Abstract
A novel spray co-precipitation method was adopted to synthesize well dispersed nanocrystalline Y2O3 powders for transparent ceramics. Several analytic techniques such as XRD, SEM, BET and UV–Vis–NIR spectrophotometer were used to determine the properties of coprecipitated powders, and the microstructure and optical properties of as-fabricated ceramics. The influences of the aging time on powders and ceramics were systematically investigated. Precursors were completely reached to yield the Y2O3 phase after being calcined at 1250 °C in air. The calcined Y2O3 powders exhibited an approximately spherical morphology with narrow size distribution and weak agglomeration, with mean particle size of ~140 nm. The co-precipitated nanopowders with an aging time of 12 h exhibited the best sintering activity due to the low agglomeration, and the in-line transmittance of Y2O3 ceramic sintered at 1800 °C for 8 h in vacuum reached to 77.2% at 1064 nm (1 mm thickness).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Yttrium Oxide (Y2O3) ceramics have various applications in high-temperature-resistance windows, solid state lasers, and scintillator field because of its excellent physical and chemical properties, such as high corrosion resistivity, high temperature stability and excellent optical transparency, etc. [1–5] However, Y2O3 transparent ceramic is hard to densification due to its high melting point. Generally, the optical quality of transparent ceramics significantly depends on the purities, morphologies, particle sizes, and especially the agglomerate state of raw powders. The severe agglomeration of nano-powders never benefits to the densification of ceramic with defect-free structure [6]. Therefore, the synthesis of nanopowders with high dispersibility and high sintering activity is crucial for the fabrication of Y2O3 transparent ceramics [7, 8].
Y2O3 nanopowders have been successfully prepared by various approaches, such as sol–gel method [9–11], hydrothermal synthesis approaches [12, 13], and co-precipitation method [14–16]. As a wet-chemistry method, co-precipitation method is generally considered as the most effective way to prepare Y2O3 powders of high sintering activity. Conventional co-precipitation method using drop-by-drop feed style is widely adopted to synthesize inorganic oxide powders. However, the synthesized Y2O3 powders become relatively large and easily agglomerated due to the big droplets deteriorated the uniformity of solution. The local concentrations of metal ions or precipitants are relatively high, which lead to the formation of quick nucleation and growth.
In order to solve the above problems, we proposed a novel co-precipitation method that the solution of precipitant was atomized and a large amount of atomized droplet into the metal salt solution. Compared with the conventional co-precipitation, the way that precipitant added the solution is different. During the spray co-precipitation, the precipitant is atomized. As the rate equation of mass transfer: transfer rate = (driving force × phase contact area)/mass transfer resistance shown, increasing the phase contact area could promote the transfer rate. The novel co-precipitation method could atomize the solution of precipitant to increase the phase contact area. The atomized droplets are benefited for homogeneous local concentration in the solution and even nucleation which are the two main reasons for fine powder.
In this research, the mixture of ammonia water and ammonium hydrogen carbonate was employed as the precipitant, and the properties of obtained Y2O3 powders under different aging times as well as the corresponding ceramics were systematically investigated.
2 Experimental procedure
2.1 Synthesis of nanocrystalline Y2O3 powders
In this research, high purity Yttrium Oxide (Y2O3, 99.99%, Alfa Aesar, Ward Hill, MA) was selected as starting material. Ammonia water (NH3·H2O, analytical grades, Aladdin chemicals, Shanghai, China) and ammonium hydrogen carbonate (NH4HCO3, analytical grades, Aladdin chemicals, Shanghai, China) were used as precipitant. Nitric acid (HNO3, analytical grades, Aladdin chemicals, Shanghai, China) was used to dissolve Y2O3 powders, and 30 mol% ([SO4 2−]/[Y3+] = 30 mol%) ammonium sulfate ((NH4)2SO4, analytical grades, Aladdin chemicals, Shanghai, China) as dispersant. Firstly, the Y(NO3)3 solution was prepared by dissolving yttria powders into nitric acid, and then diluted with distilled water until the solution concentration reached to 0.15 mol L−1. 30 mol% (NH4)2SO4 was then added into the Y(NO3)3 solution as dispersant. The mixed solution of 1.0 mol L−1 ammonium hydrogen carbonate and 1.5 mol L−1 ammonia water was used as combined precipitant.
