Abstract
In this paper, the pure In2O3 and Pd-doped In2O3 (0.5, 1.0, 2.0 and 4.0 mol%) flower-like spherical microstructures have been synthesized by a hydrothermal method. The crystal structure and surface morphology of as-prepared samples were characterized by X-ray diffraction and scanning electron microscopy. The gas sensing experiments were carried out on all the as-prepared gas sensors to hydrogen gas, and the measured results demonstrated that the Pd-doped In2O3 gas sensors exhibit enhanced gas sensing performance under the optimal working temperature of 210 °C. Especially, the 1.0 mol% Pd-doped In2O3 sensor shows the highest response to 100 ppm hydrogen gas at 210 °C, which was almost two times higher than that of pure one. Furthermore, the 1.0 mol% Pd-doped In2O3 gas sensor also shows fast response/recovery time about 4 and 7 s, respectively. Finally, the gas sensing mechanism was also discussed on the pure and Pd-doped In2O3 gas sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen gas (H2), as an environment friendly and clean energy resource, has been widely used in a variety of fields, including manufacturing, chemical processes, transportation, energy storage and household applications [1–5]. However, hydrogen belongs to the group of flammable and highly explosive gases, and safety is a special matter of considerable concern during handling and usage, especially at elevated temperatures. Furthermore, hydrogen gas is an odorless, colorless and tasteless properties, it can cause explosions when its concentration ranging from 4.65 to 93.9 vol% in air [6]. Therefore, it is very critical to develop the high sensitivity, rapid response, good reversibility and cost effective gas sensors for early detection the hydrogen leakages in industrial applications.
In the past few decades, great efforts have focused on metal oxide nanomaterials such as TiO2 [7–9], SnO2 [10, 11] ZnO [12–16], WO3 [17, 18] and In2O3 [19–22] for gas sensor applications, owing to their simplicity in synthesis, higher stability and electrical conductivity responses to different gases. However, there are still some challenges facing metal oxide-based gas sensors, for example low response, high operating temperature and slow response/recovery time, which limit their further development in practice. Therefore, different methods have been used to improve the gas sensing properties, including improving surface area [23, 24], controlling crystalline size [25, 26], doping or adding catalyst [27–29]. Among them, adding noble metal dopants into metal oxide nanostructures is considered to be an effective and simple method. For example, Moon et al. [30] fabricated the Pd decorated TiO2 tubular structures on SiO2/Si substrate combing anodization with deposition method. It is demonstrated that the sensor shows excellent hydrogen sensing properties including high response (7800 at 5050 ppm), wide detection range (1 ppm–1 %) and low operating temperature (140–180 °C). Wen group developed hydrogen sensor based on Au–Pd doped SnO2 by electroless deposition [31]. The experimental results showed that the Au/Pd/SnO2 nanocomposite film sensor exhibits a response of 1.1 % toward 134–1469 ppm hydrogen at room temperature, and has good stability and repeatability. More recently, Chang et al. prepared Pd nanoparticles decorated ZnO nanorod arrays using a unique photochemical deposition method for hydrogen detection [32]. It is found that the gas sensitivity to 500 ppm H2 can be significantly improved by around 553-fold at 260 °C, and the corresponding sensitivity can also reach to 16.9 at room temperature. To the best of our knowledge, although considerable efforts have been devoted to the study of the influence of noble metal nanoparticles to the sensing performances, few of them put forward the investigation of Pd content in indium oxides nanostructures for the hydrogen detection.
In this work, pure and Pd-doped In2O3 flower-like spherical microstructures were synthesized by a facile hydrothermal method. The crystal structure and surface morphology of as-prepared products were characterized, and the gas sensing properties were also carried out on hydrogen gas detection. The measured results revealed that the gas sensing performances were greatly affected by the concentration of Pd. Moreover, the optimum 1.0 mol% Pd-doped In2O3 sensor exhibits a higher gas sensing performance, which is one of the most promising candidates for highly sensitive hydrogen detection.
