Abstract
Nanoparticles cadmium molybdate was prepared by ultrasonic method using cadmium(II) nitrate hexahydrate and ammonium heptamolybdate tetrahydrate were used as precursor materials. To the best of authors’ knowledge, it is the first time that cadmium molybdate was synthesized by ultrasonic method. The as synthesized nanostructures were characterized by X-ray diffraction, scanning electron microscopy, X-ray energy dispersive spectroscopy, and ultraviolet–visible spectroscopy. To evaluate the catalytic properties of nanocrystalline cadmium molybdate, the photocatalytic degradation of 2-naphthol under visible light irradiation was carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Throughout last decades an important activity in the use of semiconductor oxides as photocatalysts has been observed. Since discovery of photocatalytic activity of TiO2 by UV irradiation, many works have described novel methods to synthesize photocatalysts with special textural properties in order to increase its photocatalytic activity in redox reactions. Although TiO2 anatase form isconsidered as the photocatalyst for excellence, different semiconductor oxides such as WO3, ZnO, and CdS have been proposed for photocatalytic conversion [1–3]. Recently some binary and ternary oxides with scheelite and related structures have shown photocatalytic activity under UV irradiation [4,5]. In particular, PbMoO4 with scheelite structure have gained interest as photocatalyst for the splitting of water into hydrogen and oxygen [6,7] and for the degradation of complex organic compounds [8,9]. For this reason, different soft chemistry methods have been developed to obtain PbMoO4 with specific textural properties and morphology such as co-precipitation [10], hydrothermal [11], microwave irradiation [12], and sonochemical [10–15]. We tried to extend our knowledge to apply the sonochemical technique to synthesize CdMoO4 photocatalyst. The physical phenomenon responsible for the ultrasonic process is acoustic cavitation. The ultrasonic cavitation generates a very strong stirring environment. Therefore, application of ultrasound is expanding in material science for dispersion, emulsifying, crushing, impregnation, surface treatment, synthesis and activation of nanoparticles. During the process, the rapid ultrasonic vibrations and cavitation effects cause to increase collision between the molecules which in turn enhance the chemical reactivity. The photocatalytic degradation was investigated using 2-naphthol under visible light irradiation (λ > 400 nm). The resulting degradation rates of the 2-naphthol were measured to be as high as 90 % in 360 min. In the current study, the synthesis of CdMoO4 nanoparticles is reported [16–19]. This production is done by ultrasonic solution of cadmium(II) nitrate hexahydrate Cd(NO3)3·6H2O and ammonium heptamolybdate tetrahydrate (NH4)6Mo7O24.4H2O. Besides, the effect of reaction parameters ultrasonic power on the morphology and particle size of CdMoO4 nanoparticles was investigated.
2 Experimental
2.1 Materials and characterization
All chemical reagents in this experiment were of analytical grade and used without further purification. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Spectroscopy analysis (UV–Vis) was carried out using shimadzu UV–Vis scanning UV–Vis diffuse reflectance spectrometer. Ultrasonic irradiation was accomplished with a high-intensity ultrasonic bath. The EDS analysis with 20 kV accelerated voltage was done.
2.2 Synthesis of CdMoO4 nanoparticles
CdMoO4, was synthesized ultrasonic method. Appropriate amounts of Cd(NO3)3·6H2O and ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24.4H2O) were used as the Zn and Mo sources. In a typical synthesis (0.116 g) ammonium heptamolybdate tetrahydrate and (0.202 g) of Cd(NO3)3·6H2O were dissolved in 30 ml distilled water and methanol, respectively. Then Cd(NO3)3·6H2O was heated at 60 °C for 10 min and finally the ammonium heptamolybdate tetrahydrate solution was added dropwise to this solution with pH 5–6 and solution was heated at 45 °C and finally treated with ultrasonic irradiation with different power. The white product was filtered, washed with distilled water and ethanol several times and dried in vacuum in less than 90 °C. Finally, the product was dried in vacuum at 90 °C for 2 h. Reaction conditions are listed in Table 1.
