The effect of Ca-substitution on the physical and magnetic properties of Li0.3-0.5x Zn0.4Ca x Fe2.3-0.5x O4 ferrites (x = 0.0, 0.01, 0.02, 0.03, and 0.05), prepared by the standard ceramic method, has been studied. It is found that the saturation magnetization increases up to x = 0.01 and then it decreases. On the other hand, the initial permeability decreased while the Curie temperature remained almost constant with increasing x. The coercivity and remanence ratio increased with increasing x.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Li-Zn ferrites are low-cost materials and have important properties for technological applications. It is known that the intrinsic parameters of ferrites, such as magnetization, initial permeability, Curie temperature, and resistivity depend on the chemical composition, heat treatment, and type of additive or substituted ions (Ref 1). The rectangularity of B-H loop is also found to increase with increasing the defects that hinder the magnetic domain wall motion in the grains (Ref 2). The influence of additives, such as Na2O, CaO, Sb2O3, and ZrO2 on the structure, magnetic and electrical properties of Li-Zn ferrites were previously reported (Ref 1, 3). This paper is devoted to study the effect of Ca2+ substitution, not additives, on the physical and magnetic properties of Li-Zn ferrite.
Experimental Techniques
Ferrite samples with the chemical formula Li0.3−0.5x Zn0.4Ca x Fe2.3−0.5x O4 (x = 0.0, 0.01, 0.02, 0.03, and 0.05) were prepared by the standard ceramic method. High purity oxides, 99.99%, of ZnO and Fe2O3 with Li2CO3 and CaCO3 were mixed together according to their molecular weight. The mixture of each sample was ground to a very fine powder and then presintered at 800 °C for 6 h. The presintered mixture was ground again and pressed at room temperature, under a pressure of 7 × 108 Pa, into tablet and toroidal forms. They were finally sintered at 1050 °C for 3 h and then slowly cooled to room temperature. X-ray diffraction patterns were performed using a diffractometer of type X’Pert Graphics and identified with Cu K α radiation. The theoretical x-ray density (d x) of the samples was calculated using the formula (d x = 8M/Na 3) where M is the molecular weight, N is Avogadro’s number, and a is the lattice parameter. The density (d) of each composition was measured in bidistilled water using Archimedes’ principle. The porosity percentage P (%) was calculated according to the relation P = 100(1 − d/d x)%. The magnetization (M), at room temperature, was measured using the vibrating sample technique. The magnetizing field ranged from 0.0 up to 1.4 T. A toroidal sample of each composition was used as a transformer core for measuring the initial permeability μi which was measured as a function of temperature at a constant frequency f = 10 KHz. The value of μi was calculated using Poltinnikov’s formula (Ref 4), V s = Kμi, where V s is the induced voltage in the secondary coil and K = μo n p n s I p Aω/L; n p and n s are the number of turns of the primary and secondary coils, respectively, I p is the current in the primary coil, A is the cross-sectional area of the sample, ω is the angular frequency, and L is the average path of the magnetic flux. Moreover, the coercive field and remanence were determined from the hysteresis loop. The tablet samples were used for the electrical resistivity measurements. The sample was inserted between two platinum electrodes where In-Hg was used as a contact material.
Results and Discussion
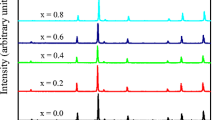
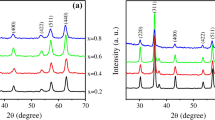
The x-ray diffraction patterns, (Fig. 1), showed that all investigated samples have single-cubic spinel phase. The d-spacing for each peak was recorded automatically and then the lattice parameter (a) was calculated from the relation
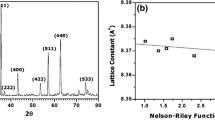
The values of the lattice parameters obtained for each reflected plane are plotted against the function F(θ) where F(θ) = (½)[(cos2θ/sinθ) + (cos2θ/θ)]; θ is the Bragg’s angle. Straight lines were obtained and the accurate values of (a) were determined from the extrapolation of these lines to F(θ) = 0 (Ref 5). The variation of the lattice parameter (a) as a function of Ca-concentration (x) is shown in Fig. 2. It can be seen that the lattice parameter slightly increases linearly with the Ca-concentration, which obeys Vegard’s law. Such an increase can be understood from the fact that we substitute Fe3+ and Li1+ ions by Ca2+ ion which has a larger ionic radius (\({r_{{\rm Fe}^{3+}}=0.64\,\AA}\), \({r_{{\rm Li}^{1+}}=0.68\,\AA}\), and \({r_{{\rm Ca}^{2+}}=0.99\,\AA}\)). It is also noticed that the value of the lattice parameter (a) for the unsubstituted sample Li0.3Zn0.4Fe2.3O4 (8.3817 Å) compares well with those reported earlier (8.378 Å (Ref 1, 6), 8.380 Å (Ref 3)).