2 Experimental
2.1 Materials
Indium (III) chloride tetrahydrate (InCl3·4H2O, ≥99.9 %) and Palladium chloride (PdCl2, ≥99.9 %) were purchased from Sigma-Aldrich Corporation. Urea (CH4N2O, ≥99.0 %) and Sodium dodecyl sulphate (SDS, ≥59.9 %) were obtained from Sinopharm Chemical Reagent Co., Ltd. Terpineol and ethyl cellulose were ordered from Tianjin Kermel Chemical Reagent Co., Ltd. All other reagents and chemicals in the experiments were of analytical grade and used without further purification.
2.2 Synthesis In2O3 spherical microspheres
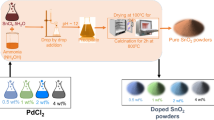
The pure and Pd-doped In2O3 flower-like spherical microspheres were prepared through hydrothermal method. In a typical process, 1.2 mmol InCl3·4H2O, 3.6 mmol SDS and 6 mmol urea were dissolved into 70 mL of distilled water magnetic string for 10 min. Then, various amounts of PdCl2 were added into the mixture and stirred for 2 h to form a homogeneous reaction solution. Subsequently, the solution was transferred into a Teflon-lined stainless steel autoclave (100 mL) and kept in an electric oven under 120 °C for 15 h. After that, the white precipitate was collected by centrifugation, washed thoroughly with ethanol, and dried at 80 °C overnight. Finally, the dried precipitates were annealed at 500 °C in a muffle furnace for 2 h with a heating rate of 3 °C min−1. In our experiments, different contents of Pd-doped In2O3 microstructures were denoted as x mol% Pd-doped In2O3, where x represented the concentration of Pd mole percentage (0, 0.5, 1.0, 2.0 and 4.0 mol%).
2.3 Characterization
The X-ray diffraction (XRD) measurements were carried out to determine the crystal structure of pure and Pd-doped In2O3 microstructures through Haoyuan X-ray diffractometer with Cu Ka1 radiation source (k = 1.5406 Å) operated at 40 kV and 120 mA in a step-scan mode in the 2θ range of 20°–80° at intervals of 0.02°. The morphology of the obtained samples was observed using scanning electron microscope (SEM, JSM-7001F, Japan) at an accelerating voltage of 15.0 kV. The gas sensing performances of the fabricated gas sensors were conducted on a commercial WS-30A system (Weisheng Instruments Co., Zhengzhou, China), and the testing glass chamber was 18 L.
2.4 Fabrication and measurement of gas sensors
In a typical process, gas sensors were fabricated according to the following steps: the prepared sample was firstly mixed with terpineol and ethyl cellulose (in a weight of 2:7:1), and ground in an agate mortar with ethanol to form slurry. The slurry was then coated onto the outer surface of an alumina tube, which was previously incorporated with two Au electrodes and platinum wires. After that, the ceramic tube was annealed at 500 °C (heating rate of 2 °C min−1) for 3 h. At last, a small Ni–Cr alloy heating coil was inserted in the center of the tube, which was used as a heater to control sensing temperature as shown in Fig. 1a. To improve the stability, the gas sensors were welded onto the pedestal and aged for 1 week by applying 5 V on the heating wire. The gas sensing properties of the sensors were measured in a computer-controlled static gas sensing test system of WS-30A with the relative humidity of 25–30 % RH in a fume hood. Figure 1b displays the work principle of gas sensing test system. V h is the heating voltage to control the operating temperature. V c provides a constant loop voltage of 5 V. The gas sensing property of gas sensor was reflected by testing output voltage of R L , which is connected in series with the gas sensor. The gas response S was defined as the ratio S = R a/R g for reducing gas, where R a and R g are the resistances measured in ambient air and target gas atmosphere, respectively. The response and recovery times are defined as the time taken by the sensor to achieve 90 % of the total resistance change in the case of adsorption and desorption.
