Abstract
This paper reviews the design and development of magnetothermally-triggered drug delivery systems, whereby magnetic nanoparticles are combined with thermally-activated materials. By combining superparamagnetic nanoparticles with lower critical solution temperature (LCST) polymers, an alternating current (AC) magnetic field can be used to trigger localized heating in vivo, which in turn causes a phase change in the host polymer to allow diffusion and release of drugs. The use of magnetic nanoparticles for biomedical applications is reviewed, as well as the design of thermally-activated polymeric systems. Current research on externally-triggered delivery is highlighted, with a focus on the design and challenges in developing magnetothermally-activated systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Magnetothermally-triggered drug delivery systems offer a novel mechanism by which release of a drug can be triggered externally to the body. Such systems could improve therapies for diseases such as cancer, diabetes, and cardiovascular diseases. While sustained release systems work well for many orally-administered drugs, localized and triggered treatments are needed for delivery of potent agents such as those used in chemotherapy. Triggered release systems generally rely on the selection of an appropriate material to carry the drug; for example, pH-responsive polymers are used in many orally-administered medications as enteric coatings to protect the drug in the acidic environment of the stomach, but release the drug in the more neutral pH of the intestines, where the drug can be more readily absorbed into the bloodstream (1). Triggered release can also be designed around the breaking of a chemical bond (through hydrolysis, for example) to free the drug. Early work to study magnetically-controlled release showed that relatively large (millimeter-sized) magnetic beads imbedded in an ethylene vinyl acetate matrix could be triggered to open pores for release through application of an oscillating magnetic field (2,3), but this way of using magnets in drug delivery found little application because there was little difference between the on and off states. More recently, advances in nanotechnology have led to investigating magnetic nanoparticles which can be heated by an alternating current (AC) magnetic field, and several drug delivery researchers have taken a renewed interest in magnetically-triggered release, although the current work focuses on magnetothermally-responsive (or magnetically-triggered, thermally-sensitive) materials. However the triggering is accomplished, the benefit of controlling drug release in either a single or multiple pulse formulation benefits the patient by reducing the total amount of drug required to reach an effective dose, reducing the frequency of administration, and assisting in sophisticated devices that include targeting, imaging and multiple modes of therapy.
Magnetic Nanoparticles in the Design of Therapeutic Systems for Cancer Treatment
Cancer is one area where magnetothermal drug delivery would provide an important new avenue of therapy. Current treatment of cancers usually involves surgery, chemotherapy, radiation, immunotherapy or a combination of these methods (4,5). In recent years, remarkable progress has been made in developing new drugs and effectively targeting to tumor tissue. For example, Herceptin®, marketed by Genentech, targets human epidermal growth factor receptors (HER2) which are overexpressed in around 25% of breast cancer tumors (6). This treatment allows more powerful chemotherapeutic agents to be used since more of the drug can be effectively delivered to breast cancer cells, while less of the drug can damage healthy cells. There has also been a resurgence in the research literature for the use of hyperthermia as a treatment technique (7–11), with magnetic fluid hyperthermia (MFH) reaching clinical study stages in Germany (12). Hyperthermia is the application of heat to preferentially kill cancer cells while having a lesser effect on healthy cells (which are less susceptible to the heat because a mature vascular system has not been developed in tumors and heat cannot be removed efficiently) (13). Effective hyperthermia temperatures are usually from 42 to 45°C, as higher temperatures cause whole cell ablation and significant necrosis in both healthy tissue and cancerous tumors.
Before this recent interest in magnetic fluid hyperthermia, the 1960s saw the initial clinical usage of hyperthermia to treat cancers, as whole body hyperthermia was accomplished using immersion water baths or heated blankets (14). However, the serious side effects of whole body heating led to application of hyperthermia as a regional treatment aimed at sections of the body where tumors are present. Even with this improvement, hyperthermia treatment of large sections of tissue lost favor to radiation and chemotherapy (plus surgery) as the standard of care for clinical treatment. Recently, due in part to the rapid advancement of nanotechnology, magnetic hyperthermia has succeeded in minimizing the area of tissue that is heated and theoretically allows cellular localization for potential treatment of even metastatic cancers.
In magnetic fluid hyperthermia treatment (MFH), magnetic nanoparticles are designed to be heated by an alternating current (AC) magnetic field that can be applied external to the body, and was first investigated by Gilchrist et al. in 1957 (11,13,15). Using superparamagnetic nanoparticles (with crystal phases—or entire particle sizes—typically below 20 nm), the applied field heats the magnets by the mechanism of Néel relaxation, or rotation of the magnetization within each particle. As long as the particles are below their Curie temperature, they are heated when the AC field is applied. Once they reach the Curie temperature, the saturation magnetization of particles drops to zero, and heating stops. Thus, if the Curie temperature can be fixed by judicious selection of particle composition and size, hyperthermia can be applied and carefully controlled to not reach excessive temperatures (16). Due to their small size, magnetic nanoparticles can localize heating to small tissues and potentially even individual cells, with addressable ligands that can target receptors present on cancer cells (such as folate receptors, which are overexpressed in many types of cancer cells, or HER2 receptors in breast cancers, as mentioned above). MFH is currently applied by injection into tumors that can be imaged with MRI, but a continuing challenge for improved therapy with nanoparticles is the development of a delivery route that can target the individual cells of metastatic cancers. Injection into the bloodstream provides a sufficient pathway for nanoparticles to reach throughout the body, but the endothelial cell layer in blood vessels remains difficult to cross and transport within the interstitial fluid in the extracellular matrix is slow. Thus, much of the work with magnetic hyperthermia has focused on either follow-up treatment to surgery, with the magnetic particles left in the region after tumor removal, or direct injection into a tumor.
With the significant improvements in technology surrounding hyperthermia, it is gaining acceptance or even preference in clinical settings, and deserves strong consideration from many insurance companies that currently favor radiotherapy and do not cover hyperthermia treatments.
In the treatment of cancers, it is normal protocol to use a combination of therapies. The choice of therapy depends on several factors (tumor size, metastases, location of tumor, and patient preference) and each has benefits and drawbacks. The side effects of radiation and chemotherapy drugs are well known, but steps have been made to reduce the effects of the treatment to healthy tissue with more precise radiation treatment, and targeted chemotherapies. Several research groups are working to create single devices that allow a combination of these modes of treatment, as numerous studies have shown that cancers are more likely to go into remission (17,18). Thus, an important improvement in cancer therapy would allow two modes of treatment with a single material.
Nanotechnology using Magnetothermally-triggered Systems as Part of a Cancer Therapy System
Recently, the National Cancer Institute set out a vision for the next advancement in cancer diagnosis, treatment and monitoring (19). Strong nanotechnology science developed in the past decade led (at least in part) to the plan laid out by the NCI (Table I). These four functions present varying degrees of difficulty to achieve on their own, and the goal of creating a single device that combines all four into a nanoscale device is particularly challenging, though not without some promising approaches. The use of nanotechnology in oncology to meet at least some of these challenges has been recently published (20).
TARGETING
Targeting has already been achieved (though not necessarily perfected) for many types of cancers, and there remain challenges to optimize transport from the bloodstream to the site of a cancerous tumor, with an emphasis on improving the targeting efficiency to reduce patient side effects even further. The strategies for targeting will likely continue to rely on antibodies, receptors expressed on the surfaces of cell membranes, and passive targeting through the leaky vasculature that is present during the growth of tumors; see also this excellent review (4). Some significant advances in targeting in the past few years may allow addressable nanoparticles that not only reach the surface of a targeted cell, but shed an outer layer to further target specific organelles within a cell (for instance to deliver siRNA) (21–23).
IMAGING AND DIAGNOSTICS
Imaging is one of the most important tools in cancer diagnostics. MRI is the standard clinical test for determining the presence, location and size of a tumor, as the T2 relaxation mode allows distinction between normal soft tissue and the more rigid tissue that make up a tumor. However, MRI has not been able to reach the resolution required to diagnose metastatic cancers or individual cancer cells. To do this, nanoscale materials are required. Gadolinium nanoparticles are the standard phase contrast agent used to improve imaging (24,25), and with targeting moieties attached, they could potentially greatly improve the detection of very small tumors. In recent years, certain magnetic nanoparticles have also been shown to act as phase contrast agents, with the added benefit that these magnets could be used for both imaging and hyperthermia therapy (26,27).