Magnetic Studies
Magnetization
Figure 3 indicates the variation of magnetization M (emu/g) vs. the applied magnetic field H(T) at the room temperature. As a normal behavior, the magnetization increases with increasing the applied magnetic field and attains its saturation value for higher fields. The saturation magnetization M s for each sample was determined by extrapolation of the magnetization curve to H = 0. The dependence of (M s) on the Ca-concentration (x) is shown in Fig. 4. It is noticed that M s increases rapidly with x and attains a maximum value at x = 0.01. Further increase of Ca-concentration leads M s to decrease. Such a result could be discussed assuming the following cation distribution:
where the brackets ( ) and [ ] denote A- and B-sites, respectively. Such cation distribution is based on the following facts:
-
1.
Li1+ ions have a strong preference to occupy the B-site (Ref 7) while Zn2+ ions have a strong preference to occupy the A-site (Ref 8).
-
2.
As for Ca-ion distribution, it was reported that Ca2+ ions strongly prefer to occupy the A-site for low Ca-concentration (Ref 6, 9). However, for high Ca-concentration, it was suggested that the Ca2+ ions are either distributed between A- and B-sites (Ref 10) or reside at the grain boundaries (Ref 11). Thus, according to the assumed cation distribution (Ca ions occupy the A-sites) increasing of the Ca-concentration from x = 0 to x = 0.01 leads Fe3+ content in B-site to increase and that in A-site to decrease. Hence the total magnetization (M s = M B − M A) increases. For x > 0.01, more Fe3+ ions migrate to B-site causing the B-B interaction to increase and hence the canting angle (θYK) establishes. It is expected that (θYK) increases with increasing Ca-concentration. Such an increase in (θYK) leads to a decrease of M s according to the equation (Ref 12) M s = M BcosθYK − M A, where M A and M B are the magnetizations of A- and B-sites, respectively. Thus our results support the occupation of Ca ions for A-sites at low Ca-concentration.
Initial Permeability
Figure 5 shows the variation of the initial permeability μi with temperature for the series Li0.3−0.5x Zn0.4Ca x Fe2.3−0.5x O4 (x = 0.0, 0.01, 0.02, 0.03, and 0.05). It is seen that the initial permeability increases with temperature up to Curie temperature T c. Near T c there is a sharp drop in μi. This result could be explained according to the Globus relation (Ref 13), which is given by
where D is the average grain size and K 1 is the anisotropy constant. According to the Globus relation, the dependence of μi on temperature results from the temperature dependence of M s and K 1. It is known that the anisotropy field usually decreases much faster with temperature than M s (Ref 14) which leads to an increase in μi. Figure 5 shows also that the rate of increase of μi with temperature decreases by increasing Ca-content. This may lead us to conclude that the Ca2+ ions improve the thermal stability of μi for the investigated samples (Ref 14) by compensating changes in M s and K 1 with temperature. The compensating mechanism is still under investigation.
Furthermore, from Fig. 5 one can conclude that μi, at room temperature, decreases with increasing Ca-concentration. This is illustrated in Fig. 6 in addition to the variation of the porosity with Ca content. It is obvious that as the porosity increases, μi decreases with increasing Ca-concentration. The decrease of μi with increasing x could be discussed using Globus relation as follows.
According to the proposed cation distribution, the amount of iron in B-site increases, which in turn increases the amount of Fe2+ in B-site. According to the one ion model, the anisotropy constant K1 has a direct relation with the amount of Fe2+. Therefore, as Ca-concentration increases, K 1 also increases. Furthermore, previous studies (Ref 1) have shown that doping Li-Zn ferrite with CaO decreases the grain size D. These two factors and the increase in porosity lead to the decrease of μi with increasing x. Moreover, the decrease of M s in the range 0.01 < × ≤ 0.05, may contribute in decreasing μi.
