Abstract
In this study, lithium-doped p-type ZnO thin film was achieved by applying optimized parameters such as surfactant concentration and annealing temperature in sol–gel method. The stable and reproducible Li-doped ZnO film was characterized by Hall-effect measurement, which revealed high hole concentration of 1019 cm−3 order. The conductivity of the ZnO film doped with optimized Li ratio (15 at%) changed from n-type to p-type. To study the effect of lithium doping, we characterized the optical, electrical and structural properties in comparison with undoped ZnO thin film fabricated by the same method. The XRD results show Li-doping could strongly improve the crystal quality and c-axis orientation of low temperature annealed ZnO thin film. The SEM observations confirm the XRD results based on size reduction. The UV–visible spectra of films show high transparency over 95 % in visible region. The PL-spectra obviously revealed that Li dopants substitute in Zn vacancies. And most deep level defects could disappear with Li doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide is a compound semiconductor with a wide direct band gap (3.37 eV). It has hexagonal wurtzite structure with lattice parameters: a = 3.249 Å and c = 5.207 Å. ZnO is an intrinsic n-type semiconductor due to its intrinsic defects, such as Zn interstitials (Zni) and O vacancies (VO). According to the first principles total energy calculations, these defects have low formation energies and therefore existence of these defects lead to stability of n-ZnO. Zinc oxide also has large exciton binding energy of 60 meV at room temperature. These properties make it a promising material for short-wavelength optoelectronic devices. Also it is a popular semiconductor due to its piezoelectricity properties, non-toxicity and low cost [1–4].
To achieve the assorted application of zinc oxide in optoelectronic devices such as photodiodes, photo detectors, LEDs, transistors and etc., it is essential to realize both high quality n- and p-types ZnO for fabricated p–n homojunctions because of better lattice matching. Hence, for widespread use of ZnO, it is indispensable to achieve the high quality, stable and reproducible p-ZnO. However the achievement of p-type ZnO is difficult because of low solubility of acceptor dopants, self-compensation of intrinsic donor defects (Zni, VO) and formation of deep level acceptors [5]. Therefore, finding a method for fabrication of high quality p-ZnO in promoting zinc oxide based homojunctions is very determining. The group 1 and 5 elements are potentially p-type ZnO dopant candidates. From group 1 elements, Li has the shallowest ionization energy and from group 5, N substituting for O appears promising. But generally dopants at cation sites in compound semiconductors produce shallower acceptor levels than dopants at anion sites [6]. In this regard the use of cations of group 1 instead of anions of group 5 is more appropriate. It seems that lithium is the best candidate for monodoping in fabricating p-type ZnO. Hence, lithium was selected among all the elements of group 1 in this work. The reported p-ZnO films are mostly grown by pulsed laser deposition (PLD) [7], RF Magnetron Sputtering [8], Metal Organic Chemical Vapor Deposition (MOCVD) [9], that all of them are complicated and expensive methods. Compared with these techniques, Sol–gel method is a low cost, simple and easily available technique to realize p-type ZnO [10]. Also sol–gel method has more advantages such as ability to uniform distribution of dopant materials in the host lattice and large area coating capability. But in the most reported p-ZnO thin films fabricated by sol–gel method (specially monodoped p-ZnO films) high temperature annealing was required, which restricted the choice of substrates to high melting temperature options such as quartz and Si substrates. In this study we achieved suitable annealing process for p-ZnO film by controlling of surfactant concentration in sol synthesis, in relative low temperature without any gas flue treatment. Different Li dopant ratio ZnO thin films were fabricated and the 15 at% lithium doped film showed the lowest resistivity among them, as confirmed by Wang et al. [11]. Therefore high hole concentration p-type ZnO thin film with optimized ratio of lithium was fabricated by sol–gel method and deposited on glass by simple spin coating technique and annealed at a relative low temperature. It seems that this concentration of Li dopant is a promising value in Li-mono doping for p-ZnO fabrication. That can be used in p–n homojunctions as a based on electro optical devices such as photodiodes, solar cells and etc. To comprehending the change of intrinsic n-type conductivity of ZnO to p-type, the effect of lithium doping on the optical, electrical, structural and morphological properties comparing with undoped ZnO thin film fabricated in the same way have been investigated by PL spectroscopy, UV–visible spectroscopy, Hall effect measurement, XRD and SEM analysis.
