads to the expansion of Mg0.2Mn0.5Zn0.3Fe2O4 lattice. As a consequence, the lattice constant increases. A slight decrease in the lattice constant for x = 0.20 is due to the migration of few Mg2+ to A-sites owing to the incorporation of Ca2+ on the B-sites. The decrease in lattice constant for x = 0.15 indicates the solubility limit. When the solubility limit is obtained no further Ca2+ is dissolved in Mg0.2Mn0.5Zn0.3Fe2O4 lattice and Ca2+ ions accumulate at the grain boundaries and thus form the thin insulating layer around the grains. The existence of Ca2Fe2O5 or CaFe2O4 phases on the grain boundaries suppresses the grain growth by hindering the mobility of grain boundary [19, 21, 24]. The Ca2+ may reside on B-sites as Ca2+ have larger ionic radius (1.14 Å) as compare to that of Fe3+ (0.64 Å) ions. The occupation probability of Ca2+ in the A-sites is very remote because the A-sites are too small for the occupation of large Ca2+. When the Ca2+ ions occupy in B-sites, the internal stresses may occur in B-sites because of their large ionic radii [19, 21, 24]. The variation of lattice constant with sintering temperatures is attributed due to the variation of cation distribution between both B-sites and A-sites. It is observed that the most intense (311) peak shifts towards the lower angle side with the increase of Ca2+ concentration at 1100 and 1200 °C, respectively which reveals the expansion of unit cell lattice due to Ca2+ substitutions. In Table 2, the crystallite size at 1100 and 1200 °C is presented. The crystallite size for Mg0.2Mn0.5Zn0.3Fe2O4 sample is higher than that of Ca2+ substituted samples 1100 °C, which is attributed due to the fact that the Ca2+ restrict the grain growth of Mg0.2Mn0.5Zn0.3Fe2O4. It is observed that the crystallite size of Ca2+ substituted samples is higher at 1200 °C than that of 1100 °C. Crystallite size increases with sintering temperature which is attributed due to the Oswald ripening. From Table 2, it is found that the grain sizes obtained from SEM are significantly larger than the crystallite sizes as determined by Debye–Scherer’s formula at both sintering temperature. The difference between abovementioned matters is attributed due to the microstructures consisting of coalesced and multi-grained structures [20]. The hopping length between the magnetic ions in the A-site and in the B-site is calculated with the help of XRD data and tabulated in Table 1. It is found that the hopping lengths (\({L_A}\) and\({L_B}\)) increase with increase of Ca2+ substitution which suggests that the magnetic ions go far away to each other. On the other hand, tetrahedral bond length \({d_{AO}}\left( {{d_{A - {O^{2 - }}}}} \right)\), the octahedral bond length \({d_{BO}}\left( {{d_{B - {O^{2 - }}}}} \right)\), tetrahedral edge \({d_{AE}}\), the octahedral edge \({d_{BE}}\) and unshared edge \({d_{BEU}}\) are calculated and presented in Table 1. Therefore, to the difference in the ionic radii of Ca2+ and Mg2+ ions, the allied parameters increases with the increase of Ca2+ ions.
3.2 Fourier transform infrared spectroscopic (FTIR) analysis
FTIR spectra of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites are shown in Fig. 2a, b sintered at 1100 and 1200 °C for 4 h, respectively. It is observed that the high frequency band \({\nu _1}\) and low frequency band \({\nu _2}\) are in the range of 559.36–549.71 and 464.84–458.35 cm−1 at 1100 and 1200 °C, respectively. Waldron [35] has reported that their presence and assigned the high frequency band \({\nu _1}\) which is attributed due to the A-site where it is produced by stretching vibrations of A-site metal–oxygen bonding, and low frequency band \({\nu _2}\) which is attributed due to the metal–oxygen bonding in the B-sites. Due to the shorter bond length of the A-site cluster than that of the B-site cluster, the \({\nu _1}\) values are higher than those of \({\nu _2}\). The values of \({\nu _1}\) and \({\nu _2}\) at 1100 and 1200 °C are presented in Table 2. The spectra showed a small shift by the substitution of Ca2+ i.e. the A-site bands are shifted from higher value (559.36 cm−1) to lower value (538.14 cm−1) at 1100 °C. On the other hand, the A-site bands are shifted from higher value (549.71 