Figure 1 shows the schematic diagram of spray co-precipitation device. An air compressor provided the driving force of the spraying process, and four fine air-filters were adapted to eliminate impurities of the compressed air. The pressure of air compressor was set as 0.8 MPa, and the diameter of atomizer was 10 μm. The combined precipitant was sprayed into the Y(NO3)3 solution at a rate of 2 mL min−1 precisely controlled by a peristaltic pump, and stirred at room temperature until the final pH of the solution was 8.0. After spraying, the precipitates were aged under different aging times ranging from 0 to 48 h to obtain Y2O3 precursors. The obtained precursors were filtrated and washed firstly with deionized water for three times, and then with alcohol for another three times. Finally, the washed precursors were dried at 60 °C for 24 h in an oven and then calcined at 1250 °C for 4 h in air to obtain nanocrystalline Y2O3 powders.
2.2 Fabrication of Y2O3 ceramics
The calcined Y2O3 powders were sieved through a 150 mesh screen and then dry-pressed into Φ 16 mm disks in a stainless steel mould under 25 MPa, followed by cold isostatic pressing under 200 MPa for 5 min. After removing the residue organics by being calcined at 800 °C for 4 h in a muffle furnace, Y2O3 green bodies were sintered at 1400–1600 °C for 4 h in air to measure the densities of sintered Y2O3 ceramics, and then vacuum sintered at 1800 °C for 8 h in a vacuum furnace under 10−3 Pa for the transmittance investigation. Finally, the sintered Y2O3 ceramics samples were annealed at 1100 °C in air for 10 h to eliminate oxygen vacancies generated during vacuum sintering, and mirror polished on both sides to 1 mm.
2.3 Characterization
The phases of Y2O3 powders with different aging times were identified by X-ray diffraction (XRD, D2, Bruker, Germany). The morphologies of calcined Y2O3 powders as well as microstructures of the sintered ceramics were performed by a scanning electron microscope (SEM, JSM-6510, JEOL, Japan). The relative densities of Y2O3 ceramics sintered under different temperatures in air were measured and calculated by the Archimedes method. The in-line transmittance of sintered Y2O3 ceramics in vacuum was obtained by a UV–Vis–NIR spectrophotometer (Lambda 950, Perkin-Elmer, Waltham, MA, America) in the wavelength range of 200–1500 nm. The specific surface area was measured by the BET method (Tristar II 3020, Micromeritics Instrument, America), and the equivalent particle size (DBET) of Y2O3 powders can be calculated according to the following equation:
where ρ is the theoretical density (5.031 g cm−3) of Y2O3 and S BET is the measured specific surface of the powder.
3 Results and discussion
3.1 Characteristics of Y2O3 powders
Figure 2 shows the XRD patterns of Y2O3 precursors under different aging times calcined at 1250 °C for 4 h, and the uncalcined Y2O3 precursor aged for 12 h was adopted for comparison. There was no diffraction peak observed from the uncalcined precursor, indicating that the precursor remained amorphous. After being calcined, the characteristic diffraction peaks corresponding to Y2O3 phase (PDF#41-1105) could be observed from all precursors under different aging times. The diffraction peaks were sharp indicated Y2O3 powers had good crystallinity. The grain sizes of calcined Y2O3 powders were calculated according to the Scherrer formula, and the corresponding results were listed in Table 1. As can be seen from Table 1, the grain sizes of powders under different aging times had no significant difference since the spraying process promoted the homogeneous nucleation and uniform growth of powders during the aging stage.
Figure 3 shows the SEM images of Y2O3 powders under different aging times calcined at 1250 °C for 4 h. All obtained nanocrystalline Y2O3 powders displayed approximately spherical shapes with round edge. The particle sizes of the powders were approximately 140 nm with a narrow size distribution. It is worth mentioning that the morphology and the particle size of the as-prepared Y2O3 powders were similar even under the different aging times. The SEM images of powders aged for 48 h had no obvious change with that of 36 h and thus was not shown here.