3 Results and discussion
3.1 Structure analysis
Figure 2a shows the XRD patterns of the pure and Pd-doped In2O3 microstructures obtained after calcination process. All of the diffraction patterns illustrate that the peaks characteristic to In2O3 with a cubic bixbyite phase (JCPDS Card No.06-0416), and the patterns show seven main reflection peaks at 30.68° (222), 35.51° (400), 38.45° (111), 44.69° (431), 51.05° (440), 60.74° (622) and 65.12° (444). However, there is no obvious diffraction peak observed for Pd-doped In2O3 microstructures, which is probably due to the low content of Pd in the samples. Figure 2b shows the high-resolution diffraction peak of In2O3 (444). The measured results reveal that the peak positions shift to lower angles with the increase of Pd content due to the lattice expansion of In2O3. The reason was attributed to the substitution In3+ ions with Pd2+ in In2O3 crystal because Pd2+ ions (0.86 nm) have a similar size to In3+ (0.8 nm) [33, 34]. Moreover, the larger size of Pd2+ ions causes the lattice expansion. As a result, the measured results provide indirect evidence that partial Pd2+ ions are doped in the lattice of In2O3.
3.2 Morphology analysis
Figure 3a–b show the typical SEM images of the obtained pure and 4.0 mol% Pd-doped In2O3 microstructures, which were composed of flower-like spherical microstructure with an average size range from 4 to 6 μm. From the insert images, we can observe that the microstructure was constructed by numerous thin nanosheets, and the thickness of nanosheets was about 25 nm. Moreover, the measured results reveals that there is no appreciable change of shape after adding Pd, which indicates that doping of Pd has little influence to the morphology of In2O3 microstructure. Meanwhile, to investigate the compositions of Pd-doped In2O3 microstructures, EDS measurement was also carried out on the 4.0 mol% Pd-doped In2O3 sample. Figure 3c displays the elemental mapping images of indium, oxygen and palladium, and the measured results clearly reveal that the spatial distribution of palladium element is homogeneous and exhibits the same uniform distribution with indium and oxygen elements, which suggests that palladium element was immobilized on the surface of indium oxide.
3.3 Gas sensing performances
In order to evaluate the potential application, gas sensors based on as-prepared pure and Pd-doped In2O3 microstructures were fabricated and their gas sensing properties were investigated. Figure 4a shows the response of all the fabricated sensors to 100 ppm hydrogen under the operating temperature varies from 140 to 360 °C. It is obvious that all the sensors exhibit an increase response at the beginning. However, further increase the operating temperature lead to the decrease of response. Moreover, the measured results demonstrate that all the sensors can reach to the maximum response at 210 °C. Consequently, an optimum working temperature of 210 °C was chosen for the remainder of the experiments. Figure 4b describes the relationship between gas sensors response and doping content of Pd to 100 ppm hydrogen. For pure In2O3 microstructure, the maximum value of response was about 1.4 at 210 °C. In contrast, the Pd-doped In2O3 sensors exhibited a relatively faster increase of response and higher maximum value under the same optimal operating temperature. Furthermore, 1.0 mol% Pd-doped In2O3 microstructure displayed the highest sensitivity of 3.6, which is almost two times higher than that of pure In2O3 at the same operating temperature. The measured results indicate that the 1.0 mol% Pd-doped In2O3 microstructure is very promising for the detection of hydrogen.
The response and recovery behavior is an important parameter that determines the gas sensing performance of gas sensor. To investigate the response and recovery time of pure and Pd-doped In2O3 gas sensors, all the as-prepared gas sensors were exposed to 100 ppm hydrogen gas (Fig. 5a). As can be seen, all the fabricated gas sensors exhibit fast response to target gas under the optimum operating temperature of 210 °C. Figure 5b displays the plots of the response and recovery time (Res./Rec. time) versus the Pd concentration with 100 ppm hydrogen gas. It can be clearly observed that the Res./Rec. time decrease with the increasing of Pd content at the initial stage, and then increases with the concentration above 0.5 and 1.0 mol%, respectively. Moreover, the measured response and recovery time for 1.0 mol% Pd-doped In2O3 sensor is only 4 and 7 s, which are shorter than the response and recovery time (11 and 12 s) of pure In2O3 gas sensor, suggesting the faster response and recovery behaviors of 1.0 mol% Pd-doped In2O3 sensor.