2 Experimental details
2.1 Undoped and Li-doped thin films preparation
Undoped zinc oxide and lithium doped zinc oxide sols were synthesized by sol–gel method and their thin films were deposited on glass substrates using spin coating technique. Zinc acetate dihydrate was used as the precursor and lithium nitrate was used as source material for lithium dopant. For preparation of two sols (ZnO and Li-doped ZnO), the precursor was dissolved in 2-methoxyethanol and stabilized by monoethanolamine at ambient temperature. The concentration of zinc acetate was 0.5 mol/L and the molar ratio of monoethanolamine to zinc acetate was kept at 4:5. The solutions were stirred for 1 h to achieve clear and homogeneous undoped ZnO sol. To prepare Li doped p-ZnO sol, 15 at% lithium was added to the similar pure ZnO homogeneous sol and stirred to get clear. The glass substrates were rinsed with Piranha solution [a strong acid that is a 3:1 mixture of sulfuric acid (H2SO4) with hydrogen peroxide (H2O2)] and cleaned with DI water and ethanol sequentially, then dried and preheated at 400 °C for 10 min before deposition. The sols were spin coated on the cleaned glass substrates (3000 rpm; 15 s) and dried at 180 °C for 30 min to evaporate the solvent. To obtain an adequate thickness (about 400 nm), this process was repeated several times. The thin films were annealed at 450 °C for 1.5 h to remove organic compounds and stabilizing the structure. Figure 1 shows the flow chart of preparation of ZnO thin films.
2.2 Characterization
The optical properties of lithium doped and undoped ZnO thin films were investigated by UV–visible spectra transmission (Perkin-Elmer) and PL spectra (Perkin-Elmer LS-5 Fluorescence Spectrometer) with an excitation wavelength of 325 nm at room temperature. The electrical properties of thin films were measured by Hall effect measurement system. Structural and morphological properties of Li-doped and undoped ZnO thin films were examined using X-ray diffraction (XRD-PANalytical PW3050/60) and scanning electron microscopy (SEM-Mira Tescan).
3 Results and discussion
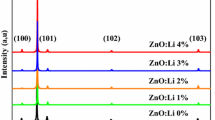
The X-ray diffraction pattern of thin films (Fig. 2) confirmed the formation of hexagonal wurtzite structure of ZnO lattice in both samples according to JCPDS Card no. 036-1451. Amorphous bumps at around 20°–30° in the diffraction spectra are attributed to glass substrates. High intensity of the strongest peak of lithium doped ZnO film (002) at 2 = 34.4924° with full-width half maximum (FWHM) of 0.2047° showed good crystal quality and strong -axis orientation. But in pure ZnO XRD pattern, the intensity of (101) peak is only a little more than others. It can be seen that the lithium doping, could greatly improve the crystalline properties of ZnO lattice, although preparation of films were at a relatively low temperature. The intensity of main peaks of X-ray diffraction patterns are comparable in Fig. 3. The lattice parameters a and c were calculated for both of the films and tabulated (Table 1). These parameters are calculated from the Bragg relation [12]:
where n is the order of diffraction, λ is the wavelength of X-ray and d is the distance between the planes. Miller indices (h, k and l) and dhkl are related to lattice parameters through the following equation [13]:
The calculated values of lattice parameters of films for the preferred directions of crystals growth are also presented in Table 1.