The agglomeration state of powders were calculated according to the following equation [16]:
where n is the agglomeration parameter, and the smaller the parameter n, the weaker the agglomeration state of powders. The calculated n values of Y2O3 powders were also listed in Table 1. It is obvious that the Y2O3 powders aged for 12 h had the smallest n value, but the n values among different aging times were small. The agglomeration states of spray precipitated powders were similar. The morphologies of the as-prepared Y2O3 powders with different aging times observed from SEM image were also similar which was in accordance with the calculated n value. This phenomenon was ascribed to the small sprayed droplet can effectively separate the nucleation and growth processes of the precursor, and thus fasten the nucleation rate. In this case, the growth rate of the crystalline grains was inhibited accordingly, and the effect of aging time on the growth of crystalline grains was exhibited as well.
In fact, the particle sizes of the powders prepared by conventional co-precipitation method were relatively large. According to the previous reports, Ikegami et al. [17] adopted titration method to synthesis Y2O3 powders, and the specific surface was only ~6 m2 g−1 after being calcined at 1200 °C in air. Liu et al. [18] demonstrated the Y2O3 powders prepared by titration co-precipitation method and calcined at as low as 1100 °C in air, and the specific surface was ~8 m2 g−1. As depicted in Table 1, the specific surface of the powders was still ~9 m2 g−1 when the calcining temperature was 1250 °C. The above comparison clearly shows that spray co-precipitation method is fit to synthesize fine powders.
3.2 Effect of sintering temperature on the densification of Y2O3 ceramics
Figure 4 shows the effects of sintering temperature on the densification behavior of Y2O3 ceramics. It can be seen that the relative densities of Y2O3 ceramics increased with the sintering temperatures increased from 1400 to 1600 °C, and the ceramics fabricated by powders aged for 12 h had the highest relative density at each sintering temperature, and the relative density reached to 99.8% after sintered at 1600 °C for 4 h in air, indicating the sintering activity of the Y2O3 powders was high. However, the variation of relative densities of Y2O3 ceramics was very small (≤2%), owing to the similar morphologies of starting powders.
Figure 5 shows the SEM images of the thermally etched surfaces of Y2O3 ceramics of different powder aging times. Both intragranular pores and intergranular pores could be observed from the surface of the all Y2O3 ceramics, due to the relative lower sintering temperature. It is obvious that the Y2O3 ceramics of which powder aged for 12 h had fewer pores, and the average grain size was about 1.88 μm, which due to the powder aged for 12 h had the smallest particle size and the lowest agglomeration degree, as is shown in Table 1 and Fig. 3. The SEM images of ceramics aged for 48 h had no obvious change with that of 36 h and thus was not shown here. The sintering aids had pivotal factors for transparent ceramics [19]. Wang et al. [20]. used γ-Al2O3 as sintering additive, the grain size was about 25 μm.
3.3 Effect of sintering temperature on optical properties of Y2O3 ceramics
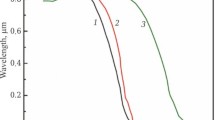
Figure 6 shows the in-line transmittance of the ceramics fabricated from powders aged for 12 h sintered at 1800 °C for 8 h in vacuum and annealed at 1100 °C for 10 h in air. The in-line transmittance of the ceramics without sintering aid reached to 77.2% at 1064 nm (1 mm thickness), which was very close to theoretical transmittance. The insert of Fig. 6 displays a photograph of the Y2O3 sample, and words behind the sample can be clearly observed by the naked eyes. However, the transmittance at the ultraviolet region was relatively low, indicating residue small pores still existed in ceramics.
4 Conclusions
NanocrystallineY2O3 powders were prepared by calcining the precursor that obtained by a novel spray co-precipitation route. All Y2O3 powders exhibited approximately spherical morphology with a narrow distribution and low agglomeration, and the mean particle size was ~140 nm. The research showed that the aging time had only slight effect on the morphology, particle size, particles size distribution and agglomeration of the Y2O3 powders, which due to uniform nucleation. The powder aged for 12 h exhibited the best dispersity. The obtained Y2O3 powders had high sintering activity and the relative density of the ceramics sintered at 1450 °C reached 95%. The relative density of the ceramics sintered at the same temperature was no significant difference. The in-line transmittance of the ceramics of aging 12 h sintered at 1800 °C for 8 h in vacuum was 77.2% at 1064 nm.