Figure 6a presents the dynamic response and recovery curves of pure and Pd-doped In2O3 gas sensors to hydrogen with concentrations ranging from 10 to 1000 ppm at 210 °C. The measured results reveal that all the as-prepared gas sensors exhibit highly sensitive and reversible responses to hydrogen with different concentrations. The response amplitude of the sensors are increased with increasing gas concentration and then they recovered to their baseline after several cycles between exposure to hydrogen gas and air, which indicate that all the fabricated sensors have good reversibility and reproducibility simultaneously. Moreover, the sensors based on Pd-doped In2O3 show much higher response than the sensor of pure In2O3. Figure 6b describes the calibration curves of the sensing performances. As can be seen, when the concentration of hydrogen is below 100 ppm, all the as-prepared gas sensors exhibit no obvious differences for the response. However, with the increasing of the hydrogen concentrations, the 1.0 mol% Pd-doped In2O3 sensor increased sharply and provides the highest sensitivity than that of others, and the measured sensitivity of 1.0 mol% Pd-doped In2O3 sensor can reach to 3.6–100 ppm hydrogen gas at 210 °C. All these measured results strongly demonstrate that the as-prepared 1.0 mol% Pd-doped In2O3 sensor is promising for gas sensing applications.
3.4 Gas sensing mechanism
As we all know, the gas-sensing mechanism of In2O3 gas sensor belongs to surface-controlled type, and chemisorbed oxygen ions on the surface plays a key role in the chemical reaction process [35]. In general, when the as-prepared pure In2O3 gas sensor was exposed to air, oxygen molecules will be adsorbed on the surface to generate chemisorbed oxygen species by trapping electrons from the conduction band of In2O3 (Fig. 7a), and it results in a decrease of carrier concentration and formation of depletion layer, which lead to a rise in the resistance of the In2O3 based sensor. Oxygen molecules adsorb on the surface of In2O3 as given in the following equations [35]:
When the sensor is transferred to hydrogen atmosphere, the hydrogen molecules interact with surface-adsorbed ionized oxygen species (O2 −, O−, O2−), and produce H2O (Fig. 7b). This process releases the captured electrons, and it causes an increase of carrier concentration and a narrower depletion layer, which result in a decrease in the electric resistance of the In2O3 sensor.
Compared to the pure In2O3 gas sensor, the Pd-doped In2O3 gas sensors exhibit significantly enhanced gas sensing performance. The improvement of gas sensing properties can be attributed to the interface between In2O3 (n-type semiconductor) and PdO (p-type semiconductor), which further enlarge the space charge layer near the interface in air (Fig. 7c) [36]. However, when the sensors were exposed to hydrogen gas, the magnified depletion layer was fading away fast, leading to bigger change in the sensor resistance and improvement of the response value, as shown in Fig. 7d. Thus, these Pd doped on the surface of In2O3 microstructures become high-performance gas sensing elements, which make the sensors more efficiently in gas detection than that of pure In2O3 microstructures.
4 Conclusions
The pure and different concentrations of Pd-doped In2O3 flower-like spherical microstructures were synthesized using a facile hydrothermal method. The crystal structure and surface morphology of as-prepared sample were characterized and gas sensing experiments were also conducted on the pure and Pd-doped In2O3 gas sensors to hydrogen detection. The results show that Pd addition can significantly improve the responses of In2O3 gas sensors to hydrogen. Moreover, the 1.0 mol% Pd-doped In2O3 sensor presents the highest sensing performance and its response is about two times higher than that of pure one to 100 ppm hydrogen under the optimum operating temperature. In additional, the 1.0 mol% Pd-doped In2O3 gas sensor also shows fast response/recovery time about 4 and 7 s, respectively. Therefore, the gas sensing performance of In2O3 microstructures could be largely enhanced by optimizing the doped Pd concentration, which is promising for early detecting the hydrogen leak in practice application.