cm−1) to lower value (522.33 cm−1) at 1200 °C. The shifting of \({\nu _1}\) and \({\nu _2}\) reconfirms the cation distribution in both B-sites and A-sites of the synthesized samples. The shift towards higher frequency can be attributed to the shifting of Fe3+ towards the O2−. The Fe3+–O2− bond distance decreases since larger ionic radii of Ca2+ occupies A-site. Similarly, \({\nu _2}\) bands are slightly shifted to higher value i.e. 460.99–464.84 cm−1 at 1100 °C and 458.35–460.14 cm−1 at 1200 °C. The following bands appeared in the spectrum such as 1003.28 cm−1 (x = 0.20), 1109.07 cm−1 (x = 0.0), 1109.07 cm−1 (x = 0.10), 1112.93 cm−1 (x = 0.15), 1165.00 cm−1 (x = 0.0), 1381.03 cm−1 (x = 0.0), 1377.17 cm−1 (x = 0.10), 1462.04 cm−1 (x = 0.0), 1479.40 cm−1 (x = 0.2) at 1100 °C. The abovementioned absorption peaks are appeared due to the deformation of C–H group [36]. The bands around 1629.85 cm−1 (x = 0.0), 1627.92 cm−1 (x = 0.10), 1627.92 cm−1 (x = 0.15), 1625.99 cm−1 (x = 0.20), 1743.65 cm−1 (x = 0.0), 2380.41 cm−1 (x = 0.0), 2380.41 cm−1 (x = 0.10), 2369.70 cm−1 (x = 0.15), 2380.41 cm−1 (x = 0.20), 2854.65 cm−1 (x = 0.0), 2854.65 cm−1 (x = 0.20), 2924.09 cm−1 (x = 0.0), 2926.01 cm−1 (x = 0.10), 2926.01 cm−1 (x = 0.15), 2924.09 cm−1 (x = 0.20), which are attributed due to the stretching and bending vibrations of H–O–H, absorbed water and atmospheric CO2 [37]. 3444.87 cm−1 (x = 0.0), 3444.87 cm−1 (x = 0.10), 3446.79 cm−1 (x = 0.15), 3437.15 cm−1 (x = 0.20), 3693.68 cm−1 (x = 0.0), 3691.75 cm−1 (x = 0.10), 3695.61 cm−1 (x = 0.15), 3693.68 cm−1 (x = 0.20), and 3774.69 cm−1 (x = 0.0), 3774.69 cm−1 (x = 0.20), 3776.62 cm−1 (x = 0.10), 3772.76 (x = 0.15), 3876.92 (x = 0.20) are attributed due to the presence of small moisture. On the contrary, the following bands appeared in the spectrum such as 1109.07 cm−1 (x = 0.0), 1109.07 cm−1 (x = 0.10), 1109.07 cm−1 (x = 0.15), 1107.12 cm−1 (x = 0.20), 1163.08 cm−1 (x = 0.0), 1438.90 cm−1 (x = 0.0) at 1200 °C. The abovementioned absorption peaks are appeared due to the deformation of C–H group [36]. The bands around 1625.99 (x = 0.0), 1624.06 cm−1 (x = 0.10), 1627.72 cm−1 (x = 0.15), 1625.99 cm−1 (x = 0.20), 1749.44 cm−1 (x = 0.0), 1755.22 cm−1 (x = 0.10), 1751.36 cm−1 (x = 0.20), 2380.41 cm−1 (x = 0.0), 2380.41 cm−1 (x = 0.10), 2380.41 cm−1 (x = 0.15), 2380.41 cm−1 (x = 0.20), 2854.65 cm−1 (x = 0.0), 2856.58 cm−1 (x = 0.10), 2856.58 cm−1 (x = 0.15), 2856.58 cm−1 (x = 0.20), 2924.09 cm−1 (x = 0.0), 2922.16 cm−1 (x = 0.10), 2924.09 cm−1 (x = 0.15), 2924.09 cm−1 (x = 0.20), 3437.15 cm−1 (x = 0.0), 3441.01 cm−1 (x = 0.10), 3444.87 cm−1 (x = 0.15), 3435.22 cm−1 (x = 0.20) which are attributed due to the stretching and bending vibrations of H–O–H, absorbed water and atmospheric CO2 [37]. 3693.68 cm−1 (x = 0.0), 3697.64 cm−1 (x = 0.10), 3693.68 cm−1 (x = 0.15), 3693.68 cm−1 (x = 0.20), 3770.84 cm−1 (x = 0.0), 3770.84 cm−1 (x = 0.10), 3770.84 cm−1 (x = 0.15), 3774.69 cm−1 (x = 0.20), and 3874.99 cm−1 (x = 0.0), 3876.92 cm−1 (x = 0.10), 3874.99 cm−1 (x = 0.20) are attributed due to the presence of small moisture.
3.3 Microstructural and elemental analysis
Figures 3a–d and 4a–d show typical SEM images and EDAX spectra of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites sintered at 1100 and 1200 °C. It is clear from SEM images that the Mg0.2Mn0.5Zn0.3Fe2O4 ferrite consists of some irregularly cubic particles at 1100 and 1200 °C. On the contrary, sharp hexagonal/plate like-shaped stack on each other are formed with the increase of Ca2+ concentration at 1100 and 1200 °C. The average grain sizes of the samples sintered at 1100 and 1200 °C is presented in Table 2. It is found that the grain size increases with increasing Ca2+ concentrations and it strongly depends on the amount of Ca2+. Ca2+ influences the morphology by forming a liquid phase during sintering and expedites the grain growth by lowering the rate of cation interdiffusion [38, 39]. It is also found that the average grain size of the synthesized samples is greater than 2.8 μm for both sintering temperatures, which is assumed to remain in the multi-domain structure [40]. EDAX confirms the presence of Mg2+, Ca2+, Mn2+, Zn2+, Fe3+, and O2− as per the initial stoichiometry.