The crystallite size of the ZnO nanoparticles can also be estimated by the Scherer formula [14]:
where D is the crystallite size, λ is the wavelength of the X-radiation, θ is the diffraction angle and β is the FWHM of the observed peak. The tension and stress of the lattice can be caused by many factors such as impurities, defects, expansion coefficient mismatch between the film and substrate and etc. This parameter can be obtained from the following equation [15]:
where c0 is the c for bulk ZnO and cij constants are elastic stiffness of ZnO (c11 = 209.76 Gpa, c12 = 121.1 Gpa, c33 = 210.9 Gpa and c13 = 105.1 Gpa). By replacing them in the above equation the following formula is obtained [15]:
Positive σ indicate the biaxial stress is tensile and if σ is negative, the biaxial stress is compressive. The obtained values of σ are also presented in Table 1. The minus sign indicates that the size of the lattice reduced in both films. The lithium doped zinc oxide nanocrystals show more compressive behavior, which ascribed for replacement of Li1+ (ionic radius 0.68 Å) instead of Zn2+ (ionic radius 0.74 Å) in the ZnO lattice [14]. According to the XRD results, it seems that although the pure ZnO thin film annealed at low temperature has not excellent crystallinity but by lithium doping, addition to control the electrical properties (expressed as follows), can also greatly improve the crystalline properties of ZnO thin films which could leads to fabricating of high quality crystals of ZnO in low temperature on more optional substrates such as easily available and low cost glass substrates.
Figure 4 shows SEM images of the surface of ZnO and ZnO: Li thin films. The SEM images clearly revealed that the size of ZnO nanoparticles reduced and the quality of thin film get better with lithium doping. The observations completely confirm the XRD results.
The electrical properties of p-ZnO thin films were investigated by Hall-effect measurement. The obtained Li-doped thin film shows p-type conductivity, with electrical resistivity of 11.62 Ωcm, Hall mobility of 0.01 cm2/Vs and hole concentration of 5.08 × 1019 cm−3. Besides Hall-effect measurements, the p-type conductivity of lithium doped ZnO film was also confirmed by the Seebeck-effect measurement. The high hole concentration of the p-ZnO film obtained in this work are better than those of the Li-doped ZnO films prepared by more expensive techniques such as dc reactive magnetron sputtering [16], pulsed laser deposition [17] and comparable to the p-type ZnO:Li films grown by spray pyrolysis [18].
UV–visible absorption spectra are shown in Fig. 5. It can be seen that the absorption peak of undoped ZnO thin film is sharper than Li-doped thin film. This phenomenon is attributed to decreasing UV absorption via adding Li in ZnO lattice [19]. The optical transmission spectra of the undoped and lithium doped ZnO films which are displayed in Fig. 6 indicate that both films have optical transparency over 95 % in the visible region. A little increase in the transition spectra in visible region was observed with lithium doping. To calculate optical band gap Eg in direct band gap semiconductors, the following equation is used [20]:
where \(h\nu\) is photon energy and α is absorption coefficient and can be calculated from thefollowing [21]:
where T is the transmittance of film and t is the film thickness. The band gap was evaluated by plotting \((\alpha h\nu )^{2}\) versus \(h\nu\) and then extrapolating the straight linear part to the curve and intercept with energy axis [22], as shown in Fig. 7. The band gaps of the undoped and Li-doped ZnO films are determined as 3.270 and 3.285 eV, respectively, that shows the band gap of the ZnO films increases with Li-doping. This result can be attributed to particle size reduction due to lithium doping in ZnO lattice [23]. This is in complete agreement with the XRD and SEM results. In addition to grain size reduction, the increase of carrier density cause broadening of the energy band and enhancement of optical band gap [24]. Linear refractive index can be achieved through the following equation [25]:
where α = 4.048 and \(\beta\) =−0.62 eV−1. The linear refractive indexes of undoped and lithium doped ZnO films were calculated and found to be 2.0206 and 2.0113 respectively. Also the optical dielectric constant \(\varepsilon_{\infty } = n^{2}\) can be calculated [25]. The dielectric constants are determined as 4.0828 and 4.0453 for ZnO and ZnO:Li thin films respectively.