References
G. Korotcenkov, S.D. Han, J.R. Stetter, Review of electrochemical hydrogen sensors. Chem. Rev. 109, 1402–1433 (2009)
X. Bevenot, A. Trouillet, C. Veillas, H. Gagnaire, M. Clement, Hydrogen leak detection using an optical fibre sensor for aerospace applications. Sens. Actuators B Chem. 67, 57–67 (2000)
M. Balat, Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 33, 4013–4029 (2008)
M. Sevilla, R. Mokaya, Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 7, 1250–1280 (2014)
A. Yilanci, I. Dincer, H.K. Ozturk, A review on solar-hydrogen/fuel cell hybrid energy systems for stationary applications. Prog. Energy Combust. Sci. 35, 231–244 (2009)
X.Q. Zeng, M.L. Latimer, Z.L. Xiao, S. Panuganti, U. Welp, W.K. Kwok, T. Xu, Hydrogen gas sensing with networks of ultra small palladium nanowires formed on filtration membranes. Nano Lett. 11, 262–268 (2010)
M. Zhang, Z. Yuan, J. Song, C. Zheng, Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sens. Actuators B 148, 87–92 (2010)
M. Sánchez, R. Guirado, M.E. Rincón, Multiwalled carbon nanotubes embedded in sol–gel derived TiO2 matrices and their use as room temperature gas sensors. J. Mater. Sci. Mater. Electron. 18, 1131–1136 (2007)
A. Esfandiar, S. Ghasemi, A. Irajizad, O. Akhavan, M.R. Gholami, The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing. Int. J. Hydrogen Energy 37, 15423–15432 (2012)
X. Yin, C. Xu, S. Li, J. Lu, Q. Wang, Sensing properties of Au-loaded SnO2 sensor for H2 and CO detection. J. Mater. Sci. Mater. Electron. doi: 10.1007/s10854-015-3718-4
M. Li, W. Yan, H. Zhu, S. Xia, W. Hao, Z. Tang, Synthesis and gas sensing properties of biomorphic SnO2 derived from loofah sponge and eggshell membrane. J. Mater. Sci. Mater. Electron. 26, 9561–9570 (2015)
X. Tian, K. Yu, X. Wang, L. Yang, J. Sun, Influence of ammonia sources on the gas sensing properties of the direct grown ZnO nanomaterials. J. Mater. Sci. Mater. Electron. 27, 4711–4722 (2016)
J. Hu, F. Gao, S. Sang, P. Li, X. Deng, W. Zhang, Y. Chen, K. Lian, Optimization of Pd content in ZnO microstructures for high-performance gas detection. J. Mater. Sci. 50, 1935–1942 (2015)
O. Lupan, G. Chai, L. Chow, Novel hydrogen gas sensor based on single ZnO nanorod. Microelectron. Eng. 85, 2220–2225 (2008)
J. Hu, F. Gao, Z. Zhao, S. Sang, P. Li, W. Zhang, X. Zhou, Y. Chen, Synthesis and characterization of Cobalt-doped ZnO microstructures for methane gas sensing. Appl. Surf. Sci. 363, 181–188 (2016)
Y. Sun, Z. Wei, W. Zhang, P. Li, K. Lian, J. Hu, Synthesis of brush-like ZnO nanowires and their enhanced gas-sensing properties. J. Mater. Sci. 51, 1428–1436 (2016)
Y. Gui, J. Zhao, H. Wang, J. Tian, H. Zhang, Microwave-assisted gas–liquid interfacial synthesis of WO3 precursor and nano-WO3 for gas-sensing application. J. Mater. Sci. Mater. Electron. doi 10.1007/s10854-016-4584-4
S. Sekimoto, H. Nakagawa, S. Okazaki, K. Fukuda, S. Asakura, T. Shigemori, S. Takahashi, A fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuators B 66, 142–145 (2000)
S. Elouali, L.G. Bloor, R. Binions, I.P. Parkin, C.J. Carmalt, J.A. Darr, Gas sensing with nano-indium oxides (In2O3) prepared via continuous hydrothermal flow synthesis. Langmuir 28, 1879–1885 (2012)
L. Xu, H. Song, B. Dong, Y. Wang, J. Chen, X. Bai, Preparation and bifunctional gas sensing properties of porous In2O3–CeO2 binary oxide nanotubes. lnorg. Chem. 49, 10590–10597 (2010)