3.4 Variation of dielectric constant
Figure 5a, b shows frequency dependent real part (\(\varepsilon '\)) of dielectric constant for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites sintered at 1100 and 1200 °C. It is observed that \(\varepsilon '\) decreases as frequency increases showing usual dielectric and it remains constant at higher frequencies. The dispersion in dielectric behavior is attributed due to the Maxwell–Wagner [41, 42] interfacial space charge polarization in agreement with Koop’s phenomenological theory [43]. The low value of \(\varepsilon '\)at high frequency is in good agreement with the previous reports [27] and the high value of \(\varepsilon '\)often at low frequency is due to the effect of heterogeneity of the ferrite samples such as pores, surfaces and layers on grains. It is observed that the value of increases with Ca2+ concentration at 1100 and 1200 °C. Figure 5c, d shows the value of \(\varepsilon '\) as function of Ca2+ concentration at different selected frequencies. As the Ca2+ concentration is increasing the value of \(\varepsilon '\) is increasing up to x = 0.10, thereafter, it displayed a decrease on further increasing Ca2+ concentration. It is known that Fe3+ ions reside on both A and B-sites in Mg0.2Mn0.5Zn0.3Fe2O4 lattice. After the substitution of Mg2+ by Ca2+, some Mg2+ ions occupy A-sites in Mg0.2Mn0.5Zn0.3Fe2O4 lattice and displace Fe3+ ions from A-sites. Therefore, the displaced Fe3+ ions migrate from A-site to B-site. As a consequence, the concentration of Fe3+ at B-site increases and the hopping conduction increases. The probability of occupying Ca2+ on A-site is very rare owing to the larger ionic radius of Ca2+ (1.14 Å). Therefore, the substitution of Ca2+ enhances the dielectric polarization, consequently the dielectric constant.
3.5 Variation of dielectric loss
Figure 6a, b shows the variation of dielectric loss tangent (tanδ) for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites with frequency sintered at 1100 and 1200 °C. From the Fig. 6a, b, it is observed that tanδ decreases rapidly at low frequencies is in accordance with the Koop’s phenomenological model [43], however, when the frequency increases the rate of decrease in tanδ turns out to be slow and becomes independent of frequency. Generally, tanδ in ferrites is originated electron hopping and charged defect dipoles [44]. In the low frequency domain, tanδ is attributed to electron hopping, while in the high frequency domain; tanδ attributes to the response of the defect dipoles to the field. The defect dipoles are formed due to change in the cation state, such as Fe3+/Fe2+, Mg2+/Mg3+, Zn2+/Zn3+ or Mn2+/Mn3+, during the heating process. With the increase of frequency, relaxation of dipoles under the influence of electric field is decreased. As result, tanδ is decreased in the high frequency domain [44]. Furthermore, it is worthwhile to mention that tanδ starts to increase at a frequency of ≥46.6 MHz for x = 0.10 when the sample sintered at 1100 °C. On the contrary, tanδ starts to increase at a frequency of ≥25.03 MHz for x = 0.10, 0.15 and 0.20 when the samples sintered at 1200 °C. At lower frequency region, the value of tanδ is high which is attributed due to the lower conductivity of grain boundary because the more energy is required for electron exchange between Fe2+ ↔ Fe3+. In the higher frequency range, tanδ is low which is attributed due to the higher conductivity of grain because the small amount of energy is required for electron transfer between Fe2+ ↔ Fe3+ [45]. The x = 0.20 sample exhibits loss peak at a frequency of ≤1 MHz when the sample sintered at 1100 °C. A maximum in tanδ is observed when \(\omega \tau =1\), where \(\omega\) is the angular frequency of the field (\(\omega =2\pi {f_{\max }})\) and \(\tau\) is the relaxation time which is inversely proportional to the hopping probability per unit time (\(\tau =\left( {{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} \right)p)\)) or \({f_{\max }}=\left( {{1 \mathord{\left/ {\vphantom {1 \pi }} \right. \kern-\nulldelimiterspace} \pi }} \right)p\) [46, 47]. The loss is attributed due to the imperfection in crystal lattice and impurities [48]. It is observed that the tanδ increases in the high frequency region with the increase of Ca2+ concentration which may be attributed due to the appearance of small pores [49]. Figure 6c, d shows the variation of tanδ as a function of Ca2+ concentration at different selected frequencies. As the Ca2+ concentration is increasing the tanδ is increasing up to x = 0.10, thereafter, it displayed a decrease with the further increase of Ca2+ concentration. The replacement of Mg2+ by Ca2+ might have been improved the DC resistivity of the ferrites. As a result, the tanδ decreases [50]. The tanδ also decreases with increasing Ca2+ concentration. As the frequency increases the tanδ increases. This phenomenon could be attributed to the microstructure with poor surface area.