The photoluminescence spectra of pure and Li-doped ZnO thin films with an excitation wavelength of 325 nm are displayed in Fig. 8. It was shown that there was a strong emission near UV and violet light region in ~395 nm (~3.13 eV) in both samples. The main peaks are attributed to radiative electron transition from conduction band to intrinsic defects such as Zn vacancy (VZn) [26]. As can be seen, the intensity of this peak decreased with lithium doping, that can be attributed to filling some of Zn vacancies with Li1+ substitutional, which leads to becoming intrinsic n-type ZnO to p-type. Also there are two weak peaks in 465 nm (2.66 eV) and in 545 nm (2.27 eV) in both of samples that ascribed to oxygen vacancy (VO) and interstitial oxygen (Oi) respectively [26, 27]. There is a sharp peak in 582 nm (2.13 eV) in undoped ZnO thin film that is faded away by Li-doping. This peak is close to the reported peak by Willander et al. [27] (2.16 eV) which is attributed to the clusters of VOZni. As can be seen, these native point defects are disappeared with Li doping. With regard to the XRD results, it seems the loss of these defects improves the crystalline properties of ZnO films and eliminates lattice disorder along c-axis. Our hypothesis is that lithium compensation occurred by Zni leads to eliminate the VOZni cluster defects. Thus, some of substitutional Li come out of their places and replaced by Zni (which are separated from VOZni clusters), and therefore, one of the important defects (VOZni clusters) in ZnO lattice is completely disappear. Because of such occurrences (compensation) the Li concentration of the doping is considered to be excessive in this work. This may be causes the formation of some interstitial Li but avoids to prevailing of self-compensation and leads to more stability of electrical properties of p-type zinc oxide films which obtained in this research. So that the p-type conductivity and electrical properties of the fabricated film still remain stable over several months (11 months).
4 Conclusion
In conclusion, p-type ZnO thin film with satisfying structural, optical and electrical properties is obtained due to optimized ratio of 15 at% Li-doping by low cost sol–gel method and spin coating deposition technique. It was compared with undoped ZnO thin film fabricated by the same method, to investigate the effect of Li-dopant (in a suitable and demonstrated concentration) on undoped ZnO optical, electrical and structural properties in related to each other. The XRD pattern of the produced thin films indicate Li-doping can strongly improve the crystallinity and c-axis orientation at low annealing temperature ZnO thin films. The SEM images reveal the size of nanocrystals of ZnO decrease with Li-doping and layer get more homogeneous. A comparative photoluminescence study between undoped and 15 at% Li-doped ZnO thin films show that by Li-doping some of the deep level defects substantially decrease. The PL and XRD results show the direct effect of disappearance of complex intrinsic defects by 15 at% Li-doping. The UV–visible spectra investigations show the band gap of ZnO increase with Li-doping that is in confirmation with XRD and SEM results based on the nanoparticles size reduction with Li addition. The Hall-effect measurement revealed high hole concentration of 5.08 × 1019 cm−3. One of the most important advantages of the p-ZnO thin film fabricated by the presented method in this paper is reproducibility and stability, due to surfactant concentration control and annealing temperature reduction.