Curie Temperature
The values of Curie temperature (T c) were determined from the extrapolation of the linear part at the sudden decrease of μi of Fig. 5 for all investigated samples. The values of T c and the rate of decrease of μi (slope of the linear part) are given in Table 1.
Table 1 illustrates that T c is almost constant (628 ± 5 K). The constancy of T c is discussed as follows. It is known that, in ferrites, T c depends mainly on the A-B interaction, which in turn is affected by the following factors:
-
1.
The magnitude and the concentration of magnetic moments existed in A- and B-sites. In our investigated system, the only moments existed those of Fe3+ ion. Moreover, the relative concentration of the moments on A- and B-sites (M A/M B) is almost constant, where for unsubstituted sample (M A/M B) = 0.353, and for the highest substituted sample (M A/M B) = 0.319.
-
2.
The distance between the A- and the B-sites where Fig. 2 shows that the change of lattice parameter with Ca-concentration, 0.025 Å/Ca content, is very small to affect T c.
Thus, according to the above factors, T c tends to be almost constant which we have found experimentally.
Table 1 indicates also that the slope of the linear part of μi(T) curve, at the sudden decrease of μi, decreases with increasing Ca2+ concentration. It was reported that the value of this slope gives a good indication about the sample homogeneity (Ref 15), the higher the slope the higher the homogeneity. Thus one concludes that the homogeneity decreases with increasing Ca-concentration. Such a result could be attributed to the increase of the porosity with Ca-concentration.
Coercivity and Squareness
The variations of the coercive field H c and the remanence ratio (B r/B s), which expresses the squareness of the hysteresis loop, for all Ca-concentrations (x) at a field H = 340 A/m are given in Table 2.
One can observe that H c increases with increasing x. Similar results were reported by Rezlescu et al. (Ref 16) for Ni-Zn ferrite doped with CaO. Table 2 shows also that the ratio (B r/B s) increases with increasing Ca-concentration (x). These results are in agreement with that of the porosity. As the porosity increases, then higher field is needed to push the domain wall, i.e., H c increases. Moreover, the behavior of M s may play an indirect role in increasing H c, specially for x > 0.01, through Brown’s relation (Ref 17) which is given by
Furthermore, it is known that the remanence B r is directly proportional to the porosity (Ref 18). Moreover, for x ≤ 0.01, the porosity increases by a factor of 67% while M s increases only by a factor of 11.5%. This leads to an increase of B r/B s. In addition, for the range 0.01 < × ≤ 0.05, the porosity continues to increase while M s decreases. This causes more increase of B r/B s.
Resistivity at Room Temperature
The dc resistivity (ρ) of all samples, at room temperature, are given in Table 3.
The table indicates that in the range 0 < × ≤ 0.02, the dc resistivity (ρ decreases with increasing Ca-concentration (x), while ρ increases again for x > 0.02. This result could be explained as follows:
It was assumed above that the Ca2+ ions occupy the A-site for Ca-concentration up to x = 0.01. Hence, some Fe3+ ions migrate from A- to the B-site. The migration of Fe3+ is accompanied by the formation of Fe2+ ions in B-site (Ref 19). Thus the ratio of Fe2+/Fe3+ ions in B-site increases. Thus the probability of hopping between Fe2+ and Fe3+ increases and hence, the resistivity decreases. For higher Ca-concentration, the effect of the porosity becomes the dominant factor and the resistivity increases.
Conclusion
Single spinel phases of Ca-substituted Li-Zn ferrite are studied. The Ca-substitution slightly increased the lattice parameter, the coercivity and the squareness of the samples. The magnetization increased for small Ca-concentration, x = 0.01, and then decreased for higher Ca content. The homogeneity of the samples and initial permeability decreased with increasing Ca-concentration while Curie temperatures are found to be almost constant.