3.6 Variation of ac conductivity
Figure 7a, b shows the variation of ac conductivity (\({\sigma _{ac}})\) for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites with frequency sintered at 1100 and 1200 °C, respectively. At low frequency region, it is observed that \({\sigma _{ac}}\) almost remains constant and it increases in the high frequencies region. The increase in \({\sigma _{ac}}\) at the high frequency region is attributed to the enhancement of electron hopping between the charge carriers. Figure 7c, d shows the variation of \({\sigma _{ac}}\) as function of Ca2+ concentration at different frequencies sintered at 1100 and 1200 °C, respectively. The samples x = 0.10 and 0.15 exhibited the highest \({\sigma _{ac}}\) at 1100 and 1200 °C. This improvement in \({\sigma _{ac}}\) is attributed due to the increase in the charge carrier’s with the increases of Ca2+ concentration [51]. It was assumed that the Ca2+ occupy the A-site [52]. Hence, some Fe3+ migrates from the A-site to the B-site. The migration of Fe3+ may be accompanied by the formation of Fe2+ ions in the B-site [53]. Thus, the ratio Fe2+/Fe3+ in the B-site increases which causes the probability of hopping between Fe2+/Fe3+ and consequently \({\sigma _{ac}}\) increases. A further increase in Ca2+ concentration leads to a decrease in \({\sigma _{ac}}\). This decrement in \({\sigma _{ac}}\) for high Ca2+ may be attributed due to the decreased conduction path as a result of a local distortion of the Mg0.25Mn0.25Zn0.5Fe2O4 lattice. The source of this distortion may be originated from the difference in the charge and ionic radii between Mg2+ and Ca2+ cations. The decreasing in \({\sigma _{ac}}\) for higher Ca2+ concentrations is possibly due to larger formation Fe2+ at higher sintering temperature. The magnitude of electron exchange interaction between Fe2+ and Fe3+ depends on the concentration of Fe2+/Fe3+ present on the crystallographically equivalent sites. When the Mg2+ is completely replaced by the Ca2+, the \({\sigma _{ac}}\) lies between the values of x = 0.10 and 0.15 samples. The sample x = 0.10 shows the highest electrical conductivity which is attributed due to the electron hopping between Fe3+ and Fe2+ increases as the migration of Fe3+ to the B-sites increases with increasing the occupancy of Ca2+ in the A-sites at 1100 °C [52]. Furthermore, the slight decrease of \({\sigma _{ac}}\) for x = 0.15 and 0.20 samples is due to a slight decrease of the electron hopping between Fe3+ and Fe2+ as a result of migration small fraction of Ca2+ to the B-sites.
3.7 Complex impedance spectroscopy
Figure 8a, c shows the variation of real part (\(Z'\)) of impedance for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites sintered at 1100 and 1200 °C. It is seen that \(Z'\) decreases as the frequency increases up to ≤0.1 MHz and attain a constant value at ≥1 MHz. The decrement in \(Z'\) with the increase of frequency indicates that the value of \({\sigma _{ac}}\) is enhanced [54]. At high frequency region, the value of \(Z'\) merged together. Therefore, at higher frequency, the merger of curves indicates the reduction of space charge polarization and a consequently barrier properties of the materials is lowered [55]. Figure 8b, d shows the imaginary part (\(Z''\)) of impedance at 1100 and 1200 °C. Samples x = 0.10, 0.15 and 0.20 samples show peak in \(Z''\) and the peak shifts to high frequency region. The shifting of peak to the lower frequency region is attributed to the presence of space charge, which is related to the charge carriers in association with the oxygen vacancies [56, 57]. The peak in \(Z''\) indicates the beginning of electrical relaxation [58].
3.8 Nyquist plots
Nyquist plots of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites sintered at 1100 and 1200 °C is presented in Fig. 9a, b, respectively. Depending on the grain, grain boundary, and grain-electrode effects three semicircles are appeared in Nyquist plots. The semicircle in low frequency region and the semicircles in the high frequency are attributed due to the grain boundary properties and the grain or bulk property [59]. It is observed that the Mg0.2Mn0.5Zn0.3Fe2O4 and x = 0.20 samples exhibit single semicircle when the samples sintered at 1100 °C for 4 h. The appearance of the single semicircle in Mg0.2Mn0.5Zn0.3Fe2O4 at 1100 °C is due to the existence of localized and non-localized conduction phenomena. Therefore the conduction process in Mg0.2Mn0.5Zn0.3Fe2O4 ferrite takes place predominantly through the grain interior only [60]. It is observed that incomplete semicircular arc with large positive slope appears in Mg0.2Mn0.5Zn0.3Fe2O4 sample at 1200 °C which is due to the very high of impedance. There is a tendency for forming two semicircles. A small semicircle at high frequencies corresponding to grain and large semicircle at low frequencies corresponding to grain boundary effect for x = 0.10 and 0.15 samples at 1100 and 1200 °C, respectively [61]. The sample x = 0.10 exhibits three semicircle at 1100 °C. As the Ca2+ concentration increases the diameter of the semicircle decreases except for x = 0.10 which indicates that the grain interior resistance reduces [62].