2.3 Photocatalytic experimental
The 2-naphthol photodegradation was examined as a model reaction to evaluate the photocatalytic activities of the CdMoO4 nanoparticles. The photocatalytic experiments were performed under an irradiation wavelength of λ > 400 nm. The photocatalytic activity of nanocrystalline cadmium molybdate obtained from sample no. 4 was studied by the degradation of 2-naphthol solution as a target pollutant. The photocatalytic degradation was performed with 0.001 g of 2-naphthol solution containing 0.1 g of CdMoO4. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photoreactor in which the vessel was 15 cm away from the visible source of 400 W Xeno lamp. The photocatalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–vis spectrometer. The 2-naphthol degradation percentage was calculated as:
where A0 and A are the absorbance value of solution at A0 and A min, respectively.
3 Results and discussion
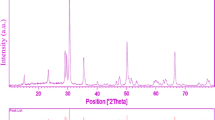
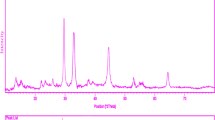
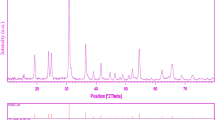
Figure 1 shows a typical XRD pattern (10° < 2θ < 80°) of CdMoO4 nanoparticles (sample 1). Based on the Fig. 1, the diffraction peaks can be indexed to pure tetragonal phase of CdMoO4 (space group I41/a, JCPDS No. 85-0888). No other crystalline phases were detected. From XRD data, the crystallite diameter (Dc) of CdMoO4 nanoparticles obtained from sample 1 was calculated to be 45 nm using the Scherer equation:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the socalled shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The effect of different dosage of sucrose and ultrasonic power on the morphology and particle size of CdMoO4 nanoparticles was investigated. Figure 2a–c shows the SEM images of CdMoO4 nanoparticles sample No 1–3, respectively. Base on the Fig. 2a, the product is mainly composed of the agglomeration nanoparticles. When ultrasonic power increased from 50 to 70 W, morphology of CdMoO4 is consist of agglomeration nanoparticle, as shown in Fig. 2b, respectively. Furthermore, increase in ultrasonic power result in increase size of product. Schematic diagram of formation of nanostructures is illustrated in Scheme 1.
The EDS analysis measurement was used to investigate the chemical composition and purity of CdMoO4 nanoparticles (sample 2, Fig. 3). According to the Fig. 4, the product consists of Cd, Mo, and O elements. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any surfactant or impurity. To investigate the optical properties of the nanocrystalline CdMoO −4 , UV–vis spectra was recorded. Figure 4 shows the UV–vis diffuse reflectance spectrum of sample no 2. Using Tauc’s formula, the band gap can be obtained from the absorption data. The energy gap (Eg) of the nanocrystalline CdMoO4 has been estimated by extrapolating the linear portion of the plot of (αhν)2 against hν to the energy axis. The Eg value of the nanocrystalline CdMoO4 calculated to be 2.85 eV. Photodegradation of methyl orange under UV light irradiation (Fig. 5a–c) was employed to evaluate the photocatalytic activity of the as-synthesized CdMoO4 (sample no. 2). No methyl orange was practically broken down after 6 h without using visible light irradiation or nanocrystalline CdMoO4. This observation indicated that the contribution of self-degradation was insignificant. The probable mechanism of the photocatalytic degradation of 2-naphthol can be summarized as follows:
Using photocatalytic calculations by Eq. (1), the 2-naphthol degradation was about 90 % after 6 h irradiation of visible light, and nanocrystalline CdMoO4 presented high photocatalytic activity (Fig. 5a). The spectrofluorimetric time-scans of 2-naphthol solution illuminated at 365 nm with nanocrystalline CdMoO4 are depicted in Fig. 5b. Figure 5b shows continuous removal of 2-naphthol on the CdMoO4 under visible light irradiation. It is generally accepted that the heterogeneous photocatalytic processes comprise various steps (diffusion, adsorption, reaction, and etc.), and suitable distribution of the pore in the catalyst surface is effective and useful to diffusion of reactants and products, which prefer the photocatalytic reaction. In this investigation, the enhanced photocatalytic activity can be related to appropriate distribution of the pore in the sponge-like nanocrystalline CdMoO4 surface, high hydroxyl amount and high separation rate of charge carriers (Fig. 5c).
