Abstract
Structure and temperature dependence of electrical properties of Ca(Bi,La)4Ti4O15–(Bi,La)4Ti3O12 (CBT–BIT–xLa) ceramics were studied. With La3+ doping content increasing, the samples exhibited diffused dielectric constant anomalies, and the Curie temperature (Tc) decreased. Meanwhile, the dielectric loss of CBT–BIT–xLa was reduced. CBT–BIT–0.8La performed an improved piezoelectric constant (d33) value of 23.4 pC/N and remained until the temperature increased to 500 °C. In addition, CBT–BIT–0.8La performed an enhanced ρdc value of 3.26 × 1015 Ω cm, at room temperature. CBT–BIT–0.8La showed clearly higher dc resistivity (ρdc) than CBT–BIT, even with increasing the temperature to 600 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Piezoelectric materials desired for techniques and devices including piezoelectric sensors should function at high temperatures without failure. Due to their distinctive microstructure, high fatigue resistance, large remnant polarization (2Pr) and high Curie temperature (Tc), the intergrowth bismuth layer-structured ferroelectrics (BLSFs) have been given abroad attention for high temperature applications [1–4]. In BLSFs, the high Curie temperature is closely related to Bi3+ at A-site with lone pair of 6s 2 electrons [3–5]. For high temperature vibration sensing applications, the sensitivity of these techniques and devices throughout the temperature ranges is significantly influenced by the temperature dependence of electrical properties including piezoelectric properties and resistivity [6]. However, the temperature stability of BLSFs-based materials is the major problem for applications in electronic devices at high temperature. The piezoelectric constant values of BLSFs are usually less than 20 pC/N and the depoling temperatures could be lower than 400 °C for most of pure intergrowth BLSFs [3, 7, 8]. Piezoelectric materials with high piezoelectric constant usually perform high sensitivity [6, 9]. In addition, high temperature stability of piezoelectric properties is of great help for techniques and devices to retain the same sensitivity throughout the temperature range. However, with the increase of temperature, the dc resistivity of BLSFs would appreciably decrease rapidly. Since the minimum useful frequency of a sensor is inversely proportional to the electrical resistivity, the rapidly decreased resistivity of BLSFs restrains the application of BLSFs in high temperature sensors [6, 10]. These drawbacks of intergrowth BLSFs will restrict their potential applications. Lots of efforts have been paid to improve the electric properties of intergrowth BLSFs. A-site modification and high-valence B-site doping have been proven to be effective approaches to improve electric properties of intergrowth BLSFs. Wang et al. reported the substitution of V5+ for Ti4+ in SrBi4Ti4O15–Bi4Ti3O12 (SBT–BIT) could increase 2Pr and d33 values to 32.5 μC/cm2 and 18.3 pC/N [8]. Yi et al. [3] have reported La3+-modified Bi3TiNbO9–Bi4Ti3O12 (BTN–BIT) performed an increased piezoelectric constant (d33) value of 16.6 pC/N.
According to the previous report, CaBi4Ti4O15–Bi4Ti3O12 (CBT–BIT) performed a high curie temperature (Tc) value of 750 °C, but the dielectric loss is also very high and piezoelectric properties are relatively low [8]. The further information about A- or B-site modification of CBT–BIT intergrowth ceramics is favorable for the improvement in electric properties of CBT–BIT. Meanwhile, the temperature dependence of piezoelectric and dc resistivity properties of A- or B-site modification of CBT–BIT ceramics has not been systematically investigated yet. In the present study, the influences of La3+ doping on the temperature dependence of electric properties of CBT–BIT ceramics were studied. The results also show La3+ doping leads to a remarkable increase in the piezoelectric properties and dc resistivity properties of CBT–BIT.
2 Experimental procedures
Ca(Bi,La)4Ti4O15–(Bi,La)4Ti3O12 (CaBi8−xLaxTi7O27: CBT–BIT–xLa, x = 0, 0.4, 0.8, 0.12) powders were prepared by traditional solid-state reaction method. The stoichiometric mixtures of analytical grade CaCO3, Bi2O3, TiO2 and La2O3 were ball-milled and calcined at 850 °C in alumina crucibles for 2 h. The resultant powders were pressed into disks, sintered at 1150 °C for 2 h and cooled naturally. The phase identification and the microstructure characterization were examined on a Rigaku D/max2550 V X-ray diffractometer (XRD) with Cu Kα radiation. Temperature dependent dielectric constant (εr) and tan δ was measured by an HP4284A LCR meter. Temperature dependent dc resistivity was measured by a HP4339B high-resistivity meter. The piezoelectric constant (d33) was measured using a ZJ-3A quasi-static d33 meter.