3.9 Variation of complex permeability
Figure 10 show the real part (\({\mu '_i}\)) of complex permeability of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites sintered at (a) 1100 and (c) 1200 °C. The \({\mu '_i}\) spectra show a complicated variation with the variation of Ca2+ concentration at both sintering temperatures. The value of \({\mu '_i}\) for x = 0.10, significantly decreases as compared to that of Mg0.2Mn0.5Zn0.3Fe2O4 at 1100 °C for 4 h. The value of \({\mu '_i}\) for x = 0.15 is higher than x = 0.10 but it is low as compared to Mg0.2Mn0.5Zn0.3Fe2O4 which means that the magnetic state of Mg0.2Mn0.5Zn0.3Fe2O4 is slightly influenced by the presence of Ca2+. When the doping level is less than x = 0.15, the amount of Ca2+ appears to be too low to give homogeneous liquid sintering. At x = 0.15, the presence of a few large faceted crystallites gives a higher value of \({\mu '_i}\). The liquid phase is formed with intragranular porosity at higher Ca2+ concentration, and \({\mu '_i}\) decreases. When the Mg2+ is completed substituted by the Ca2+, the value of \({\mu '_i}\) is the lowest one as compared to Mg0.2Mn0.5Zn0.3Fe2O4 sample. The sample x = 0.10 exhibits the minimum value of \({\mu '_i}\) at 1200 °C. The value of \({\mu '_i}\) for x = 0.15 substantially decreases as compared to that of Mg0.2Mn0.5Zn0.3Fe2O4 ferrite. But the value of \({\mu '_i}\) for x = 0.15 is higher than that of x = 0.10 but it is low as compared to that of Mg0.2Mn0.5Zn0.3Fe2O4. When the Mg2+ is completed replaced by the Ca2+, the value of \({\mu '_i}\) is the lowest one as compared to that of x = 0.10 and x = 0.15. In general, complex permeability of polycrystalline ferrites is strongly dependent on grain size, porosity etc. [63]. It is well know that the permeability of polycrystalline ferrites is the combined effect spin rotation and domain wall motion. At low frequency, the contribution of spin rotation is smaller than domain wall motion which is due to the reversible motion of domain walls in the case of a weak magnetic field [63]. The contribution of domain wall motion is negligible at low frequency [64, 65]. The \({\mu '_i}\) for polycrystalline ferrite due to the domain wall motion can be delineated as.
where \({M_s}\) is the saturation magnetization, \(D\) the average grain diameter, and \({\gamma _{DW}}\) the domain wall energy [66]. It is observed that the \({\mu '_i}\) remains constant up to a certain frequency called resonance frequency, and then exhibits a shallow peak before falling to low value for Mg0.2Mn0.5Zn0.3Fe2O4 sample when the sample sintered at 1100 and 1200 °C [67]. It is observed that \({\mu '_i}\) remains constant up to a certain frequency and then falling rapidly to relatively low values for x = 0.10, 0.15 and 0.20, respectively. It is also observed that the natural resonance peak gradually disappears for the Ca2+ substituted samples, which exhibits excellent higher frequency properties of the ferrites. It is found that the value of \({\mu '_i}\) significantly reduces with the increases of Ca2+ concentration for both sintering temperature which is attributed due to lower saturation magnetization and increased inner stress or magneto crystalline anisotropy. When Ca2+ entered the Mg0.2Mn0.5Zn0.3Fe2O4 lattice; it forced a larger fraction of Fe3+ to occupy B-sites. Therefore, the magnetic dilution of the A-sites increases. The A-site to B-site interaction is much weaker than the B-site to B-site interaction when the concentration of Ca2+ on A-sites increased to a certain value. Therefore, the weakened interaction between the B-site and A-site lattices divides B-site lattice into two sublattices with a canted magnetic moment, which expedites the decrease in the saturation magnetization [25, 68, 69]. Due to the intragranular pores magnetic properties deteriorates [70]. The intragranular pores pin the domain walls motion. Moreover, the Ca2+ induces an increase of the crystalline anisotropy which results in the decrease of \({\mu '_i}\) to a smaller value than that of the sample without Ca2+ [19]. For a higher amount of Ca2+, the decrease of the magnetization and \({\mu '_i}\), as well as the increase of the resistivity suggest that Ca2+ are dissolved in Mg0.2Mn0.5Zn0.3Fe2O4 lattice beyond their solubility limit; secondary phases Ca2Fe2O5 and CaFe2O4 with magnetically compensated sublattices appear at the boundaries. At the same time, the segregation process is enhanced with increasing Ca2+ concentration, resulting in a depletion of the ferromagnetic phase in Ca2+. Apart from the reduction in saturation magnetization, the value of \({\mu '_i}\) for Mg0.2Mn0.5Zn0.3Fe2O4 ferrite with the increase of Ca2+ concentration may be partly due to the presence of a Ca2Fe2O5 and CaFe2O4 compound [19, 21, 24]. The value of \({\mu '_i}\) at 1200 °C is much better than that of 1100 °C which is attributed due to the increased grain size [71] as well as the decrement of magnetic anisotropy. Magnetic anisotropy decreases due to the reduction of the internal stresses and crystal anisotropy when the sintering temperature increases [72]. The decrement of magnetic anisotropy may results minimum impediment for the domain walls motion. So that \({\mu '_i}\) increases with increase in sintering temperature. The values of \({\mu '_i}\) the samples at a particular frequencies sintered at different temperatures are shown in Table 3. Figure 10b, d shows the variation of magnetic loss (tanδ) with frequency at 1100 and 1200 °C. The tanδ arises due to the lag of domain walls motion with respect to the applied alternating magnetic field and imperfections in the synthesized ferrite lattice [73]. The values of tanδ are observed to decrease initially with frequency (≤105 Hz), reaching a minimum value (≤1), and then become almost constant up to 1 MHz. The value of tanδ decreases with increasing frequency because the domain wall motion cannot follow the applied field at a certain frequency. The increase in tanδ at ≥1 MHz indicates that the beginning of a possible resonance [74]. When the resonance occurs, there is a rapid increase in tanδ because the maximum energy is transferred from the applied field to the lattice. It is clear from Fig. 10b, d that the tanδ at 1200 °C is higher than that of 1100 °C which is attributed due to the increased Zn2+ loss. The loss of Zn2+ creates defects in the synthesized samples lattice. The values of tanδ of the samples at a particular frequencies sintered at different temperatures are presented in Table 3.