Photocatalytic 2-naphthol degradation of CdMoO4 nanoparticles obtained from sample no. 2 under visible light (a), fluorescence spectral time scan of 2-naphthol illuminated at 365 nm with CdMoO4 nanoparticles (b), and reaction mechanism of 2-naphthol photodegradation over CdMoO4 under visible light irradiation
4 Conclusions
CdMoO4 nanoparticles have been successfully synthesized through an ultrasonic method. We investigated the effect of dosage of ultrasonic power on the morphology and particle size of CdMoO4 nanoparticles. SEM results indicated that the size and morphology of the products could were found to be greatly influenced by the aforementioned parameters. CdMoO4 nanoparticles were characterized by XRD, UV–Vis, EDS, and SEM. When as prepared nanocrystalline cadmium molybdate was utilized as photocatalyst, the percentage of 2-naphthol degradation was about 90 % after 360 min irradiation of visible light.
References
S. Gupta, M. Tripathi, High Energy Chem. 46, 1 (2012)
X.S. Yang, H. Kim, Appl. Mech. Mater. 110, 3781 (2012)
D. Sanchez-Martinez, A. Martinez-de la Cruz, E. Lopez-Cuellar, Solid State Sci. 12, 60 (2010)
Z. Shan, Y.W.H. Ding, F. Huang, J. Mol. Catal. A Chem. 302, 54 (2009)
H. Kato, N. Matsudo, A. Kudo, Chem. Lett. 33, 1216 (2004)
Y. Shimodaira, H. Kato, H. Kobayashi, A. Kudo, Chem. Soc. Jpn. 80, 885 (2007)
A. Kudo, M. Steinberg, A.J. Bard, A. Campion, M.A. Fox, T.E. Mallouk, S.E. Webber, J.M. White, Catal. Lett. 5, 61 (1990)
J. Bi, L. Wu, Y. Zhang, Z. Li, J. Li, X. Fu, Appl. Catal. B Environ. 91, 135 (2009)
G.J. Xing, R. Liu, C. Zhao, Y.L. Li, Y. Wang, G.M. Wu, Ceram. Int. 37, 2951 (2011)
W.S. Wang, L. Zhen, C.Y. Xu, W.Z. Shao, Z.L. Chen, J. Alloys Compd. 529, 17 (2012)
D. Li, Y.F. Zhu, Cryst. Eng. Commun. 14, 1128 (2012)
L.R.N. Hou, L. Lian, L. Zhang, J.Y. Li, Mater. Lett. 109, 306 (2013).
H.W. Liu, L. Tan, Ionics. 16, 57 (2010)
D. Zhu, K. Ki, X. Chen, T. Ying, J. Optoelectron. Adv. Mater. 5, 403 (2011)
Q.L. Dai, G.G. Zhang, P. Liu, J. Wang, J.K. Tang, Inorg. Chem. 51, 9232 (2012)
J. Lin, Z. Zeng, Q. Wang, Inorg. Chim. Acta 408, 59 (2013)
D.B. Hernandez-Uresti, l.C.A. Martinez-de, L.M. Torres-Martinez, Res. Chem. Intermed. 38, 817 (2012)
T.H. Kim, V. Rodríguez-González, G. Gyawali, S.H. Cho, T. Sekino, S.W. Lee, Catal. Today 9, 14 (2012)
A. Sun, D. Wang, Z. Wu, X. Zan, J. Alloys Compd. 505, 588 (2010)
Acknowledgments
Authors are grateful to council of University of South Tehran for providing financial support to undertake this work.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khademolhoseini, S., Zakeri, M., Rahnamaeiyan, S. et al. A simple sonochemical approach for synthesis of cadmium molybdate nanoparticles and investigation of its photocatalyst application. J Mater Sci: Mater Electron 26, 7303–7308 (2015). https://doi.org/10.1007/s10854-015-3358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3358-8