LOCALIZED THERAPY
Nanoparticles are ideal for localized therapy, at least as far as their transportability in vivo. The NCI goal of localizing cancer therapy can be achieved using nanoparticles for localized chemotherapy, radiation, immunotherapy or hyperthermia, or combinations of these therapies. All of these therapies have potential for use in a nanoplatform for cancer treatment, although radiation therapies lack the benefit of delayed triggering, so small doses of radiation would be delivered during the transport of the device to the affected tissue or cells. Hyperthermia can be activated in vivo using a magnetic field or by photothermal therapy using infrared light to heat gold nanoshells (28,29), and delivery of a therapeutic drug could be achieved using a temperature responsive coating. A pH-responsive coating could also be used to trigger release as the nanodevice is brought into the cytoplasm of a cell (16).
One drawback for delivery of chemotherapeutics using a nanoscale device is that the payload of drug is quite small, often to just a few molecules of a therapeutic agent. Fortunately in cancer chemotherapy, most of the agents are highly potent, and effective targeting to a cancer cell will overcome this drawback. The same drawback (that the particles are so small) is also a challenge for either magnetically- or photo-triggered hyperthermia. The amount of heat generated depends on several factors. For magnetic hyperthermia, this includes the strength and frequency of an applied magnetic field, the depth of the particle in tissue, the particle size and composition, and the concentration of particles in the tissue (30–32). Hyperthermia therapy has been shown to be effective on a macroscale (in tumor tissue) (33), but more work is needed to understand heat transfer and the cellular mechanisms by which hyperthermia works to stop the growth of cancers to develop effective magnetic nanoparticle systems to target metastatic cancers.
CONFIRMATION OF SUCCESS
Perhaps the most challenging of the four components of the cancer treatment nanodevice described by the NCI is including functionality in the nanoplatform that allows noninvasive reporting of the success of treatment. This requires either the incorporation of a sensor into the nanoplatform, or using it in conjunction with a medical instrument to probe the tissue and cells that were targeted and treated. One potential method would be to use high field MRI which can detect the presence of ATP in tissue (34), and when combined with targeted nanoparticles that act as phase contrast agents, images before and after the therapy could be used to confirm that cellular metabolism in the tumor has decreased. Because many magnetic nanoparticles are good phase contrast agents (as mentioned in the discussion of imaging), they have great potential for use as the core of a nanodevice that meets all four goals set out by NCI.
While the motivation for development of magnetothermally-triggered drug delivery systems has been motivated by the need for novel cancer treatments, this type of triggering can be applied in numerous medical applications (e.g., triggered release of antibiotics from implant surfaces, or of calcium channel agonists from scaffolds for bone tissue engineering) as well as non-pharmaceutical consumer applications. Now that one application for magnetothermal drug delivery has been described, some novel methods for drug delivery using a controllable trigger external to the body are discussed below.
TRIGGERING MECHANISMS EXTERNAL TO THE BODY
Triggered drug release can be achieved by many mechanisms, but it is quite challenging to place a device in vivo with drug release activated at the will of the patient or a physician by a trigger external to the body. Pressure-induced rupture of microcapsules was one of the first reliable techniques to release a liquid, but the size of liquid-loaded microcapsules precludes their use for many in vivo applications; however, this mechanism has some limited potential for subdermal implanted devices. Other mechanisms are well known to modulate the behavior of polymers and have been utilized for controlled release, notably with the use of enteric coatings for pH-sensitive release for oral formulations where an internal trigger (the pH change from the stomach to the intestines) causes a swelling response in polymers such as poly(acrylic acid), marketed as Eudragit® for pharmaceutical coatings (35). More sophisticated nano-scale systems also utilize pH to trigger release, but take advantage of much smaller changes in pH between fluid in the extracellular matrix, endosomes and cell cytoplasm; these materials are under development to target specific organelles or increase the uptake of medicine inside cells. However, modulating pH cannot be accomplished with an external trigger; thus, further discussion will be limited to photo-, electronic- ultrasound- and magnetic-responsive designs.
For drug delivery to be activated at the will of a patient or physician, an external stimulus must be used to trigger release. The main systems that have been investigated for external triggering include devices with electronic interfaces (which, by their nature, require some invasive procedures to install or interact with the device) and phototherapies. Ultrasound- and magnetically-activated drug delivery systems have also begun to receive considerable attention in the scientific literature.
Electronic-, Ultrasonic- and Photo-Triggered Devices
Electronically-triggered drug delivery devices, such as insulin pumps, are well known and highly reliable, especially for chronic diseases (36,37). They require an interface between the patient and the delivery device, and often include a biosensing surface that allows detection of physiological substances, such as glucose or hormones, in addition to a reservoir of drug that can be administered automatically through a closed control loop, or by the patient or a doctor through an open control loop. While reliability and precise control are some of the strongest features of electronic devices, they require a battery or other power source, and the current size requirements of this power source prevent these devices from reaching nanoparticle sizes. Thus, electronic devices cannot be easily designed to target individual cells.
Ultrasound, applied on the order of 1 MHz can cause disruptions in tissue as well as drug carriers. Because ultrasound can penetrate through deep tissue, it can be applied externally to trigger release, although regional application may limit the effectiveness if the drug carrier is not localized to a certain area. Notably, pulsatile delivery of insulin has been shown using a barrier of methylene chains surrounding a poly(2-hydroxyethyl methacrylate) hydrogel crosslinked with poly(ethylene glycol dimethacrylate) (38,39). It is thought that cavitation of dissolved gases leads to increased diffusivity when ultrasound is applied. The group of Prausnitz has also done pioneering work in the use of ultrasound for biomedical applications, including ultrasound in drug delivery to increase the permeability of the cell membrane (40). Ultrasound has also been shown as a way to trigger release of doxorubicin from Pluronic® liposomes using subsecond pulses at a frequency of 20 kHz (41). Additional research on biomedical applications of ultrasound in drug delivery has been summarized in recent papers (42,43). Ultrasonic triggering offers a reasonable option for pulsatile delivery of a wide range of medications, particularly if targeted to a specific region.
Photodynamic therapies have advanced rapidly as a non- or minimally-invasive method to trigger release after a carrier has been injected or implanted into the body (44,45). Light-activated materials have received considerable attention as a way to trigger drug release, particularly with the creation of gold nanoshells that are designed to heat upon the application of a near infrared (NIR) beam of light (29). Because tissue is largely transparent in this region, NIR light can be effective at reaching even deep tissues. Nano-sized particles are critical to the successes observed with this technique, as is the ability to coat the surfaces of nanoparticles with a thin layer of gold (46). The ability to use nano-scale carriers allows these materials to target specific cells using classical targeting techniques: antibodies, viruses or various proteins or ligands. Once targeted, light activates heating of the gold nanoshells, which in turn can cause a phase change in a thermally responsive coating to deliver medication. An alternate material for photo-activated release was reported by Babincova et al., as they developed magnetoliposomes based on dipalmitoyl phosphatidyl choline (a natural phospholipid) that were triggered to heat using a laser light (47).Although the payload of any individual particle is small, by effective targeting, the amount of drug needed for treatment is significantly reduced over i.v. injections or oral delivery of medicine, thus greatly improving the effectiveness of treatment while minimizing patient side effects. Though not a focus of this paper, photothermal therapy, which uses light-activated heating for localized hyperthermia, has shown great promise for cancer treatment, and the light-activated heating can trigger drug release in much the same way as magnetic field-activated heating, which is described below.
Magnetically-Triggered Devices
A fourth method for external triggered release, magnetically-modulated release can be accomplished by either (1) the oscillatory motion of magnets imbedded in a polymer host to mechanically force openings for drug diffusion, or (2) magnetic heating of nanoparticles imbedded in a thermally-responsive polymer. In the case of oscillating magnets, on/off control over release rates has been difficult to achieve (2,3,48), and if the magnets are reduced to the nanoscale, the mechanical force exerted by the particles becomes so small that the mechanical forces caused by the magnetic field are not strong enough to overcome the elastic modulus of the polymer to open pores for release. In one recent report, though, ferrogels have been synthesized using magnetite nanoparticles of different sizes; the gels loaded with 500 nm particles showed the greatest modulation of release, with release stopped when a static magnet was applied to align the magnets and release starting when the magnet was removed (49). This same research group has shown that a high frequency AC magnetic field can cause bursts of drug release in ferrogels with release occurring during the application of the AC field (50). Despite this recent success, magnetothermally-triggered release, where an AC magnetic field heats nanoparticles to trigger release from a thermosensitive material, is the focus of this paper. Both photodynamic and magnetothermal systems utilize a triggering source that can be applied externally to cause heating, with a thermoresponsive polymer or lipidic structure designed to release the drug as it is heated above physiological temperatures.