Q. Lu, C. Wang, S. Liu, M. Ren, Continuous pearl-necklace-shaped In2O3 ceramic nanofibers: preparation, characterization and gas sensing properties. Mater. Trans. 52, 1206–1210 (2011)
T.V. Belysheva, E.A. Kazachkov, E.E. Gutman, Gas sensing properties of In2O3 and Au-doped In2O3 films for detecting carbon monoxide in air. J. Anal. Chem. 56, 676–678 (2001)
L. Liu, S. Li, X. Guo, L. Wang, L. Liu, X. Wang, The fabrication of In2O3 nanowire and nanotube by single nozzle electrospinning and their gas sensing property. J. Mater. Sci.: Mater. Electron. 27, 5153–5157 (2016)
L. Xu, B. Dong, Y. Wang, X. Bai, J. Chen, Q. Liu, H. Song, Porous In2O3: RE (RE) Gd, Tb, Dy, Ho, Er, Tm, Yb) nanotubes: electrospinning preparation and room gas-sensing properties. J. Phys. Chem. C 114, 9089–9095 (2010)
Y. Lü, W. Zhan, Y. He et al., MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 6, 4186–4195 (2014)
C. Liangyuan, S. Bai, G. Zhou, D. Li, A. Chen, C. Liu, Synthesis of ZnO–SnO2 nanocomposites by microemulsion and sensing properties for NO2. Sens. Actuators B 134, 360–366 (2008)
K. Suematsu, Y. Shin, Z. Hua et al., Nanoparticle cluster gas sensor: controlled clustering of SnO2 nanoparticles for highly sensitive toluene detection. ACS Appl. Mater. Interfaces 6, 5319–5326 (2014)
A. Chen, X. Huang, Z. Tong, S. Bai, R. Luo, C. Liu, Preparation, characterization and gas-sensing properties of SnO2–In2O3 nanocomposite oxides. Sens. Actuators B 115, 316–321 (2006)
S. Wang, B. Xiao, T. Yang, P. Wang, C. Xiao, Z. Li, R. Zhao, M. Zhang, Enhanced HCHO gas sensing properties by Agloaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2, 6598–6604 (2014)
S. Kundu, P.K. Warran, S.M. Mursalin, M. Narjinary, Synergistic effect of Pd and Sb incorporation on ethanol vapour detection of La doped tin oxide sensor. J. Mater. Sci. Mater. Electron. 26, 9865–9872 (2015)
Z.A. Ansari, S.G. Ansari, T. Ko, J. Oh, Effect of MoO3 doping and grain size on SnO2-enhancement of sensitivity and selectivity for CO and H2 gas sensing. Sens. Actuators B Chem 87, 105–114 (2002)
J. Moon, H.P. Hedman, M. Kemell et al., Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B Chem. 222, 190–197 (2016)
X. Wen, M. Wang, C. Wang, J. Jiang, Electroless plated SnO2–Pd–Au compositethin film for room temperature H2 detection. Electrochim. Acta 56, 6524–6529 (2011)
C.-M. Chang, M.-H. Hon, I.-C. Leu, Outstanding H2 sensing performance of Pd nanoparticle-decorated ZnO nanorod arrays and the temperature-dependent sensing mechanisms. ACS Appl. Mater. Interfaces 5, 135–143 (2012)
Y. Chang, J. Xu, Y. Zhang et al., Optical properties and photocatalytic performances of Pd modified ZnO samples. J. Phys. Chem. C 113, 18761–18767 (2009)
L. Liu, T. Zhang, S. Li, L. Wang, Y. Tian, Preparation, characterization, and gas-sensing properties of Pd-doped In2O3 nanofibers. Mater. Lett. 63, 1975–1977 (2009)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51205274), Shanxi Scholarship Council of China (Grant No. 2013-035), Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province ([2014]95), Shanxi Province Science Foundation for Youths (Grant No. 2013021017-2).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, L., He, X., Liang, Y. et al. Synthesis and gas sensing properties of palladium-doped indium oxide microstructures for enhanced hydrogen detection. J Mater Sci: Mater Electron 27, 11331–11338 (2016). https://doi.org/10.1007/s10854-016-5257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5257-z