3 Results and discussion
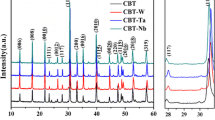
Figure 1 shows the XRD patterns of CBT–BIT–xLa ceramics, which represents the enlarged view of the (118) and (201) peaks. The shift in the positions of (118) and (201) peaks for CBT–BIT–xLa is attributed to the addition of La3+ in the A sites, and suggests the increase of lattice parameters, which we had discussed in detail elsewhere [11]. Meanwhile, with increasing La3+ doping content, the split between the (201) and (021) peaks get weaker and finally disappears when x = 1.2, indicating a structural symmetry transition from orthorhombic to pseudotetragonal.
The variation of dielectric constant (εr) of CBT–BIT–xLa ceramics as a function of temperature at 100 kHz is shown in Fig. 2. It can be seen that there are two dielectric peaks at two different temperatures in CBT–BIT–xLa (the lower temperature as Tm1, the higher one as Tm2 or Tc of CBT–BIT) which are related to the phase transition caused by BIT blocks and the phase transition from ferroelectric to paraelectric [8]. The dielectric peaks of CBT–BIT–xLa samples are gradually broadened, and Tm1 and Tm2 decrease. Since La3+, Bi3+ and Ca2+ are randomly distributed on A-site in the structure of CBT–BIT–xLa, the redistribution of A-site cations and the increase of lattice parameters caused by La3+ doping may enhance the positional disorder of cations. The cationic disordering could induce random fields, impede the development of long range polar ordering and lead to competition as well as coexistence between long-range and short-range polar order, resulting in the formation of PNRs which have different transition temperatures [4, 12–15], and the broadening of dielectric peaks.
Figure 3 shows the difference between the two dielectric peak temperatures (∆T: Tm1 − Tm2), and the insert shows the variation of Tm1 and Tm2 of CBT–BIT–xLa. The Tm1 and Tm2 of CBT–BIT are 675 and 750 °C, respectively. With increasing the La3+ content, both Tm1 and Tm2 decrease, the difference between the two dielectric peak temperatures of CBT–BIT–xLa is increased from 75 °C (x = 0) to 106 °C (x = 1.2). When x = 1.2, Tm1 = 466 °C and Tm2 = 571 °C. It is evident that Bi3+ with 6s 2 lone pair electrons has great influence on the dielectric peak temperature [4]. The substitution of La3+ for Bi3+ could decrease Tc significantly. However, the dielectric peak around Tm2 becomes much lower than that around Tm1, and the temperature difference between the two peaks in CBT–BIT–xLa becomes bigger with increasing La3+ content, which is different from that in BNT–BIT–xLa reported by Yi et al. [16]. That could be caused by the variation of the structure symmetry of CBT–BIT–xLa. With increasing La3+ content, CBT–BIT–0.8La and CBT–BIT–1.2La perform a pseudotetragonal–tetragonal phase transition at Tm2 while CBT–BIT and CBT–BIT–0.4La perform an orthorhombic–tetragonal phase transition.
The dielectric loss of CBT–BIT–xLa decreases with increasing La3+ content. The thermal motion of oxygen vacancies caused by the volatilization of Bi2O3 can be the predominant reason for the increase of dielectric loss at the temperature range from 100 to 400 °C. And the rapid increase of dielectric loss at higher temperature range can be caused by the movement of ferroelectric domain walls. The substitution of Bi3+ by La3+ can decrease concentrations of oxygen vacancies and electrons caused by the Bi2O3 volatilization, and reduce the dielectric loss caused by the motion of these free charge carriers. The domain wall pinning caused by oxygen vacancies is also reduced.