BIOLOGICAL AND BIOMEDICAL APPLICATIONS OF MAGNETIC NANOPARTICLES: DRUG TARGETING, MRI AND BIOSEPARATIONS
Magnetic nanoparticles have been used or are under investigation for several biomedical and biological applications (51–54). By applying a static magnetic field over a targeted region of the body, magnetic nanoparticles have shown promise at localizing therapeutics in vivo (54–56), with important advances such as imbedded magnetic seeds used to improve the entrapment efficiency (57). Recent work has shown that superparamagnetic iron oxide nanoparticles can be combined with a biodegradable gel to prolong the local delivery of dexamethasone as an anti-inflammatory agent (58). Magnetic nanoparticles have also proven to greatly improve gene transfection when complexed with DNA to offer an alternative to gene guns and viral vectors for gene therapy (59), and they can be used in tissue engineering by attaching to cell membranes and applying an oscillating field to condition the cells for tissue growth (60,61). Magnetic separations have been achieved by binding antibodies to magnetic nanoparticles to isolate and purify compounds for bioseparations (62); similarly, magnetic nanoparticles have been investigated as a way to detoxify blood, with targeting agents used to attach poisons and an ex vivo shunt used for the magnetic separation step (63). Similarly, highly specific bioseparations have been proven effective when magnetic nanoparticles are combined with binding ligands and passed through a flow chamber in a magnetic field. Two additional applications of magnetic nanoparticles are relevant for development of a system to meet the criteria laid out by NCI: their use as MRI contrast agents (64,65) and their use in magnetic hyperthermia, which has progressed through human clinical trials in Germany (12).
CONCEPTUAL DESIGN OF MAGNETOTHERMAL DELIVERY SYSTEM
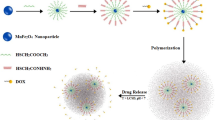
Two basic events are required for magnetothermal delivery: magnetic heating (or magnetic hyperthermia) and a thermally-responsive or -rupturable coating. One conceptual design of a magnetothermally-triggered device is shown in Fig. 1. Here, magnetic nanoparticles that are designed with the proper composition and size to achieve heating using an AC magnetic field are placed inside a thermally-responsive hydrogel. Once heated, the hydrogel changes conformation to open pores (in this case, thermosensitive grafts collapse, opening pathways for an imbedded drug to escape). The polymer can also be coated directly onto the nanoparticle, or a self-assembled structure (e.g., micelles, liposomes or polymersomes) can be devised to encapsulate the nanoparticle (66,67). For biomedical applications, the coating must rupture or open to release a drug when heated above physiological temperatures.
As discussed above, some of the leading technologies to externally trigger release in a non-invasive way are photothermal and magnetothermal systems. The focus of this paper is on magnetic nanoparticles and their combination with thermally-responsive carriers. As discussed further below, these systems can be designed to meet all four of the NCI goals for a nanoplatform for cancer treatment. The two components required for a magnetothermally-triggered device (magnetic nanoparticles and thermally-responsive materials) merit further discussion, as the selection and design of magnetic nanoparticles can optimize the heating while minimizing the exposure time for activation. Low Curie temperature magnetic nanoparticles should also be considered, as well as the biocompatibility or toxicity of the materials. Similarly, the design of a thermoresponsive carrier requires careful selection of materials and optimization of the structure to achieve an on/off delivery system. With a good system design, magnetothermally-triggered release can offer a novel mechanism by which drug delivery can be activated outside the body.
Magnetic Nanoparticle Selection and Design
Two mechanisms can be responsible for magnetic heating: Brownian and Néel relaxation (31). For magnets with large crystals (greater than 20 nm, depending on composition), eddy currents can cause significant non-specific heating, as has been used for decades in induction heating of metals (68). For nanoparticles Brownian relaxation refers to whole particle spinning as the particle attempts to align with the applied magnetic field. Néel relaxation is the decay of the magnetic moment inside the nanoparticle, and predominates for smaller nanoparticles or when the nanoparticles are imbedded in a material that restricts their free motion (such as a hydrogel). The mechanisms of magnetic heating are described in greater detail by Rosensweig (31), and some of the mathematics are included in the section on heat transfer below. If eddy current heating can be avoided, then only the nanoparticles will be specifically heated by the AC magnetic field, allowing the designer greater control over the power output and temperatures reached when the pulsed field is applied.
Magnetic nanoparticles commonly investigated for hyperthermia application are generally iron oxides, with magnetite being the primary material investigated. Several of these are biocompatible (or even found in biological systems), so they can be introduced into medical devices with relative ease, while the cytotoxic behavior of other materials have also been investigated (69–72). Beyond magnetite, several additional magnetic materials have also been investigated (Table II). Each of these materials can be used in magnetic hyperthermia, but some have unique properties that improve the heating, add in safety factors, or provide a second functionality for biomedical devices. High specific absorption ratios, SARs, are desirable because heat can be more effectively generated with smaller concentrations or lower magnetic fields (although the particle/crystal size also plays a role). Others, such as manganese ferrite, have good properties as MRI contrast agents, so they could be used in the development of a system that combines magnetic hyperthermia, magnetothermally-controlled release and imaging.
The Curie temperature is an important property for magnetic materials, as this indicates the temperature where the material will stop heating and reach steady state, even if the AC magnetic field is applied for a long period of time (81,82). Most large scale magnets will continue to heat upon application of an AC magnetic field to quite high temperatures. For example, pure iron has a Curie temperature of nearly 800°C and cobalt has a Curie temperature over 1100°C (73). While these temperatures will certainly allow for fast heating, for safety reasons in an in vivo application, materials with Curie temperatures just above physiological temperature are desired. The Curie temperature can be tuned by the choice of metal composition, but is also dependent on the crystal (or nanoparticle) size (83,84). The effect of nanoparticle size and shape has been investigated for manganese, iron and cobalt nanoparticles (85,86). Because nanoparticles are normally made of only single crystals, the synthesis procedure to make nanoparticles, which controls the particle size, also controls the Curie temperature and heating properties of the magnet. A few groups are working to optimize nanoparticles with low Curie temperatures, but this could be an important area of research for biomedical applications.
Heating magnetic nanoparticles depends on properties of both the particles and the applied field (87). One device used for creating an AC magnetic field uses an electric coil to focus the magnetic field within, as pictured in Fig. 2 for a 4-turn coil and a slotted coil, with space to insert a Petri dish. Variable voltage and capacitors allow the magnitude of the field to be adjusted as a resonant frequency is determined for each setting. In Fig. 2, thermal infrared images are shown to collect temperature data, but T-type thermocouples can also be placed in AC magnetic fields. In most cases, higher field intensities generate more heat. For example, cobalt ferrite nanoparticles are heated much more efficiently as the field intensity is increased from 127 to 634 Gauss (Fig. 3— see Ref. (75)). The applied frequency is also important, as researchers have generally focused on pulsed frequencies in the range of 50 kHz to 10 MHz to achieve heating. Nanoparticle composition and size impact the SAR values as well; for example, manganese ferrite nanoparticles were synthesized with nominal diameters ranging from 5.3 to 12.1 nm, with the heating rate optimized for the 10.5 nm particles (76). An additional consideration for further development of devices triggered by an AC magnetic field is the tolerance of the human body to the applied field: what intensities are allowable and for what periods of time will greatly impact the design of magnetic materials for hyperthermia or magnetically-triggered delivery systems.
Magnetic hyperthermia coils and infrared temperature images of nanoparticle solutions: A 4-turn test-tube coil, B infrared image of cobalt ferrite nanoparticle solution heated in a centrifuge tube, C Petri dish coil, and D side view infrared image of cobalt ferrite nanoparticle solution heated in a Petri dish.
Effect of magnetic field intensity on heating profiles for cobalt ferrite nanoparticles exposed to 266 kHz AC field. [Reprinted from (72) with permission from Elsevier.].
Magnetic Heating: Néel Relaxation and Pennes’ Bio-Heat equation
The mechanisms for heating magnetic nanoparticles with an AC magnetic field include three types of loss processes (hysteresis losses, and Néel and Brownian relaxation). The relative contribution of each process depends strongly on the crystal size and composition of the particles. Nanoparticles with core diameters of less than 20 nm or so, as used in most magnetic fluid hyperthermia applications, are single-domain particles, meaning that they consist of only a single organized crystal. In such small nanoparticles, magnetization relaxation is governed by a combination of the external rotation (or Brownian) and internal (or Néel) diffusion of the particle’s magnetic moment (88), with negligible contribution of hysteresis loss. A mathematical expression (31) to determine the combined effect of Néel and Brownian relaxation times (τN and τB, respectively) on the effective relaxation time, τ, is given by:
for monodispersed particles. When superparamagnetic nanoparticles are placed in an alternating current magnetic field, the total energy dissipated, P, is calculated as determined by Rosensweig (31) by:
where χ 0 is the equilibrium susceptibility of the magnetic particles, and ω is the applied frequency of the magnetic field (rad/s). Additionally, μ o is the magnetic permeability and H is the intensity or amplitude of the magnetic field. The development of further equations required for analysis is included in references (31,32).