References
A. Ikesue, Y.L. Aung, Nat. Photonics 2, 12 (2008)
L. Zhang, H. Yang, X.B. Qiao, T.Y. Zhou, Z.Y. Wang, J. Zhang, D.Y. Tang, D.Y. Shen, Q.T. Zhang, J. Eur. Ceram. Soc. 35, 8 (2015)
L. Zhang, Y. Li, X. Li, H. Yang, X. Qiao, T. Zhou, Z. Wang, J. Zhang, D. Tang, J. Alloys Compd. 639, 244 (2015)
B. Song, C.C. Tuan, X.G. Huang, L.Y. Li, K.S. Moon, C.P. Wong, Mater. Lett. 166, 12 (2016)
B. Song, C. Sizemore, L.Y. Li, X.G. Huang, Z.Y. Lin, K.S. Moon, C.P. Wong, J. Mater. Chem. A 3, 43 (2015)
A.V. Ragulya, Adv. Appl. Ceram. 107, 3 (2008)
R.P. Yavetskiy, D.Y. Kosyanov, V.N. Baumer, A.G. Doroshenko, A.I. Fedorov, N.A. Matveevskaya, A.V. Tolmachev, O.M. Vovk, J. Rare Earths 32, 4 (2014)
T.G. Deineka, A.G. Doroshenko, P.V. Mateychenko, A.V. Tolmachev, E.A. Vovk, O.M. Vovk, R.P. Yavetskiy, V.N. Baumer, D.S. Sofronov, J Alloys Compd. 508, 1 (2010)
M. Hajizadeh-Oghaz, R.S. Razavi, M. Barekat, M. Naderi, S. Malekzadeh, M. Rezazadeh, J. Sol–Gel. Sci. Technol. 78, 3 (2016)
S.Q. Xu, J. Li, C.Y. Li, Y.B. Pan, J.K. Guo, J. Am. Ceram. Soc. 98, 9 (2015)
M. Wang, R.Z. Zuo, S.S. Qi, L.D. Liu, J. Mater. Sci. Mater. Electron. 3, 23 (2012)
P.A. Tanner, L. Fu, Chem. Phys. Lett. 470, 1–3 (2009)
M. Kabir, M. Ghahari, M.S. Afarani, Ceram. Int. 40, 7 (2014)
D. Kumar, M. Sharma, O.P. Pandey, Opt. Mater. 36, 7 (2014)
Y.F. Liu, X.Y. Qin, H.X. Xin, C.J. Song, J. Eur. Ceram. Soc. 33, 13–14 (2013)
S. Fadaie, M.M. Kashani-Motlagh, A. Maghsoudipour, B. Faridnia, J. Mater. Sci. Mater. Electron. 24, 3 (2013)
T. Ikegami, J.G. Li, T. Mori, Y. Moriyoshi, T. Ikegami, J.G. Li, Y. Moriyoshi, J. Am. Ceram. Soc. 85, 7 (2002)
B. Liu, J. Li, R. Yavetskiy, M. Ivanov, Y. Zeng, T. Xie, H. Kou, S. Zhuo, Y. Pan, J. Guo, J. Eur. Ceram. Soc. 35, 8 (2015)
H.J. Wu, T.C. Lu, N. Wei, Z.W. Lu, X.T. Chen, Y.B. Guan, Y. Zhao, J.Q. Qi, Q.W. Shi, X.M. Xie, W. Zhang, J. Mater. Sci. Mater. Electron. 4, 26 (2015)
Z.Y. Wang, L. Zhang, H. Yang, J. Zhang, L.X. Wang, Q.T. Zhang, J. Mater. Sci. Mater. Electron. 27, 4 (2016)
Acknowledgements
This work was supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Research and Innovation Program for College Graduates of Jiangsu Province (KYZZ16_0231) and National Natural Science Foundation of China (51402133, 51302115 and 11274144).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, L., Zhou, T. et al. A novel spray co-precipitation method to prepare nanocrystalline Y2O3 powders for transparent ceramics. J Mater Sci: Mater Electron 28, 4684–4689 (2017). https://doi.org/10.1007/s10854-016-6108-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6108-7