Abstract
Pure lead titanate (PbTiO3) nanoparticles were successfully synthesized by novel sol–gel method with the aid of Pb(CH3COO)2·3H2O, Ti(OC4H9)4 (TNBT), and glucose without adding external surfactant. Moreover, glucose plays role as reducing agent, and natural template in the synthesis PbTiO3 nanoparticles. The structural, morphological and optical properties of as obtained products were characterized by techniques such X-ray diffraction, energy dispersive X-ray microanalysis, scanning electron microscopy, and ultraviolet–visible spectroscopy. To evaluate the photocatalyst properties of nanocrystalline lead titanate, the photocatalytic degradation of methyl orange under ultraviolet light irradiation was carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Physical properties and potential applications of nanostructures and nanomaterial have been studied intensively [1–3]. This interest results from the special properties of materials at the nanoscale, such as a large surface-to-volume ratio and increased surface activity, as compared with that of the bulk material. The properties of bulk materials usually depend on the size of the primary particles. Thus, the control of particle size and morphology plays a crucial role in the manufacturing process [4–8]. PbTiO3 (PT) with tetragonal perovskite structure at room temperature is a ferroelectric compound with Curie temperature of 490 °C, high pyroelectric coefficient, high spontaneous polarization, and low dielectric constant [9]. PT is widely applied in electronics such as multilayer capacitor, resonators, and ultrasonic transducers due to a large pyroelectric coefficient and a relatively low permittivity. The crystalline structure of PT was determined by neutron diffraction and presented tetragonal symmetry [10–14]. Lead titanate when combined with other oxides can form a series of solid solution materials such as Pb(Zr, Ti)O3(PZT) and (Pb, La)(Zr, Ti)O3(PLZT), with potential applications in sensors, capacitors and non-volatile memories. Some authors have investigated PT materials modified by various additives [15, 16]. Lead titanate (PT) ceramics modified by rare earth elements and alkaline earth elements are extremely good for high frequency applications [17, 18]. The properties of these ceramics are strongly related to stoichiometry. The presences of intermediate phases can significantly damage the electrical properties [19]. The formation of lead deficient phases delay and even hinder the formation of perovskite [20]. Sirera and Calzada [21], upon obtaining pure and doped PT powders, observed the emergence of a pyrochlore phase which disappeared with the increase of the treatment temperature. Several methods have been used to obtain lead titanate powders, such as high-energy milling, sol–gel method, co-precipitation and hydrothermal synthesis, besides the traditional solid state reaction of mixed oxides [22–27]. In this report, for the first time, we had presented the preparation of PbTiO3 nanoparticles by novel sol–gel method at 700 °C in the presence of glucose without adding external surfactant, capping agent or template. A green approach for PbTiO3 nanoparticles synthesis by utilizing natural template permits the reaction to proceed usually in milder conditions. Although existing chemical approaches have effectively produced well-defined PbTiO3 nanoparticles, these processes are generally costly and include the employ of toxic chemicals. The photocatalytic degradation was investigated using methyl orange (MO) ultraviolet light irradiation [3, 28].
2 Experimental
2.1 Characterization
X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10°< 2θ < 80°. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Spectroscopy analysis (UV–Vis) was carried out using shimadzu UV–Vis scanning UV–Vis diffuse reflectance spectrometer. The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope.
2.2 Synthesis of PbTiO3 nanoparticles
All the chemicals used in this method were of analytical grade and used as received without any further purification. At first, 1.1 g of lead (II) acetate, 1.1 g of glucose, and 0.5 ml acetylacetonate were dissolved in 10 ml distilled ethanol and stirred for 15 min. Then, 1 ml of tetra-n-butyl orthotitanate was added drop wise into solution. Afterwards, the final mixed solution was kept stirring to form a gel at 90 °C. Finally, the obtained product was placed in a conventional furnace in air atmosphere for 150 min and calcine at 700 °C. After thermal treatment, the system was allowed to cool to room temperature naturally, and the obtained precipitate was collected.
2.3 Photocatalysis experiments
The methyl orange (MO) photodegradation was examined as a model reaction to evaluate the photocatalytic activities of the lead titanate nanoparticles. The photocatalytic experiments were performed under an irradiation ultraviolet light. The photocatalytic activity of nanocrystalline lead titanate obtained was studied by the degradation of methyl orange solution as a target pollutant. The photocatalytic degradation was performed with 150 ml solution of methyl orange (0.0005 g) containing 0.1 g of PbTiO3. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photoreactor in which the vessel was 15 cm away from the visible source of 400 W mercury lamps. The photocatalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–Vis spectrometer. The methyl orange (MO) degradation percentage was calculated as:
where A0 and A are the absorbance value of solution at A0 and A min, respectively.