The energy generated by magnetic nanoparticles is transmitted as heat through the medium in which the particles are imbedded. For hyperthermia therapy, Pennes’ bioheat equation applies (89,90). When listed in cylindrical geometry, the energy balance becomes:
This equation can be solved to determine temperature profiles for magnetic nanoparticles placed within a cylindrical core of tissue, where ρ 1 represents the tissue density, c 1 is the specific heat capacity, T is temperature, t is time, k 1 is the thermal conductivity of the tissue, ω b1 is the volumetric perfusion rate of blood through the tissue, c b1 is the heat capacity of blood, and T art is the temperature of blood in an artery. The left side of the equation represents the increase in internal energy of the tissue (which can be discretized and solved by numerical methods to determine the temperature profile as a function of radius and height, r and z). The first and second terms on the right side of the equation represent conductive heat transfer in the radial and axial directions, respectively, while the third term represents heat transfer by convection to blood perfused through the tissue and is dependent on the flow rate and temperature of blood, which is usually taken as equal to the core body temperature. Using these equations to model hyperthermia heating of tissue, temperature profiles for a cylinder can be estimated as a function of nanoparticle loading concentration, power generation from the AC magnetic field and tissue properties. For example, these equations have been solved as a function of time for a cylindrically-shaped tissue with imbedded nanoparticles surrounded by tissue with no nanoparticles (Fig. 4, (32)). By performing these calculations, the time required for application of an AC magnetic field and the maximum temperature reached at various positions within the tissue can be determined at steady state. These models are extremely helpful in designing systems that will provide sufficient heat for hyperthermia, but modifications of this model can also be used to estimate the heat dissipation in magnetothermal drug delivery systems where the magnetic nanoparticles are placed within hydrogels or self-assembled lipidic structures.
Transient temperature distribution for a cylindrical tumor with R = 3/7 and Z = 1 surrounded by healthy tissue from R = 3/7 to R = 1 at a 0 s b 100 s c 200 s d 1,000 s. Here, an isothermal boundary condition of T = 37°C at R = 1, R = −1, Z = 0 and Z = 1 was used, with zero heat loss due to blood perfusion in the tissue (ω b = 0.001 g cm−3 s−1). Model parameters are fully described in reference (32).
Design of Thermoresponsive Polymeric Carriers
Polymer selection and design is critical to the development of magnetothermally-triggered systems. Since magnetic nanoparticles can deliver a localized heat source, the polymer should be designed to phase separate or change conformations when heated. Polymers displaying a lower critical solution temperature (LCST) in aqueous solutions have been investigated by numerous researchers for thermo-sensitive drug delivery (91–93). Most notable is poly(N-isopropylacrylamide), or PNIPAAm, which phase separates from water around it’s LCST of 32°C. Hydrogels based on this polymer can trap a drug, with release triggered by two possible mechanisms: swelling-controlled release or squeezing-controlled release (Fig. 5). In swelling-controlled release, the drug diffuses out of the hydrogel as it swells (91), which would be at low temperatures for PNIPAAm; this is not ideal for combination with hyperthermia, as heating from nanoparticles imbedded in the gel will cause release to slow or stop. In squeezing-controlled release, an already hydrated gel collapses rapidly when heated, exuding water and much of the imbedded drug with the water (94–96). Here, macroporous hydrogels offer large pores for rapid diffusion during the gel collapse (97). Because squeezing-based hydrogel system would release a drug when heated, they are preferred over swelling-controlled systems for the development of magnetothermally-triggered drug delivery systems. However, when a hydrogel is placed in an aqueous environment (such as the human body), the gel will swell, leaving the mesh open for drug diffusion and release even before the triggering event, so a squeezing-controlled release system is not ideal for a system with a delayed triggering event. Thus, a squeezing-controlled release mechanism could be problematic, particularly for small (nano-) sized devices (where diffusion could exhaust the drug before triggering), or in the design of systems to deliver potent drugs (as even a small amount released prior to triggering could have undesired toxic effects). To do avoid drug release prior to triggering, other polymer designs are required.
The ideal carrier system for magnetothermally-triggered release would have minimal drug release at physiological temperatures (37°C) and have an LCST slightly above physiological temperature (40 to 45°C). The LCST of a poly(alkylacrylamide) gel, such as PNIPAAm, can be altered by the judicious selection of comonomers that impart an increase or decrease in the polymer hydrophilicity (98,99). In general, adding hydrophilic comonomers to the hydrogel increases the LCST; thus, gels that combine NIPAAm with hydrophilic comonomers such as acrylic acid or acrylamide have been developed with LCSTs in the range of 35 to over 50°C, depending on the ratio of monomers used in the polymerization (99–101). Other polymers that display some thermosensitivity near physiological conditions include hydroxypropyl cellulose (102), Pluronic® triblock copolymer surfactants and block copolymers (103,104), poly(dimethylaminoethyl methacrylate) (105) and elastin-like peptides (106).
Ideally, in a magnetothermally-triggered drug delivery system, the drug should be kept sequestered in the carrier during the transit to a localized area, with diffusion activated only when triggered by heating caused by an AC magnetic field. Two such polymer designs that can achieve this goal are hydrogels grafted with thermoresponsive oligomers (as depicted in Fig. 5c) and hydrogels filled with thermoresponsive polymers. In both cases, the thermoresponsive materials fill the mesh space of the hydrogel, blocking or slowing diffusion when below the LCST, but collapsing to open space for drug diffusion when heated. These systems are under investigation and have shown promise as positive thermoresponsive systems to release when heated. For example, grafting oligo(NIPAAm) or oligo(NIPAAm-co-acrylamide) onto a poly(2-hydroxyethyl methacrylate) hydrogel network has shown that drug diffusivities are increased with a temperature rise, although the off state of these systems requires more work to optimize, as shown in Fig. 6 (99). Further designs must be considered to optimize the on/off drug release pattern, with parameters such as crosslink ratio, oligomer molecular weight, composition of LCST copolymers, and grafting density being critical.
Drug diffusion coefficients at 12, 25 and 37°C measured during release of theophylline or inulin for three hydrogel structures: non-responsive PHEMA, negative thermoresponsive PNIPAAm, and positive thermoresponsive P(NIPAAm-g-NIPAAm). Experimental details are included in reference (96). Figure is reprinted from (96) with permission from Elsevier.
Nanoparticle-Hydrogel Composite Materials
Magnetothermally-triggered carriers can be designed as implantable devices with nanoparticles loaded inside along with a drug, or as entirely nano-scale devices with a hydrogel layer coating individual magnetic nanoparticles. Both of these systems offer great potential for triggered delivery. Implantable devices can be placed in a desired location, such as the surface of an implant, with treatment triggered locally for delivery of multiple pulses of medication. The synthesis of the larger-scale devices is more straightforward, as nanoparticles can be incorporated during the polymerization or crosslinking step (48,107,108), or could be adsorbed onto the polymer surface afterwards (109). During incorporation, nanoparticles may aggregate, which can affect the thermal response of the materials (110). In the case of gel-coated nanoparticles, the great advantage is that the entire device can be localized to a particular site using traditional targeting moieties, with drug release triggered after a period of time is allowed for the coated nanoparticles to reach particular cells. Synthesis procedures for coating nanoparticles are more challenging, as agglomerations must be avoided during the coating step. Approaches that have been investigated for creating nano-scale coated magnetic nanoparticles include surface-initiated polymerizations (111,112), electrojet encapsulation (113), and ionic coupling of chitosan to surfactant groups attached to magnetite (114). An additional challenge with coated nanoparticles is that the amount of drug that can be incorporated in the nano-scale carrier is quite small, so a single pulse of drug is normally all that can be triggered.