3.10 Relative quality factor
Figure 11a, b shows the relative quality factor (RQF) for Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites as function of frequency sintered at 1100 and 1200 °C. From Fig. 11a, b, it is observed that RQF shows a peak at particular frequencies for both sintering temperature. The peak values of RQF shows exactly similar behaviour to that of \({\mu '_i}\) since it is proportional to the RQF. It is seen that RQF deteriorates beyond 1 MHz for both sintering temperatures, i.e., the tanδ is minimum up to 1 MHz, and then it rises rapidly for all synthesized simples. Deterioration of RQF is clearly observed with the increase of sintering temperature. The maximum value of RQF for Mg0.2Mn0.5Zn0.3Fe2O4 is obtained sintered at 1100 and 1200 °C which is probably attributed due to the growth of less imperfections and defects sintered at relatively lower sintering temperature [74]. It is observed that the maximum value of RQF decreases with the increase of Ca2+ concentration. The lower RQF for Ca2+ substituted Mg0.2Mn0.5Zn0.3Fe2O4 ferrites might be due to higher hysteresis loss, which might arise from its porous structure. The decrement in maximum RQF is probably attributed to increase in tanδ [73]. The peak in RQF shifts to higher frequency range for x = 0.10 and for x = 0.15 and 0.20 shift to the lower frequency range with respect to Mg0.2Mn0.5Zn0.3Fe2O4 sample when the samples sintered at 1100 °C for 4 h. For x = 0.10 and 0.15 samples, RQF shifts to higher frequency domain and on the contrary, RQF shifts to the lower frequency domain for x = 0.20 with respect to Mg0.2Mn0.5Zn0.3Fe2O4 at 1200 °C. The x = 0.20 sample exhibits the highest RQF as compared to that of x = 0.10 and 0.15 samples at 1200 °C. This improvement in RQF is attributed due to the increased electrical resistivity. It is also seen that RQF is higher at 1200 °C than that of at 1100 °C. The maximum RQF values for the samples sintered at different temperatures are tabulated in Table 3.
4 Conclusions
In the present investigation, we have successfully synthesized Mg–Ca–Mn–Zn ferrites by solid state sintering at 1100 and 1200 °C. XRD and FTIR analyses revealed that the samples are found to be formed spinel phase. The lattice parameter increased due to the difference of ions radius between Mg2+ and Ca2+. The SEM images revealed that the average grain size increases with Ca2+ which may be attributed due to the fact that Ca2+ influences the microstructure by forming a liquid phase during sintering process and expedites the grain growth by lowering the rate of cation interdiffusion. The EDAX spectra confirm the presence of all elements as per initial stoichiometry. Low frequency dielectric dispersion is attributed to the Maxwell–Wagner model and Koops phenomenological theory. Permittivity increases with Ca2+ concentration at 1100 and 1200 °C. The samples x = 0.10 and 0.15 exhibits highest conductivity at 1100 and 1200 °C, respectively because the electron hopping between Fe3+ and Fe2+ ions increases. Complex impedance study indicates that the conduction process is due to the grain and grain boundary effect only. Permeability reduces significantly with the increase of Ca2+ because Ca2+ reduces the saturation magnetization as well as increases the inner stress or crystal magnetic anisotropy. Finally, it can be said that Ca2+ substitution effectively tunes the structural, microstructural and electromagnetic properties of Mg0.2Mn0.5Zn0.3Fe2O4 ferrite.