3 Results and discussion
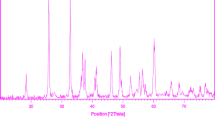
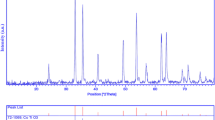
Figure 1 shows a typical XRD pattern (10° < 2θ < 80°) of PbTiO3 nanoparticles. Based on the Fig. 1, the diffraction peaks can be indexed to pure tetragonal phase of PbTiO3 (space group P4/mmm, JCPDS No. 75-0438). No other crystalline phases were detected. From XRD data, the crystallite diameter (Dc) of PbTiO3 nanoparticles was calculated to be 40 nm using the Scherer equation:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The morphology of the nanoparticles was investigated using SEM which demonstrates uniform nanoparticles with spherical shape homogenously distributed all over the sample, as it could be clearly observed in Fig. 2. The PbTiO3 nanoparticles with particle size of about 45–55 nm were observed. The EDS analysis measurement was used to investigate the chemical composition and purity of PbTiO3 nanoparticles. According to the Fig. 3, the product consists of Pb, Ti, and O elements. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any surfactant or impurity. The room temperature UV–Vis absorption spectra of PbTiO3 nanoparticles were also measured in the range of 300–600 nm. Figure 4 shows the diffuse reflection absorption spectra of the PbTiO3 nanoparticles calcined at 700 °C. The figure indicates that the PbTiO3 nanoparticles shows absorption maxima at 338 nm, the direct optical band gap estimated from the absorption spectra for the PbTiO3 nanoparticles is shown in Fig. 5. An optical band gap is obtained by plotting (αhν)2 versus hν where α is the absorption coefficient and hν is photon energy. Extrapolation of the linear portion at (αhν)2 = 0 gives the band gaps of 2.8 eV for perovskite PbTiO3 material. Photodegradation of methyl orange under UV light irradiation (Fig. 6a–c) was employed to evaluate the photocatalytic activity of the as-synthesized PbTiO3. No methyl orange was practically broken down after 60 min without using UV light irradiation or nanocrystalline PbTiO3. This observation indicated that the contribution of self-degradation was insignificant. The probable mechanism of the photocatalytic degradation of methyl orange can be summarized as follows:
Using photocatalytic calculations by Eq. (1), the methyl orange degradation was about 97 % after 60 min irradiation of UV light, and nanocrystalline PbTiO3 presented high photocatalytic activity (Fig. 6a). The spectrofluorimetric time-scans of methyl orange solution illuminated at 510 nm with nanocrystalline PbTiO3 are depicted in Fig. 6b. Figure 6b shows continuous removal of methyl orange on the PbTiO3 under UV light irradiation. It is generally accepted that the heterogeneous photocatalytic processes comprise various steps (diffusion, adsorption, reaction, and etc.), and suitable distribution of the pore in the catalyst surface is effective and useful to diffusion of reactants and products, which prefer the photocatalytic reaction. In this investigation, the enhanced photocatalytic activity can be related to appropriate distribution of the pore in the nanocrystalline PbTiO3 surface, high hydroxyl amount and high separation rate of charge carriers (Fig. 6c). Furthermore, this route is facile to operate and very suitable for industrial production of PbTiO3 nanoparticles. In addition, this process can be versatile to easily synthesize other titanium based perovskite oxides. The synthesis pathway of PbTiO3 nanoparticles is shown in Scheme 1.
a Photocatalytic methyl orange degradation of PbTiO3 nanoparticles under ultraviolet light, b fluorescence spectral time scan of methyl orange illuminated at 510 nm with PbTiO3 nanoparticles and c reaction mechanism of methyl orange photodegradation over PbTiO3 nanoparticles under ultraviolet light irradiation
4 Conclusions
In this work, lead titanate nanoparticles were successfully synthesized by a novel sol–gel method at 700 °C for 150 min. The stages of the formation of PbTiO3, as well as the characterization of the resulting compounds were done using X-ray diffraction and energy dispersive X-ray spectroscopy. The products were analyzed by scanning electron microscopy (SEM), and ultraviolet–visible (UV–Vis) spectroscopy to be round, about 45–55 nm in size and Eg = 2.8 eV. When as-prepared nanocrystalline lead titanate oxide was utilized as photocatalyst, the percentage of methyl orange degradation was about 97 % after 60 min irradiation of UV light. This result suggests that as-obtained nanocrystalline lead titanate as favorable material has high potential to be used for photocatalytic applications under UV light.