Some important considerations and challenges for the effective design of nanoparticle-hydrogel composites includes fixing the nanoparticle within the gel so that only the drug is released and ensuring that the particles are well-dispersed to generate a uniform temperature rise through the hydrogel. Due to the size of nanoparticles required for magnetic hyperthermia (5–10 nm), they may be able to diffuse within a hydrogel structure (with mesh sizes that often approach 5 nm), particularly if a macroporous structure is used. Large molecular weight drugs, such as proteins, may be similar in size to the nanoparticles, which may require that the nanoparticles be chemically linked to the polymer. However, in most cases physical entrapment is adequate to prevent the nanoparticles from leaving a host hydrogel (108). Uniform dispersion of nanoparticles within a hydrogel is also essential to optimizing the performance. Because magnetic nanoparticles have a tendency to agglomerate, the surfactants used to form an aqueous dispersion must be carefully selected. It has been found that much of the thiol chemistry used in developing surfactants for gold nanoparticles are also suitable for several magnetic materials; two surfactants that have been employed include 11-mercaptoundecanoic acid and dimercaptosuccinic acid (75,115). Even after nanoparticles are dispersed in water, they have been shown to agglomerate during the free radical polymerizations used to embed them in hydrogels.
CURRENT RESEARCH ON MAGNETOTHERMALLY-TRIGGERED SYSTEMS
Among groups working on the development of magnetothermally triggered-drug release, approaches have varied. In many cases, magnetite nanoparticles are the sole focus as a heating source, but some groups are investigating a wider range of magnetic materials, with the hopes of optimizing the heat output, enabling combination of magnetic hyperthermia with imaging techniques, developing materials with a limiting Curie temperature as a safety measure for use in vivo, or a combination of these goals (13,18,77,82). For instance, Lao and Ramanujan studied the effect of magnetic field strength on heating in hydrogel-magnetite composite materials (116). Other groups are focused on development of hydrogels, liposomes, micelles or other advanced structures that are thermally sensitive and can be triggered to release a drug by magnetic hyperthermia. The Hilt group has shown that magnetic hyperthermia could be used to heat in thermosensitive hydrogels with imbedded Fe3O4 nanoparticles, and showed that the presence of the nanoparticles had little impact on the LCST behavior of the hydrogels (117,118); they have also shown that short pulses of an AC magnetic field can cause rapid shrinking in thermosensitive hydrogels, resulting in similarly short bursts of drug release due to the magnetothermal heating (119). Meledandri and Brougham reported two methods to synthesize magnetoliposomes with the goal of combining contrast agents with drug delivery (120). Advances in the development of instruments to deliver pulsed magnetic fields with tunable parameters to optimize heating have been reported (75,121). Other important work has been done to develop biodegradable materials (122), and study the toxicity of magnetic nanomaterials (69,123), but there remain numerous challenges to be addressed by multidisciplinary teams including physicists and engineers, as well as biologists and medical researchers.
Liposomes and micelles also have a rich history for their use in drug delivery systems, and a number of investigators have created magnetoliposomes or other self-assembled nano-structures that can incorporate magnets (124,125). By judicious selection of the amphiphilic structures used to design these materials (including micelles, liposomes and polymersomes), they can be destabilized by heating, to release a drug. Thus, self-assembled magnetothermal drug delivery systems using these small structures offer an alternate pathway to nano-sized, targetable carriers. The magnetoliposomes of Babincova et al. that were photothermally triggered using laser light could be easily transformed into magnetothermal devices. Anti-HER2 immunoliposomes have also been studied as a way to combine immunotherapy with hyperthermia (17), but the systems (so far) lack a thermosensitive trigger to release the antibodies. Further work in this area has focused on the development of biodegradable micelles with LCST behavior (126) and block copolymers that form thermoresponsive micelles (127). Because these systems are formed through self-assembly and stabilized by strong thermodynamic interactions between hydrophilic or hydrophobic regions, one potentially difficult challenge to using these systems for effective delivery is the requirement that they disassemble upon localized heating (at least long enough to release the drug).
CONCLUSIONS
Magnetothermally-triggered drug delivery holds promise as a novel method for triggering events inside the body in a non-invasive manner, as they can be made small enough to be injectable and activated with an external AC field. This adds a significant new tool for pharmaceutical researchers, even though there are a number of technical challenges to understand and optimize the material behavior and design challenges for developing biocompatible, targetable, nano-sized systems that can be used effectively in vivo with a magnetic field applied to generate the sufficient heating with minimal patient discomfort.
References
N. A. Peppas, Y. Huang, M. Torres-Lugo, J. H. Ward, and J. Zhang. physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2:9–30 (2000). doi:10.1146/annurev.bioeng.2.1.9.
E. R. Edelman, L. Brown, J. Taylor, and R. Langer. In vitro and in vivo kinetics of regulated drug release from polymer matrices by oscillating magnetic fields. J. Biomed. Mater. Res. 21:339–353 (1987). doi:10.1002/jbm.820210307.
E. R. Edelman, and R. Langer. Optimization of release from magnetically controlled polymeric drug release devices. Biomaterials. 14:621–626 (1993). doi:10.1016/0142-9612(93)90182-2.
L. Brannon-Peppas, and J. O. Blanchette. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 56:1649–1659 (2000). doi:10.1016/j.addr.2004.02.014.
A. El-Aneed. An overview of current delivery systems in cancer gene therapy. J. Control Release. 94:1–14 (2004). doi:10.1016/j.jconrel.2003.09.013.
Genentech Biooncology. Herceptin: Trastuzumab, www.genentech.com, 2008.
K. L. Ang, S. Venkatraman, and R. V. Ramanujan. Magnetic PNIPA hydrogels for hyperthermia applications in cancer therapy. Mater. Sci. Eng. 27:347–351 (2007). doi:10.1016/j.msec.2006.05.027.
S. W. Lee, S. Bae, Y. Takemura, I.-B. Shim, T. M. Kim, J. Kim, H. J. Lee, S. Zurn, and C. S. Kim. Self-heating characteristics of cobalt ferrite nanoparticles for hyperthermia application. J. Magn. Magn. Mater. 310:2868–2870 (2007). doi:10.1016/j.jmmm.2006.11.080.
J. K. Yang, J. H. Yu, J. Kim, and Y. H. Choa. Preparation of superparamagnetic nanocomposite particles for hyperthermia therapy application. Mater. Sci. Eng. A. 449–451:477–479 (2007). doi:10.1016/j.msea.2006.02.336.
D. C. F. Chan, D. B. Kirpotin, and P. A. Bunn Jr. Synthesis and evaluation of colloidal magnetic iron oxides for the site-specific radiofrequency-induced hyperthermia of cancer. J. Magn. Magn Mater. 122:374–378 (1993). doi:10.1016/0304-8853(93)91113-L.
A. Jordan, R. Scholz, P. Wust, H. Fähling, and R. Felix. Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 201:413–419 (1999). doi:10.1016/S0304-8853(99)00088-8.
P. Wust, U. Gneveckow, M. B. Johannsen, D. Ãhmer, T. Henkel, F. Kahmann, J. Sehouli, R. Felix, J. Ricke, and A. Jordan. Magnetic nanoparticles for interstitial thermotherapy—feasibility, tolerance and achieved temperatures. Int. J. Hypertherm. 22:673–685 (2006). doi:10.1080/02656730601106037.
A. K. Gupta, and M. Gupta. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021 (2005). doi:10.1016/j.biomaterials.2004.10.012.
O. S. Nielsen, M. Horsman, and J. Overgaard. A future for hyperthermia in cancer treatment? Eur. J. Cancer 37:1587–1589 (2001). doi:10.1016/S0959-8049(01)00193-9.
R. K. Gilchrist, R. Medal, W. D. Shorey, R. C. Hanselman, J. C. Parrot, and C. B. Taylor. Selective inductive heating of lymph nodes. Ann. Surg. 146:596–606 (1957). doi:10.1097/00000658-195710000-00007.
E. Roux, M. Francis, F. M. Winnik, and J.-C. Leroux. Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs. Int. J. Pharm. 242:25–36 (2002). doi:10.1016/S0378-5173(02)00183-7.
A. Ito, Y. Kuga, H. Honda, H. Kikkawa, A. Horiuchi, Y. Watanabe, and T. Kobayashi. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 212:167–175 (2004). doi:10.1016/j.canlet.2004.03.038.
T. K. Jain, J. Richey, M. Strand, D. L. Leslie-Pelecky, C. A. Flask, and V. Labhasetwar. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials 29:4012–4021 (2008). doi:10.1016/j.biomaterials.2008.07.004.
National Cancer Institute, www.nano.cancer.gov, 2005.
K. B. Hartman, L. J. Wilson, and M. G. Rosenblum. Detecting and treating cancer with nanotechnology. Mol. Diagn. Ther. 12:1–14 (2008).