The dielectric relaxor behavior is further characterized by the modified Curie–Weiss law [17]: ln(1/εr − 1/εm) = γ ln(T − Tm) + C where εm is the value of the dielectric constant at Tm2(CBT–BIT) and γ (1 ≤ γ ≤ 2) is defined as the degree of diffuseness in ferroelectric–paraelectric transition. For normal ferroelectrics, γ = 1, while for an ideal relaxor, γ = 2.
With La3+ doping content increasing, the samples exhibit diffused dielectric constant anomalies around Tm2. The linear fitting and the value of γ is shown in Fig. 4. It can be seen that the γ value increases when x ≤ 0.8, gets a maximum value of 1.81 when x = 0.8, and then decreases to 1.62. The change of the diffuseness exponent γ symbolizes the variations on the dielectric relaxor behavior of CBT–BIT–xLa and the diffuse phase transition in the structure. In general, the relaxor behavior is closely related to the cationic disordering caused by the different kinds of cations on the same crystallographic site [14, 17–20]. With the content of La3+ increasing to 0.8, the cationic disordering may be enhanced, resulting in the increase of diffusion degree of phase transition. However, as we mentioned above, the structure symmetry of CBT–BIT–xLa is also improved. CBT–BIT–1.2La performs a pseudotetragonal–tetragonal phase transition at Tm2. The variation of symmetry probably causes a slight suppression of dielectric relaxor behavior and decreased diffuseness exponent.
Temperature dependence of piezoelectric properties of CBT–BIT–xLa ceramics is shown in Fig. 5. With increasing temperature, the CBT–BIT ceramics show excellent resistance to the thermal induced depolarization. The d33 value of CBT–BIT ceramics remains 13 pC/N until 670 °C, decreased slightly around 675 °C (Tm1 of CBT–BIT), and stands great until 720 °C (Tm2 of CBT–BIT). With the La3+ content increasing, the samples show enhanced piezoelectric constants but decreased depolarization temperatures. The optimal properties are obtained at the composition with x = 0.8, which exhibits piezoelectric constant d33 value of 23.4 pC/N and remains 23.4 pC/N until the temperature rises to 500 °C. Compared with the pure CBT–BIT, CBT–BIT–xLa may produce more complicated PNRs with different transition temperatures, because of the heterogeneous distribution of La3+ in BIT–xLa part and CBT–xLa part. Therefore, the substitution of La3+ for Bi3+ could cause the piezoelectric properties of CBT–BIT–xLa thermally unstable well below the Curie point. It could result in significantly decreased phase transition temperatures and the rapid thermal degradation of piezoelectric properties.
Figure 6 shows the temperature dependence of dc resistivity (ρdc) of CBT–BIT–xLa (x = 0 and 0.8) ceramics. At room temperature, CBT–BIT shows a high ρdc value of 8.79 × 1013 Ω cm, while CBT–BIT–0.8La performs an enhanced ρdc value of 3.26 × 1015 Ω cm. The ρdc of CBT–BIT–0.8La ceramics has been raised remarkably compared to CBT–BIT ceramics, even when the temperature rises to 600 °C. This is probably attributed to decreased concentrations of free charge carriers. The motion of oxygen vacancies and free electrons plays an important role in the variation of ρdc of intergrowth BLSFs in the temperature range from 75 to 600 °C [21, 22]. As reported before, the bond strength of La–O is stronger than that of Bi–O [23, 24]. La3+ doping can decrease the concentration of oxygen vacancies and electrons which is likely to be caused by the volatilization of Bi2O3. The high resistivity is beneficial to piezoelectric applications of intergrowth BLSFs at high temperature.
4 Conclusions
Structure and temperature stability of electric properties of CBT–BIT–xLa ceramics were studied. A phase transition from orthorhombic to pseudotetragonal was observed by XRD analysis when x ≥ 0.8. With increasing x, the samples exhibited diffused dielectric constant anomalies. Meanwhile, due to La3+ doping, the dielectric loss of CBT–BIT–xLa was reduced. CBT–BIT–xLa performed an enhanced d33 value of 23.4 pC/N when x = 0.8 and remained until the temperature increased to 500 °C. At room temperature, CBT–BIT shows a high ρdc value of 8.79 × 1013 Ω cm, while CBT–BIT–0.8La performs an enhanced ρdc value of 3.26 × 1015 Ω cm. Moreover, CBT–BIT–0.8La also performed clearly (about ten times) higher ρdc than CBT–BIT, even at high temperature.