References
Rezlescu N., Rezlescu E. (1995) The Influence of Additives on the Material Parameters of Li-Zn Ferrites, Phys. Stat. Sol. (a) 147:553–562 (in English)
Kim H.T., Im H.B. (1982) Effects of Bi2O3 and Nb2O5 on the Magnetic Properties of Ni-Zn Ferrites and Lithium Ferrites, IEEE Trans. Magnet. 18(6):1541–1543 (in English)
Bermejo E., le Mercier T., Quarton M. (1995). Microstructure and Physicochemical Studies of Pure and Zinc-Substituted Lithium Ferrites Sintered Above 1000°C, J. Am. Ceram. Soc., 78(2):365–368 (in English)
Poltinnikov S.A.J. (1966) Some Magnetic Properties of Nickel-Cadmium Ferrite, Sov. Phys. Solid Stat. 8:1144–1149 (in English)
B.D. Cullity, Elements of X-ray Diffraction, Second printing, Addison-Wesley Publishing Company, Inc, USA, 1959
N. Rezlescu and E. Rezlescu, Effects of Addition of Na2O, Sb2O3, CaO, and ZrO2 on the Properties of Lithium Zinc Ferrite, J. Am. Ceram. Soc., 1996, 79(8), p 2105–2108 (in English)
White G.O., Patton C.E. (1978) Magnetic Properties of Lithium Ferrite Microwave Materials, J. Magn. Magn. Mater. 9:299–317 (in English)
Xu Z.C. (2003) Magnetic Anisotropy and Mössbauer Spectra in Disordered Lithium-Zinc Ferrites, J. Appl. Phys. 93(8):4746–4749 (in English)
Upadhyay R.V., Baldha G.J., Kulkarni R.G. (1986) Mössbauer study of the Ca x Co1−x Fe2O4 system, J. Magn. Magn. Mater. 61:109–113 (in English)
Chhaya S.D., Pandya M.P., Chhantbar M.C., Modi K.B., Baldha G.J., Joshi H.H. (2004) Study of Substitution Limit, Structural, Bulk Magnetic and Electrical Properties of Ca2+ Substituted Magnesium Ferrite, J. Alloys Comp. 377:155–161 (in English)
Lipare A.Y., Vasambekar P.N., Vaingankar A.S. (2003) Ac Susceptibility Study of CaCl2 Doped Copper–Zinc Ferrite System, Bull. Mater. Sci. 26(5):493–497 (in English)
Suryawanshi S.S., Deshpande V.V., Deshmukh U.B., Kabur S.M., Chaudhari N.D., Sawant S.R. (1999) XRD Analysis and Bulk Magnetic Properties of Al3+ Substituted Cu-Cd Ferrites, Mater. Chem. Phys. 59:199–203 (in English)
Jain G.C., Das B.K., Khanduja R.S., Gupta S.C. (1976) Effect of Intragranular Porosity of Initial Permeability and Coercive Force in a Manganese Zinc Ferrite, J. Mater. Sci. 11:1335–1338 (in English)
Sankpal A.M., Kakatkar S.V., Chaudhari N.D., Patil R.S., Sawant S.R., Suryavanshi S.S. (1998) Initial Permeability Studies on Al3+ and Cr3+ Substituted Ni-Zn Ferrites, J. Mater. Sci. Mater. Electron. 9:173–179 (in English)
Globus A., Pascard H., Cagan V.J. (1977) Distance Between Magnetic Ions and Fundamental Properties in Ferrites, J. De Phys. Colloque 38(4):C1-163–C1-168 (in English)
Rezlescu E., Rezlescu N., Pasnicu C., Craus M.L., Popa D.D. (1992) The Influence of Additives on the Properties of Ni-Zn Ferrite Used in Magnetic Heads, J. Magn. Magn. Mater. 117:448–454 (in English)
Coey J.M.D. (1996) Rare Earth Permanent Magnetism, 1st ed., John Wiley and Sons, New York, p 220
Bozorth R.M. (1963) Ferromagnetism, 7th ed., Van Nostrand Company, Inc, Princeton, New Jersey, p 824
Miyata N (1961) Electric Conduction of Ferrites Containing Fe2+-ions, J. Phys. Soc. (Jpn) 16:206–208 (in English)
Author information
Authors and Affiliations
Corresponding author
Additional information
A. A. Sattar is on leave from Physics Department, Faculty of Science, Ain Shams University, Cairo, Egypt
Rights and permissions
About this article
Cite this article
Sattar, A., El-Sayed, H. & Agami, W. Physical and Magnetic Properties of Calcium-Substituted Li-Zn Ferrite. J. of Materi Eng and Perform 16, 573–577 (2007). https://doi.org/10.1007/s11665-007-9080-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-007-9080-9