E. M. Kolonko, and L. L. Keissling. Polymeric domain that promotes cellular internalization. J. Am. Chem Soc. 130:5626–5627 (2008). doi:10.1021/ja8001716.
N. Murthy, J. Campbell, N. Fausto, A. S. Hoffman, and P. S. Stayton. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J. Control Release 89:365–374 (2003). doi:10.1016/S0168-3659(03)00099-3.
R. E. Vanden-Broucke, B. G. DeGeest, S. Bonne, M. Vinken, T. Van Haecke, H. Heimberg, E. Wagner, V. Rogiers, S. C. DeSmedt, J. Demeester, and N. N. Sanders. Prolonged gene silencing in hepatoma cells and primary hepatocytes after small interfering RNA delivery with biodegradable poly(beta-amino esters). J. Gene Med. 10:783–794 (2008). doi:10.1002/jgm.1202.
D. L. Buckley, P. J. Drew, S. Mussurakis, J. R. T. Monson, and A. Horsman. Microvessel density in invasive breast cancer assessed by dynamic Gd-DTPA enhanced MRI. J. Magn. Reson. Imaging 7:461–464 (1997). doi:10.1002/jmri.1880070302.
J. L. Bridot, A. C. Faure, S. Laurent, C. Riviere, C. Billotey, B. Hiba, M. Janier, V. Josserand, J. L. Coll, L. Vander Elst, R. Muller, S. Roux, P. Perriat, and O. Tillement. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 129:5076–5084 (2007). doi:10.1021/ja068356j.
V. S. Kalambur, B. Han, B. E. Hammer, T. W. Shield, and J. C. Bischof. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology 16:1221–1233 (2005). doi:10.1088/0957-4484/16/8/041.
S. Mornet, F. Vasseur, F. Grasset, and E. Duguet. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 14:2161–2175 (2004). doi:10.1039/b402025a.
D. P. O’Neal, L. R. Hirsch, N. J. Halas, J. D. Payne, and J. L. West. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 209:171–176 (2004). doi:10.1016/j.canlet.2004.02.004.
S. R. Sershen, S. L. Westcott, N. J. Halas, and J. L. West. Independent optically addressable nanoparticle-polymer optomechanical composites. Appl. Phys. Lett. 80:4609–4611 (2002). doi:10.1063/1.1481536.
R. Hergt, W. Andra, C. G. d’Ambly, I. Hilger, W. A. Kaiser, U. Richter, and H. G. Schmidt. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans. Magn. 34:3745–3754 (1998). doi:10.1109/20.718537.
R. E. Rosensweig. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 252:370–374 (2002). doi:10.1016/S0304-8853(02)00706-0.
C. Zhang, D. T. Johnson, and C. S. Brazel. Numerical study on the multi-region bio-heat equation to model Magnetic Fluid Hyperthermia (MFH) using low curie temperature nanoparticles. IEEE Transactions on Nanobioscience in press—VOL (2008) page.
A. Jordan, R. Scholz, P. Wust, H. Fahling, J. Krause, W. Wlodarczyk, B. Sander, T. Vogl, and R. Felix. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int. J. Hypertherm. 13:587–605 (1997). doi:10.3109/02656739709023559.
V. Simonenko, H. Pageant, D. Fei, and T. C. Ng. In vitro and in vivo monitoring of 5-fluorosytosine metabolism with 19F-NMR spectroscopy after adenoviral transduction of cells with bifunctional yeast CDUPRT fusion gene. Molec. Ther. 9:S230 (2004).
M. Dittgen, M. Durrani, and K. Lehmann. Acrylic polymers—a review of pharmaceutical applications. STP Pharma Sciences 7:403–437 (1997).
J. T. Santini, M. J. Cima, and R. Langer. A controlled-release microchip. Nature 397:335–338 (1999). doi:10.1038/16898.
A. Nisar, N. Afzulpurkar, B. Mahaisavariya, and A. Tuantranont. MEMS-based micropumps in drug delivery and biomedical applications. Sens. Actuators, B, Chem. 130:917–942 (2008). doi:10.1016/j.snb.2007.10.064.
C. S. Kwok, P. D. Mourad, L. A. Crum, and B. D. Ratner. Surface modification of polymeric slab surfaces with self-assembled monolayer and its characterization with multi-surface-analytical techniques. Biomacromolecules 1:139–148 (2000). doi:10.1021/bm000292w.
C. S. Kwok, P. D. Mourad, L. A. Crum, and B. D. Ratner. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J. Biomedical Materials Research 57:151–164 (2001). doi:10.1002/1097-4636(200111)57:2<151::AID-JBM1154>3.0.CO;2-5.
R. K. Schlicher, H. Radhakrishna, T. P. Tolentino, R. P. Apkarian, V. Zarnitsyn, and M. R. Prausnitz. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 32:915–924 (2006). doi:10.1016/j.ultrasmedbio.2006.02.1416.
G. A. Husseini, and W. G. Pitt. The use of ultrasound and micelles in cancer treatment. Journal of Nanoscience and Nanotechnology 8:2205–2215 (2008). doi:10.1166/jnn.2008.225.
G. A. Husseini, N. Y. Rapoport, D. A. Christensen, J. D. Pruitt, and W. G. Pitt. Kinetics of ultrasonic release of doxorubicin from pluronic P105 micelles. Colloids Surf, B Biointerfaces 24:253–264 (2002). doi:10.1016/S0927-7765(01)00273-9.
S. L. Huang. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 60:1167–1176 (2008). doi:10.1016/j.addr.2008.03.003.
I. Roy, T. Y. Ohulchanskyy, H. E. Pudavar, E. J. Bergey, A. R. Oseroff, J. Morgan, T. J. Dougherty, and P. N. Prasad. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J. Am. Chem. Soc. 125:7860–7865 (2003). doi:10.1021/ja0343095.
P. Shum, J. M. Kim, and D. H. Thompson. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 53:273–284 (2001). doi:10.1016/S0169-409X(01)00232-0.
A. M. Gobin, M. H. Lee, N. J. Halas, W. D. James, R. A. Drezek, and J. L. West. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nanoletters 7:1929–1934 (2007). doi:10.1021/nl070610y.
M. Babincova, D. Leszczynska, P. Sourivong, and P. Babinec. Picosecond laser pulses mediated drug release from magnetoliposomes. Cell. Mol. Biol. Lett. 4:625–630 (1999).
K. S. Carroll, J. B. McKinney, D. T. Johnson, and C. S. Brazel. Development of magnetothermal responsive systems for tumor treatment. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 30:82–83 (2003).
T.-Y. Liu, S.-H. Hu, D.-M. Liu, and S.-Y. Chen. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 22:5974–5978 (2006). doi:10.1021/la060371e.
S.-H. Hu, T.-Y. Liu, D.-M. Liu, and S.-Y. Chen. Controlled pulsatile drug release from a ferrogel by a high-frequency magnetic field. Macromolecules 40:6786–6788 (2007). doi:10.1021/ma0707584.
M. Shinkai. Review: functional magnetic particles for medical application. J. Biosci. Bioeng. 94:606–613 (2002).
A. Ito, M. Shinkai, H. Honda, and T. Kobayashi. Review: medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 100:1–11 (2005). doi:10.1263/jbb.100.1.
Q. A. Pankhurst, J. Connolly, S. K. Jones, and J. Dobson. Applications of magnetic nanoparticles in biomedicine. J. Phys., D, Appl. Phys. 36:R167 (2003). doi:10.1088/0022-3727/36/13/201.
J. Dobson. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 67:55–60 (2006). doi:10.1002/ddr.20067.
H. Chen, A. D. Ebner, M. D. Kaminski, A. J. Rosengart, and J. A. Ritter. Analysis of magnetic drug carrier particle capture by a magnetizable intravascular stent—2: parametric study with multi-wire two-dimensional model. J. Magn. Magn. Mater. 293:616–632 (2005). doi:10.1016/j.jmmm.2005.01.080.
J. Yang, S.-B. Park, H.-G. Yoon, Y.-M. Huh, and S. Haam. Preparation of poly ε-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int. J. Pharm. 324:185–190 (2006). doi:10.1016/j.ijpharm.2006.06.029.
A. J. Rosengart, M. D. Kaminski, H. Chen, P. L. Caviness, A. D. Ebner, and J. A. Ritter. Magnetizable implants and functionalized magnetic carriers: a novel approach for noninvasive yet targeted drug delivery. J. Magn. Magn. Mater. 293:633–638 (2005). doi:10.1016/j.jmmm.2005.01.087.