References
M. Najafi, H. Haratizadeh, M. Ghezellou, J. Nanostruct. 5, 129 (2015)
S. Khademolhoseini, M. Zakeri, S. Rahnamaeiyan, M. Nasiri, R. Talebi, J. Mater. Sci. Mater. Electron. 26, 7303 (2015)
F.S. Ghoreishi, V. Ahmadi, M. Samadpourc, J. Nanostruct. 3, 453 (2013)
S. Moshtaghi, D. Ghanbari, M. Salavati-Niasari, J. Nanostruct. 5, 169 (2015)
Z. Khayat Sarkar, F. Khayat Sarkar, Int. J. Nanosci. Nanotechnol. 7, 197 (2011)
A. Ghasemi, A.M. Davarpanah, M. Ghadiri, Int. J. Nanosci. Nanotechnol. 8, 207 (2012)
A. Rahdar, M. Aliahmad, Y. Azizi, J. Nanostruct. 5, 145 (2015)
J. Safaei-Ghomi, S. Zahedi, M. Javid, M.A. Ghasemzadeh, J. Nanostruct. 5, 153 (2015)
F.C.D. Lemos, E. Longo, E.R. Leite, D.M.A. Melo, A.O. Silva, J. Solid State Chem. 177, 1542 (2004)
H. Fujisawa, M. Okaniwa, H. Nonomura, M. Shimizu, H. Niu, J. Eur. Ceram. Soc. 24, 1641 (2004)
H. Nonomura, M. Nagata, H. Fujisawa, M. Shimizu, H. Niu, K. Honda, Appl. Phys. Lett. 86, 163106 (2005)
S. Clemens, T. Schneller, R. Waser, A. Rudiger, F. Peter, S. Kronholz, T. Schmitz, S. Tiedke, Appl. Phys. Lett. 87, 142904 (2005)
P.S. Pizani, E.R. Leite, F.M. Pontes, E.C. Paris, J.H. Rangel, E.J.H. Lee, E. Longo, P. Delega, J.A. Varela, Appl. Phys. Lett. 77, 824 (2000)
G. Rosenman, D. Shur, Ya.E. Krasik, A. Dunaevsky, J. Appl. Phys. 88, 6109 (2000)
G. Shirane, R. Pepinsky, Acta Crystallogr. 9, 131 (1956)
S. Tang, Y. Deng, S.Z. Zhan, J. Thermal Anal. Calorimetry 104, 653 (2011)
F.M. Pontes, J.H.G. Rangel, E.R. Leite, E. Longo, J.A. Varela, E.B. Araújo, J.A. Eiras, J. Mater. Sci. 36, 3565 (2001)
R. Tickoo, R.P. Tandon, N.C. Mehra, P.N. Kotru, Sci. Eng. B 94, 1 (2002)
J. Tartaj, C. Moure, P. Durán, Ceram. Int. 27, 741 (2001)
J. Tartaj, J.F. Fernández, M.E. Villafuerte-Castrejón, Mater. Res. Bull. 36, 479 (2001)
R. Sirera, M.L. Calzada, Mater. Res. Bull. 30, 11 (1995)
D. Bersani, P.P. Lottici, A. Montenero, S. Pigoni, G. Gnappi, J. Non-Cryst. Solids 490, 192 (1995)
M.L. Calzada, L. DelOlmo, J. Non-Cryst. Solids 121, 413 (1990)
K. Ishikawa, N. Okada, K. Takada, T. Nomura, T. Hagino, J. Appl. Phys. 33, 3495 (1994)
A. Safari, Y.H. Lee, A. Halliyal, R.E. Newnham, Am. Ceram. Soc. Bull. 66, 668 (1987)
C.E. Millar, L. Pedersen, W.W. Wolny, Ferroelectrics 133, 271 (1992)
K. Hirakata, W.E. Rhine, M.J. Cima, Am. Ceram. Soc. 79, 1002 (1996)
M. Riazian, J. Nanostruct. 4, 433 (2014)
Acknowledgments
Authors are grateful to council of University of Borujerd for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Shabanalizadeh, S., Abedini, A., Alborzi, A. et al. Green synthesis and characterization of lead titanate nanoparticles and its photocatalyst application. J Mater Sci: Mater Electron 27, 2589–2593 (2016). https://doi.org/10.1007/s10854-015-4062-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-4062-4