References
T. Wei, C.Z. Zhao, C.P. Li, Y.B. Lin, X. Yang, H.G. Tan, J. Alloy Compd. 577, 728–733 (2013)
T. Wei, C.P. Li, Q.J. Zhou, Y.L. Zou, L.S. Zhang, Mater. Lett. 118, 92–95 (2014)
Z.G. Yi, Y.X. Li, Y. Liu, Phys. Status Solidi A 208, 1035–1040 (2011)
Z.G. Yi, Y.X. Li, J.T. Zeng, Q.B. Yang, D. Wang, Y.Q. Lu, Q.R. Yin, Appl. Phys. Lett. 87, 202901 (2005)
T. Sivakumar, M. Itoh, Eur. J. Inorg. Chem. 2011, 5343–5346 (2011)
S.J. Zhang, F.P. Yu, J. Am. Ceram. Soc. 94, 3153 (2011)
H. Zhang, H. Yan, M.J. Reece, J. Appl. Phys. 107, 104111 (2010)
W. Wang, D. Shan, J.B. Sun, X.Y. Mao, X.B. Chen, J. Appl. Phys. 103, 044102 (2008)
K. Kim, S.J. Zhang, G. Salazar, X.N. Jiang, Sensor. Actuat. A Phys. 178, 40 (2012)
R.C. Turner, P.A. Fuierer, R.E. Newnham, T.R. Shrout, Appl. Acoust. 41, 299 (1994)
L.J. Fei, Z.Y. Zhou, S.P. Hui, X.L. Dong, Y.S. Li, Mater. Lett. 156, 165 (2015)
P.Y. Fang, H.Q. Fan, J. Li, F. Liang, J. Appl. Phys. 107, 064104 (2010)
J. Zhu, X.B. Chen, J.H. He, J.C. Shen, J. Solid State Chem. 178, 2832–2837 (2005)
C. Karthik, N. Ravishankar, M. Maglione, R. Vondermuhll, J. Etourneau, K.B.R. Varma, Solid State Commun. 139, 268 (2006)
Y. Liu, R.L. Withers, X.Y. Wei, J.D. Fitz, Gerald. J. Solid State Chem. 180, 858 (2007)
Z.G. Yi, Y.X. Li, Y. Wang, Q.R. Yin, Appl. Phys. Lett. 88, 152909 (2006)
C.L. Diao, J.B. Xu, H.W. Zheng, L. Fang, Y.Z. Gu, W.F. Zhang, Ceram. Int. 39, 6991–6995 (2013)
B.R. Kannan, B.H. Venkataraman, Ceram. Int. 40, 16365–16369 (2014)
C.L. Diao, H.W. Zheng, Y.G. Zhang, Z. Chen, L. Fang, Ceram. Int. 40, 13827–13832 (2014)
H. Li, Q. Meng, D. Gong, H. Tian, Z. Zhou, J. Eur. Ceram. Soc. 34, 4185–4192 (2014)
Z.G. Yi, Y.X. Li, Z.Y. Wen, S.R. Wang, J.T. Zeng, Q.R. Yin, Appl. Phys. Lett. 86, 192906 (2005)
Z.G. Yi, Y.X. Li, Y. Wang, Q.R. Yin, Appl. Phys. Lett. 88, 162908 (2006)
M. Roy, I. Bala, S.K. Barbar, S. Jangid, P. Dave, J. Phys. Chem. Solids 72, 1347–1353 (2011)
Z.G. Yi, Y.X. Li, Q.B. Yang, Q.R. Yin, Ceram. Int. 34, 735–739 (2008)
Acknowledgments
The authors acknowledge the financial support by the National Natural Science Foundation of China (Grant No. 11304334).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fei, L., Zhou, Z., Hui, S. et al. Effects of La3+ substitution on structure and temperature dependence of electrical properties of CaBi4Ti4O15–Bi4Ti3O12 ceramics. J Mater Sci: Mater Electron 26, 6843–6847 (2015). https://doi.org/10.1007/s10854-015-3299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3299-2