N. Butoescu, O. Jordan, A. Petri-Fink, H. Hofmann, and E. Doelker. Co-encapsulation of dexamethasone 21-acetate and SPIONs into biodegradable polymeric microparticles designed for intra-articular delivery. J. Microencapsul. 25:339–350 (2008). doi:10.1080/02652040801999551.
J. Dobson. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 13:283–287 (2006). doi:10.1038/sj.gt.3302720.
J. Dobson, S. H. Cartmell, A. Keramane, and A. J. El Haj. Principles and design of a novel magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning, and dynamic in vitro screening. IEEE Transactions on Nanobioscience 5:173–177 (2006). doi:10.1109/TNB.2006.880823.
S. Hughes, A. J. El Haj, and J. Dobson. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med. Eng. Phys. 27:754–762 (2005). doi:10.1016/j.medengphy.2005.04.006.
M. Zborowski, L. P. Sun, L. R. Moore, P. S. Williams, and J. J. Chalmers. Continuous cell separation using novel magnetic quadrupole flow sorter. J. Magn. Magn. Mater. 194:224–230 (1999). doi:10.1016/S0304-8853(98)00581-2.
H. Chen, A. D. Ebner, D. Bockenfeld, J. A. Ritter, M. D. Kaminski, X. Liu, D. Rempfer, and A. J. Rosengart. A comprehensive in vitro investigation of a portable magnetic separator device for human blood detoxification. Phys. Med. Biol. 52:6053–6072 (2007). doi:10.1088/0031-9155/52/19/023.
H. Choi, S. R. Choi, R. Zhou, H. F. Kung, and I.-W. Chen. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Acad. Radiol. 11:996–1004 (2004). doi:10.1016/j.acra.2004.04.018.
J.-F. Lutz, S. Stiller, A. Hoth, L. Kaufner, U. Pison, and R. Cartier. One-pot synthesis of PEGylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules 7:3132–3138 (2006). doi:10.1021/bm0607527.
D. E. Discher, V. Ortiz, G. Srinivas, M. L. Klein, Y. Kim, D. Christian, S. Cai, P. Photos, and F. Ahmed. Emerging applications of polymersomes in delivery: from molecular dynamics to shrinkage of tumors. Progr. Polymer. Sci. 32:838–857 (2007).
Y.-C. Chang, S.-W. Chang, and D.-H. Chen. Magnetic chitosan nanoparticles: studies on chitosan binding and adsorption of Co(II) ions. React. Funct. Polym. 66:335–341 (2006). doi:10.1016/j.reactfunctpolym.2005.08.006.
Induction Atmospheres, What is induction heating, http://www.inductionatmospheres.com/induction_heating.html (2008).
S. Dandamudi, and R. B. Campbell. The drug loading, cytotoxicty and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials 28:4673–4683 (2007). doi:10.1016/j.biomaterials.2007.07.024.
A. K. Gupta, and M. Gupta. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 26:1565–1573 (2005). doi:10.1016/j.biomaterials.2004.05.022.
H. Yin, H. P. Too, and G. M. Chow. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials 26:5818–5826 (2005). doi:10.1016/j.biomaterials.2005.02.036.
D.-H. Kim, K.-M. Kim, K.-N. Kim, I.-B. Shim, and Y.-K. Lee. In vitro and in vivo toxicity of CoFe2O4 nanoparticles for application to magnetic hyperthermia. NSTI Nanotech 2:748–751 (2007). doi:10.1038/nnano.2007.397.
C. B. Cullity. Introduction to magnetic materials. Addison-Wesley, New York, NY, 1972.
O. Bretcanu, E. Verné, M. Cöisson, P. Tiberto, and P. Allia. Magnetic properties of the ferrimagnetic glass–ceramics for hyperthermia. J. Magn. Magn. Mater. 305:529–533 (2006). doi:10.1016/j.jmmm.2006.02.264.
D.-H. Kim, D. E. Nikles, D. T. Johnson, and C. S. Brazel. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 320:2390–2396 (2008). doi:10.1016/j.jmmm.2008.05.023.
D.-H. Kim, Y. Thai, D. E. Nikles, C. S. Brazel. Optimized heat generation of MnFe2O4 nanoparticles for magnetic hyperthermia using multifunctional nanoparticles. IEEE Trans. Magn. in press, (2008).
A. A. Kuznetsov, V. G. Leontiev, V. A. Brukvin, G. N. Vorozhtsov, B. Y. Kogan, O. A. Shlyakhtin, A. M. Yunin, O. I. Tsybin, and O. A. Kuznetsov. Local radiofrequency-induced hyperthermia using CuNi nanoparticles with therapeutically suitable Curie temperature. J. Magn. Magn. Mat. SCAMC-06 311:197–203 (2007).
H. G. Bagaria, D. T. Johnson, C. Srivastava, G. B. Thompson, M. Shamsuzzoha, D. E. Nikles. Formation of FePt nanoparticles by organometallic synthesis. J. Appl. Phys. 101:paper 104313 (2007).
D. Weller, A. Moser, L. Folks, M. E. Best, W. Lee, M. F. Toney, M. Schwickert, J.-U. Thiele, and M. F. Doerner. High K-u materials approach to 100 Gbits/in(2). IEEE Trans. Magn. 36:10–15 (2000). doi:10.1109/20.824418.
R. M. Bozorth. Ferromagnetism; IEEE, New York, NY.
C. M. Burton, and A. E. Walker. The RF thermoseed—a thermally self-regulating implant for the production of brain lesions. IEEE Trans. Biomed. Eng. 18:104–109 (1971). doi:10.1109/TBME.1971.4502810.
T. Todaka, T. Kishino, and M. Enokizono. Low curie temperature material for induction heating self-temperature controlling system. J. Magn. Magn. Mater. 320:E702–E707 (2008). doi:10.1016/j.jmmm.2008.04.146.
H. Bagaria, and D. T. Johnson. Analytical and numerical solution to a concentric sphere model and optimization for magnetic fluid hyperthermia treatment. Int. J. Hyperthermia 21:57–75 (2005). doi:10.1080/02656730410001726956.
R. C. O’Handley. Modern magnetic materials: principles and applications. Wiley, New York, 1999.
C. Liu, and Z. J. Zhang. Size-dependent superparamagnetic properties of Mn spinel ferrite nanoparticles synthesized from reverse micelles. Chem. Mater. 13:2092–2096 (2001). doi:10.1021/cm0009470.
R. Evans, U. Nowak, F. Dorfbauer, T. Shrefl, O. Mryasov, R. W. Chantrell, and G. Grochola. The influence of shape and structure on the curie temperature of Fe and Co nanoparticles. J. Appl. Physi. 99:08G703 (2006).
G. Glockl, R. Hergt, M. Zeisberger, S. Dutz, S. Nagel, and W. Weitschies. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys., Condens. Matter 18:S2935–S2949 (2006). doi:10.1088/0953-8984/18/38/S27.
M. I. Shliomis, A. F. Pshenichnikov, K. I. Morozov, and Y. Shurubor. Magnetic properties of ferrocolloids. J. Magn. Magn. Mater. 85:40–46 (1990). doi:10.1016/0304-8853(90)90013-G.
A. Shitzer, and R. C. Eberhart (eds.), Heat Transfer in Medicine and Biology. Plenum, New York, 1985.
H. Arkin, L. X. Xu, and K. R. Holmes. Recent developments in modeling heat transfer in blood perfused tissues. IEEE Trans. Biomed. Eng. 41:97–107 (1994). doi:10.1109/10.284920.
C. S. Brazel, and N. A. Peppas. Pulsatile local delivery of thrombolytic and antithrombotic agents using poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. J. Controlled Release 39:57–64 (1996). doi:10.1016/0168-3659(95)00134-4.
A. Chilkoti, M. R. Dreher, D. E. Meyer, and D. Raucher. Targeted drug delivery by thermally responsive polymers. Adv. Drug deliv. Rev. 54:613–630 (2002). doi:10.1016/S0169-409X(02)00041-8.
T. Okano. Molecular design of temperature-responsive polymers as intelligent materials. Adv. Polym. Sci. 110:179–197 (1993). doi:10.1007/BFb0021133.
A. Gutowska, J. S. Bark, I. C. Kwon, Y. H. Bae, Y. Cha, and S. W. Kim. Squeezing hydrogels for controlled oral drug delivery. J. Control Release 48:141–148 (1997). doi:10.1016/S0168-3659(97)00041-2.