References
O.M. Hemeda, N.Y. Mostafa, O.H.Abd. Elkader, M.A. Ahmed, J. Magn. Magn. Mater. 364, 39–46 (2014)
R. Valenzuela, Phys. Res. Int. 2012, 1–9 (2012)
N.M. Deraz, A. Alarifi, S.A. Shaban, J. Saudi Chem. Soc. 14, 357–362 (2010)
A. Goldman, Handbook of Modern Ferromagnetic Materials. (Kluwer Academic Publishers, Boston, 1999)
J. Fan, F.R. Sale, J. Eur. Ceram. Soc. 20, 2743–2751 (2000)
Z. Pedzich, M.M. Bucko, M. Krolikowski, M. Bakalarska, J. Babiarz, J. Eur. Ceram. Soc. 24, 1053–1056 (2004)
N.M. Deraz, A. Alarifi, Int. J. Electrochem. Sci. 7, 6501–6511 (2012)
H. Mohseni, H. Shokrollahi, I. Sharifi, Kh. Gheisari, J. Magn. Magn. Mater. 324, 3741–3747 (2012)
U.R. Ghodake, N.D. Chaudhari, R.C. Kambale, J.Y. Patil, S.S. Suryavanshi, J. Magn. Magn. Mater. 407, 60–68 (2016)
H. Rikukawa, U. Kihara, M. Torii, IEEE Trans. Magn. 18, 1538–1540 (1982)
S.S. Gorelik, B.E. Levin, L.M. Letyuk, A.P. Nikol’skii, Sov. Phys. J. 10, 14–17 (1967)
S.A.S. Ebrahimi, Z.P. Fard, Key Eng. Mater. 336–338, 699–702 (2007)
J. Fan, W. Li, H. Zhao, X. Zhang, Z. Zhang, Adv. Mater. Res. 680, 31–34 (2013)
M. Kolenbrander, P. Van Der Zaag, J. de Phys. IV Coll. 07(C1), C1-195–C1-196 (1997)
P.J. van der Zaag, M. Kolenbrander, M.Th. Rekveldt, J. Appl. Phys. 83, 6870–6872 (1998)
B.D. Prasad, H. Nagabhushana, K. Thyagarajan, B.M. Nagabhushana, D.M. Jnaneshwara, S.C. Sharma, C. Shivakumara, N.O. Gopal, S.C. Ke, R.P.S. Chakradhar, J. Magn. Magn. Mater. 358–359, 132–141 (2014)
P.P. Hankare, S.D. Jadhav, U.B. Sankpal, S.S. Chavan, K.J. Waghmare, B.K. Chougule, J. Alloys Compd. 475, 926–929 (2009)
V.G. Harris, A. Geiler, Y.J. Chen, S.D. Yoon, M.Z. Wu, A. Yang, Z.H. Chen, P. He, P.V. Parimi, X. Zuo, C.E. Patton, M. Abe, O. Acher, C. Vittoria, J. Magn. Magn. Mater. 321, 2035–2047 (2009)
C. Pasnicu, D. Condtjracre, E. Luca, Phys. Status Solidi (a) 76, 145–150 (1983)
S.D. Chhaya, M.P. Pandya, M.C. Chhantbar, K.B. Modi, G.J. Baldha, H.H. Joshi, J. Alloys Compd. 377, 155–161 (2004)
S.F. Wang, Y.F. Hsu, Y.X. Liu, C.K. Hsieh, J. Magn. Magn. Mater. 394, 470–476 (2015)
E. Rezlescu, L. Sachelarie, P.D. Popa, N. Rezlescu, IEEE Trans. Magn. 36, 3962–3967 (2000)
R. Ali, M.A. Khan, A. Mahmood, A.H. Chughtai, A. Sultan, M. Shahid, M. Ishaq, M.F. Warsi, Ceram. Int. 40, 3841–3846 (2014)
H. Hirazawa, S. Kusamoto, H. Aono, T. Naohara, K. Mori, Y. Hattori, T. Maehara, Y. Watanabe, J. Alloys Compd. 461, 467–473 (2008)
H.M. Zaki, S.A. Heniti, J. Nanosci. Nanotech. 12, 7126–7131(2012)
A.M. Escamilla-Pérez, D.A. Cortés-Hernández, J.M. Almanza-Robles, D. Mantovani, P. Chevallier, J. Magn. Magn. Mater. 374, 474–478 (2015)
Md.D. Rahaman, Md. Dalim Mia, M.N.I. Khan, A.K.M. Akther Hossain, J. Magn. Magn. Mater. 404, 238–249 (2016)
H.E. Scherrer, H. Kisker, H. Kronmuller, R. Wurschum, Nanostruct. Mater. 6, 533–538 (1995)
B.D. Cullity, Elements of X-ray Diffraction (Addison Wesley Publ. Co. Inc., Reading, 1956), p. 42
M. Junaid, M.A. Khan, F. Iqbal, G. Murtaza, M.N. Akhtar, M. Ahmad, I. Shakir, M.F. Warsi, J. Magn. Magn. Mater. 419, 338–344 (2016)
Z. Karimi, Y. Mohammadifar, H. Shokrollahi, Sh. Khameneh Asl, Gh. Yousefi, L. Karimi, J. Magn. Magn. Mater. 361, 150–156 (2014)
M.A. Amer, M. El Hiti, J. Magn. Magn. Mater. 234, 118–125 (2001)
K. Standley, Oxide Magnetic Materials (Clarendon, Oxford, 1974), p. 97
J.R. Macdonald, E. Barsoukov, Impedance Spectroscopy: Theory, Experiment and Applications, 2nd edn. (Wiley, Hoboken, 2005)
R.D. Waldron, Phys. Rev. 99, 1727–1735 (1955)
B.J. Evans, S. Hafner, J. Phys. Chem. Solids 29, 1573–1588 (1968)
M.G. Naseri, E.B. Saion, H.A. Ahangar, A.H. Shaari, M. Hashim, J. Nanomater. 2010, 907686 (2010)
T. Tsutaoka, J. Appl. Phys. 93, 2789–2798 (2003)
R.L. Coble, T.K. Gupta, in Sintering and Related Phenomena, ed. by G.C. Kuczynski, C.F. Gibbon (Gordon & Breach, New York, 1967), p. 423