References
J.C. Fan, K.M. Sreekanth, Z. Xie, S.L. Chang, K.V. Rao, Prog. Mater Sci. 58, 874 (2013)
Q.X. Xia, K.S. Hui, K.N. Hui, D.H. Hwang, J. Singh, Y.R. Cho, S.K. Lee, W. Zhou, Z.P. Wan, C.-N. Ha, C.-N.H. Thuc, Y.G. Son, Mater. Lett. 78, 180 (2012)
J.-W. Kang, Y.-S. Choi, B.-H. Kim, N.-Y. Kim, C.W. Tu, S.-J. Park, Scr. Mater. 39, 84–85 (2014)
R. Swapna, M.C.S. Kumar, Ceram. Int. 39, 1799 (2013)
T.-H. Yang, J.-M. Wu, Acta Mater. 60, 3310 (2012)
C.H. Park, S.B. Zhang, S.-H. Wei, Phys. Rev. B 66, 073202 (2002)
B. Xiao, Z. Ye, Y. Zhang, Y. Zeng, L. Zhu, B. Zhao, Appl. Surf. Sci. 253, 895 (2006)
K.-C . Chiu, Y.-W. Kao, J.-H. Jean, J. Am. Ceram. Soc. 93, 1860 (2010)
W. Liu, S.L. Gu, J.D. Ye, S.M. Zhu, Y.X. Wu, Z.P. Shan, R. Zhang, Y.D. Zheng, S.F. Choy, G.Q. Lo, X.W. Sun, J. Cryst. Growth 310, 3448 (2008)
H. Nian, S.H. Hahn, K.-K. Koo, E.W. Shin, E.J. Kim, Mater. Lett. 63, 2246 (2009)
D. Wang, J. Zhou, G. Liu, J. Alloys Compd. 481, 802 (2009)
H. Sarma, K.C. Sarma, IJSRP4, 1 (2014)
A. Jalar, M.A.A. Hamid, L. Karkeng, R. Shamsudin, Int. J. Electrochem. Sci. 8, 6767 (2013)
M. Ardyanian, N. Sedigh, Bull. Mater. Sci. 37, 1309 (2014)
C. Li, X.C. Li, P.X. Yan, E.M. Chong, Y. Liu, G.H. Yue, X.Y. Fan, Appl. Surf. Sci. 253, 4000 (2007)
Y.J. Zeng, Z.Z. Ye, W.Z. Xu, D.Y. Li, J.G. Lu, L.P. Zhu, B.H. Zhao, Appl. Phys. Lett. 88, 062107 (2006)
J.G. Lu, Y.Z. Zhang, Z.Z. Ye, Y.J. Zeng, H.P. He, L.P. Zhu, J.Y. Huang, L. Wang, J. Yuan, B.H. Zhao, X.H. Li, Appl. Phys. Lett. 89, 112113 (2006)
J.M. Bian, X.M. Li, C.Y. Zhang, L.D. Chen, Q. Yao, Appl. Phys. Lett. 84, 3783 (2004)
L. Guojie, S. Sambasivam, K. Saes Byul, P. Sung Wook, C.C. Byung, J.J. Hyun, J. Byeong Eog, J. Korean Phys. Soc. 59, 2770 (2011)
J. Xie, Q. Lu, Q. Chen, J. Mater. Sci. Mater. Electron. 26, 2669 (2015)
S.T. Tan, B.J. Chen, X.W. Sun, W.J. Fan, H.S. Kwok, X.H. Zhang, S.J. Chua, J. Appl. Phys. 98, 1 (2005)
O. Lupan, T. Pauporté, L. Chow, B. Viana, F. Pellé, L.K. Ono, B.R. Cuenya, H. Heinrich, Appl. Surf. Sci. 256, 1895 (2010)
P. Gupta, M. Ramrakhiani, Open Nanosci. J. 3, 15 (2009)
C.Y. Tsay, W.T. Hsu, Ceram. Int. 39, 7425 (2013)
M. Kashif, U. Hashim, M.E. Ali, K.L. Foo, S.M. Usman Ali, J. Nanomater. 2013, 478942 (2013)
T.P. Rao, M.C.S. Kumar, J. Alloys Compd. 509, 8676 (2011)
M. Willander, O. Nur, J.R. Sadaf, M.I. Qadir, S. Zaman, A. Zainelabdin, N. Bano, I. Hussain, Materials (Basel) 3, 2643 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagheri, N., Ara, M.H.M. & Ghazyani, N. Characterization and doping effects study of high hole concentration Li-doped ZnO thin film prepared by sol–gel method. J Mater Sci: Mater Electron 27, 1293–1298 (2016). https://doi.org/10.1007/s10854-015-3888-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3888-0