M. Bikram, A. M. Gobin, R. E. Whitmire, and J. L. West. Temperature-sensitive hydrogels with SiO2–Au nanoshells for controlled drug delivery. J. Control Release 123:219–227 (2007). doi:10.1016/j.jconrel.2007.08.013.
R. Yoshida, Y. Kaneko, K. Sakai, T. Okano, Y. Sakurai, Y. H. Bae, and S. W. Kim. Positive thermosensitive pulsatile drugs release using negative thermosensitive hydrogels. J. Control Release 32:97–102 (1994). doi:10.1016/0168-3659(94)90229-1.
S.-X. Cheng, J. T. Zhang, and R.-X. Zhuo. Macroporous poly(N-isopropylacrylamide) hydrogels with fast response rates and improved protein release properties. J. Biomed. Mater. Res. 67A:96–103 (2003). doi:10.1002/jbm.a.10062.
H. Feil, Y. H. Bae, J. Feijen, and S. W. Kim. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 26:2496–2500 (1993). doi:10.1021/ma00062a016.
I. Ankareddi, and C. S. Brazel. Synthesis and characterization of grafted thermosensitive hydrogels for heating activated controlled release. Int. J. Pharm. 336:241–247 (2007). doi:10.1016/j.ijpharm.2006.11.065.
J. Y. P. Chen, L.-M. Yang, L.-L. Shi, and H.-J. Luo. Synthesis and properties of poly(N-isopropylacrylamide-co-acrylamide) hydrogels. Macromol. Symp. 225:103–112 (2005). doi:10.1002/masy.200550709.
C. S. Brazel, and N. A. Peppas. Thermo- and chemo-mechanically responsive poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. Macromolecules 28:8016–8020 (1995). doi:10.1021/ma00128a007.
S. G. Hirsch, and R. J. Spontak. Temperature-dependent property development in hydrogels derived from hydroxypropylcellulose. Polymer 43:123–129 (2002). doi:10.1016/S0032-3861(01)00608-5.
M. D. Determan, J. P. Cox, S. Seifert, P. Thiyagarajan, and S. K. Mallapragada. Synthesis and characterization of temperature and pH-responsive pentablock copolymers. Polymer 46:6933–6946 (2005). doi:10.1016/j.polymer.2005.05.138.
K. H. Bae, S. H. Choi, S. Y. Park, Y. Lee, and T. G. Park. Thermosensitive pluronic micelles stabilized by shell cross-linking with gold nanoparticles. Langmuir 22:6380–6384 (2006). doi:10.1021/la0606704.
S. H. Cho, M. S. Jhon, S. H. Yuk, and H. B. Lee. Temperature-induced phase transition of poly(N,N-dimethylaminoethyl methacrylate-co-acrylamide). J. Polym. Sci. B: Polym. Phys. 35:595–598 (1997). doi:10.1002/(SICI)1099-0488(199703)35:4<595::AID-POLB7>3.0.CO;2-P.
D. S. Hart, and S. H. Gehrke. Thermally associating polypeptides designed for drug delivery produced by genetically engineered cells. J. Pharm. Sci. 96:484–516 (2007). doi:10.1002/jps.20755.
I. Ankareddi, M. L. Hampel, M. K. Sewell, D.-H. Kim, and C. S. Brazel. Temperature controlled grafted polymer network incorporated with magnetic nanoparticles to control drug release induced by an external magnetothermal trigger. NSTI Nanotech 2:431–434 (2007).
M. K. Sewell, K. D. Fugit, I. Ankareddi, C. Zhang, M. L. Hampel, D.-H. Kim, and C. S. Brazel. Magnetothermally-triggered drug delivery using hydrogels with imbedded cobalt ferrite, iron platinum or manganese ferrite nanoparticles. PMSE Preprints 98:694–695 (2008).
C.-Y. Lin, and K.-C. Ho. Synthesis of superparamagnetic magnetite nanoparticles for thermoresponsive drug delivery. NSTI Nanotech 2:405–408 (2007).
H. Pardoe, W. Chua-anusorn, T. G. St. Pierre, and J. Dobson. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater. 225:41–46 (2001). doi:10.1016/S0304-8853(00)01226-9.
G. D. Fu, S. C. Wuang, F. J. Xu, K. G. Neoh, and E. T. Kang. Functionalization via surface-initiated living radical polymerizations. Polymer Preprints 48(1):693–694 (2007).
B. Korth, M. Judd, B. Wong, and J. Pyun. Synthesis of core-shell magnetic nanoparticles using controlled/living radical polymerization. Polymer Preprints 46:437 (2005).
K.-H. Roh, and J. Lahann. Anisotropic encapsulation of superparamagnetic nanocrystals in polymeric biphasic nanocolloids. Polymer Preprints 48(1):209–210 (2007).
Y. Wu, J. Guo, W. Yang, C. Wang, and S. Fu. Preparation and characterization of chitosan-poly(acrylic acid) polymer magnetic microspheres. Polymer 47:5287–5294 (2006). doi:10.1016/j.polymer.2006.05.017.
H. G. Bagaria, E. T. Ada, M. Shamsuzzoha, D. E. Nikles, and D. T. Johnson. Understanding mercapto ligand exchange on the surface of FePt nanoparticles. Langmuir 22:7732–7737 (2006). doi:10.1021/la0601399.
L. L. Lao, and R. V. Ramanujan. Magnetic and hydrogel composite materials for hyperthermia applications. J. Mater. Sci., Mater. Med. 15:1061–1064 (2004). doi:10.1023/B:JMSM.0000046386.78633.e5.
R. A. Frimpong, and J. Z. Hilt. Synthesis and temperature response analysis of magnetic-hydrogel nanocomposites. J. Biomed. Mater. Res. 80A:1–6 (2007). doi:10.1002/jbm.a.30962.
N. S. Satarkar, and J. Z. Hilt. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomaterialia 4:11–16 (2008). doi:10.1016/j.actbio.2007.07.009.
N. S. Satarkar, and J. Z. Hilt. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control Release 130:246–251 (2008). doi:10.1016/j.jconrel.2008.06.008.
C. J. Meledandri, and D. F. Brougham. Optimisation of magnetoliposomes for biomedical multi-tasking; potential dual contrast agents and drug delivery vehicles. NSTI Nanotech 2:335–338 (2007).
R. Hergt, S. Dutz, R. Muller, and M. Zeisberger. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. J. Phys., Condens. Matter 18:S2919–S2934 (2006). doi:10.1088/0953-8984/18/38/S26.
J. Chatterjee, Y. Haik, and C. Jen Chen. Biodegradable magnetic gel: synthesis and characterization. Colloid Polym. Sci. 281:892–896 (2003). doi:10.1007/s00396-003-0916-z.
D.-H. Kim, S.-H. Lee, K.-N. Kim, K.-M. Kim, I.-B. Shim, and Y.-K. Lee. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J. Magn. Magn. Mater. 293:287–292 (2005). doi:10.1016/j.jmmm.2005.02.078.
M. Gonzales, and K. M. Krishnan. Synthesis of magnetoliposomes with monodisperse iron oxide nanocrystal cores for hyperthermia. J. Magn. Magn. Mater. 293:265–270 (2005). doi:10.1016/j.jmmm.2005.02.020.
B.-S. Kim, J.-M. Qiu, J.-P. Wang, and T. A. Taton. Magnetomicelles: Composite nanoparticles and cross-linked amphiphilic block copolymers. Nanoletters 5:1987–1991 (2005). doi:10.1021/nl0513939.
M. Nakayama, T. Okano, T. Miyazaki, F. Kohori, K. Sakai, and M. Yokoyama. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control Release 115:46–56 (2006). doi:10.1016/j.jconrel.2006.07.007.
H. Wei, X.-Z. Zhang, Y. Zhou, S.-X. Cheng, and R.-X. Zhuo. Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate). Biomaterials 27:2028–2034 (2006). doi:10.1016/j.biomaterials.2005.09.028.
Acknowledgments
Dr. Brazel acknowledges the J. William Fulbright Commission for supporting his sabbatical in the United Kingdom. The University of Alabama’s Alton Scott Memorial Fund supported research in the Magnetic Biomaterials research working group. Discussions with David Nikles, Hitesh Bagaria and Dong-Hyun Kim, collaborators at UA, have also been particularly helpful in developing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brazel, C.S. Magnetothermally-responsive Nanomaterials: Combining Magnetic Nanostructures and Thermally-Sensitive Polymers for Triggered Drug Release. Pharm Res 26, 644–656 (2009). https://doi.org/10.1007/s11095-008-9773-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9773-2