P.J. van der Zaag, P.J. van der Valk, M.Th. Rekveldt, Appl. Phys. Lett. 69, 2927–2929 (1996)
J.C. Maxwell, Electricity and Magnetism, (Oxford University Press, London, 1873), p. 328
K.W. Wagner, Am. Phys. 40, 817–855 (1913)
C.G. Koops, Phys. Rev. 83, 121–124 (1953)
S.N. Dolia, P.K. Sharma, M.S. Dhawan, S. Kumar, A.S. Prasad, A. Samariya, S.P. Pareek, R.K. Singhal, K. Asokan, Y.T. Xing, M. Alzamora, E. Saitovitach, Appl. Surf. Sci. 258, 4207–4211 (2012)
M.R. Bhandare, H.V. Jamadar, A.T. Pathan, B.K. Chougule, A.M. Shaikh, J. Alloys Compd. 509, L113–L118 (2011)
A.A. Birajdar, S.E. Shirsath, R.H. Kadam, S.M. Patange, D.R. Mane, A.R. Shitre, Ceram. Int. 38, 2963–2970 (2012)
S. Bhagawat, P. Rao, J. Appl. Phys. 3, 1–6 (2013)
J. Rout, R. Choudhary, S. Shannigrahi, H. Sharma, J. Electron. Mater. 44, 3811–3818 (2015)
S. Rasheed, H.S. Aziz, R.A. Khan, A.M. Khan, A. Rahim, J. Nisar, S.M. Shah, F. Iqbal, A.R. Khan, Ceram. Int. 42, 3666–3672 (2016)
Q. Xia, H. Su, T. Zhang, J. Li, G. Shen, H. Zhang, X. Tang, J. Appl. Phys. 112, 043915 (2012)
S. Mollah, K. Som, K. Bose, B.K. Chaudhuri, J. Appl. Phys. 74, 931–937 (1993)
R. Andoulsi, K.H. Naifer, M. Ferid, Ceram. Int. 39, 6527–6531 (2013)
A.A. Sattar, H.M. El-Sayed, W.R. Agami, J. Mater. Eng. Perform. 16, 573–577 (2007)
S.T. Assar, H.F. Abosheiasha, J. Magn. Magn. Mater. 374, 264–272 (2015)
K.M. Batoo, Physica B 406, 382–387 (2011)
A. Kumar, B. Singh, R. Choudhary, A.K. Thakur, Mater. Chem. Phys. 99, 150–159 (2006)
K.M. Batoo, J. Phys. Chem. Solids 72, 1400–1407 (2011)
B. Tiwari, R. Choudhary, J. Alloys Compd. 493, 1–10 (2010)
M.A. Rahman, A.K.M. Akther Hossain, Phys. Scr. 89, 8 (2014)
K.M. Batoo, M.S. Ansari, Nanoscale Res. Lett. 7, 112–126 (2012)
J.R. Macdonald, Impedance Spectroscopy Emphasizing Solid Materials and System, 3rd edn. (Wiley, New York, 1987)
R.S. Devan, Y.D. Kolekar, B.K. Chougule, J. Phys. 18, 9809–9821 (2006)
A. Goldman, Modern Ferrite Technology, (Marcel Dekker Inc., New York, 1993)
A. Globus, P. Duplex, IEEE Trans. Magn. 2, 441–445 (1966)
T.Y. Byun, S.C. Byeon, K.S. Hong, IEEE Trans. Magn. 35, 3445–3447 (1999)
A. Globus, P. Duplex, M. Guyd, IEEE Trans. Magn. 7, 617–622 (1971)
N. Bloembergen, Proc. IRE 44, 1259–1269 (1956)
K. Praveena, K. Sadhana, R. Sandhya, S.R. Murthy, H.L. Liu, Ceram. Int. 42, 8869–8877 (2016)
G.K. Joshi, A.Y. Khot, S.R. Sawant, Solid State Commun. 65, 1593–1595 (1988)
A.K.M. Akther Hossain, T.S. Biswas, S.T. Mahmud, T. Yanagida, H. Tanaka, T. Kawai, J. Magn. Magn. Mater. 321, 81–87 (2009)
A. Beitollani, M. Hoor, J. Mater. Sci. 14, 477–482 (2003)
A. Verma, T.C. Goel, R.G. Mendiratta, J. Magn. Magn. Mater. 210, 274–278 (2010)
A.K. Singh, T.C. Goel, R.G. Mendiratta, O.P. Thakur, C. Prakash, J. Appl. Phys. 92, 3872–3876 (2002)
A.K.M. Akther Hossain, M.L. Rahman, J. Magn. Magn. Mater. 323, 1954–1962 (2011)
Acknowledgements
The authors are thankful to the Experimental Solid State Physics Laboratory of Bangladesh University of Engineering and Technology (BUET) for allowing us to carry out this research. The authors would also like to thank to the authorities of the Center for Advanced Research in Sciences (CARS), University of Dhaka for allowing us to use the Scanning Electron Microscope (JEOL-JSM-6490LA), X-ray diffractometer (RIGAKU Ultima IV, Japan) and Fourier Transform Infrared (Shimadzu FTIR 8400 S) spectroscopy facilities, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maleque, R., Rahaman, M.D. & Akther Hossain, A.K.M. Influence of Ca2+ ions substitution on structural, microstructural, electrical and magnetic properties of Mg0.2−xCaxMn0.5Zn0.3Fe2O4 ferrites. J Mater Sci: Mater Electron 28, 13185–13200 (2017). https://doi.org/10.1007/s10854-017-7154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7154-5