Abstract
This paper describes the synthesis of CaCu3Ti4O12 (CCTO) with different morphologies like nanoparticles and nanorod prepared through modifies sol–gel method by using different surfactants (CTAB, SDS, PVP, and PVA). This study aimed to investigate the effects of different surfactant such as CTAB, SDS, PVP, and PVA and chelate agent on the morphology, particle size, and crystal structure of final products. It was found that the size and morphology of the products could be greatly influenced by the aforementioned parameters. To the best of authors’ knowledge, this is the first report on the synthesis of CCTO nanostructure with different morphologies in the presence of oxalic acid as a chelate agent. The as-synthesized products were characterized by powder X-ray diffraction, scanning electronic microscopy, transmission electron microscope, and spectra energy dispersive analysis of X-ray techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor nanoparticles have attracted extensive attention in the past two decades because of their size-dependent novel electronic and optical properties originating from surface and quantum confinement effects [1–6]. Recently, many perovskite-like oxides ACu3Ti4O12 nanostructures (A = Ca, La2/3,Bi1/2Na1/2, etc.) with body-centered cubic crystal structure, have drawn considerable theoretical and experimental studies [6–9]. There is considerable interest in a family of isostructural compounds ACu3Ti4O12, where A = Ca, La2/3, Na1/2Y1/2, etc. [7, 10, 11]. All possess a perovskite-related crystal structure where the TiO6 octahedra are sufficiently in-phase tilted to produce a square planar environment for Cu. To date, all of these phases are cubic and centric space group Im-3, at least at room temperature. CaCu3Ti4O12 (CCTO) is the most studied compound from this family since fixed (radio) frequency measurements on single crystals [12], and ceramics [13–15], show a “so-called” giant permittivity (>1000) over a wide temperature range, ~150–400 K. There has been much speculation on the origin of the giant permittivity, especially for single crystals; however, a combination of impedance spectroscopy [16], microcontact measurements across single grain boundaries [17], and Kelvin probe microscopy [18, 19], has shown CCTO ceramics to be electrically heterogeneous with semiconducting grains and insulating grain boundaries. Such an electrical microstructure is consistent with an internal barrier layer capacitor; the “giant” permittivity observed from fixed frequency measurements is an extrinsic effect associated with thin insulating grain boundaries. Low temperature impedance spectroscopy and fixed frequency measurements show the bulk permittivity of CCTO to be ~100. Here we report the synthesis and characterization of CaCu3Ti4O12 (CCTO) through the modify sol–gel method. Besides, several experiments were performed in order to investigate the effect of surfactants such as such as cetyltrimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS), polyvinyl pyrrolidone (PVP), and polyvinyl alcohol (PVA) and chelate cation (oxalic acid) on the morphology, particle size, and crystal structure of final products.
2 Experimental
2.1 Chemicals and equipment
The X-ray diffraction (XRD) pattern of the products was recorded by a Rigaku D-max C III XRD using Ni filtered Cu Kα radiation. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Transmission electron microscope (TEM) images were obtained on a Philips EM208S transmission electron microscope with an accelerating voltage of 100 kV. The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. Fourier transform infrared (FT-IR) spectra were recorded on Shimadzu Varian 4300 spectrophotometer in KBr pellets.
2.2 Synthesis of CaCu3Ti4O12 nanostructures
The oxalic acid, Ca(CH3COO)2.4H2O, Cu(CH3COO)2. H2O, ethanol, and titanium (IV) isopropoxide were purchased from Merck Company and used without further purification. At first, an appropriate amount of titanium (IV) isopropoxide, copper (II) acetate, and calcium (II) acetate (corresponding to Ti:Cu:Ca = 4:3:1) were dissolved stoichiometrically in ethanol in three separate A, B, and C beakers, respectively. Then, oxalic acid as chelating agent was dissolved in ethanol and added to the A solution under constant stirring. Afterwards, the calcium (II) acetate and copper (II) acetate solution was mixed with A solution under stirring at room temperature. Subsequently, the final mixed solution was kept stirring to form a gel at 90 °C. Finally, the obtained product was placed in a conventional furnace in air atmosphere for 3.5 h, and calcinated at 1040 °C (Table 1). The synthesis pathway of CaCu3Ti4O12 nanostructures is shown in Scheme 1.
3 Result and discussion
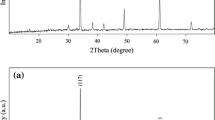
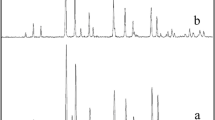
X-ray diffraction patterns of CCTO nanostructures in the presence of oxalic acid (sample no 6) and its absence (sample no 2) are shown in Figs. 1a, b, respectively. The XRD pattern of the as-synthesized CaCu3Ti4O12 without oxalic acid (Fig. 1a) indicates the formation of cubic phase of CaCu3Ti4O12 (space group lm-3, JCPDS No. 75-2188). The XRD pattern of the CaCu3Ti4O12, in the presence of oxalic acid as chelating agent is shown in Fig. 1b. The XRD pattern in Fig. 1a indicates the formation of pure cubic phase of CaCu3Ti4O12 (JCPDS No. 75-2188), with the calculated cell constants a = b = c = 7.3910 Å. According to XRD data, the crystallite diameters (Dc) of CaCu3Ti4O12 nanoparticles obtained from sample 2 and 6 are calculated to be 30 and 37 nm using the Scherer Eq. (1), respectively [20]:
where β is the breadth of the observed diffraction line at its half intensity maximum, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. Chelating agents are used in inorganic chemistry to prevent particle agglomeration by reducing condensation reactions in liquid phase synthesis. To investigate the effect of oxalic acid as chelating agent on the surface morphology of CaCu3Ti4O12 nanostructures several experiments were performed. SEM images of CCTO in the absence of oxalic acid and its presence are shown in Figs. 2 and 3, respectively. The typical SEM images of CCTO synthesized in the presence of different surfactant such as CTAB, SDS, PVP, and PVA and without oxalic acid as a chelate agent are shown in Fig. 2a–d, respectively. According to the Fig. 2a, product mainly consists of agglomeration nanoparticles. With change of the surfactant from CTAB to SDS, PVP, and PVA, the nanoparticles become densely agglomerated and particle size was increased to create microstructure, as shown in Fig. 2b–d, respectively. As a result, the optimum surfactant for synthesis of CCTO nanostructures in the absence of oxalic acid is CTAB (sample 1). SEM images of CCTO nanostructures in the presence of oxalic acid as a chelate agent (sample 5–8) are shown in Fig. 3a–d. In comparison with samples 1–4 which are in the absence of oxalic acid (Fig. 2a–d), product mainly consists of microstructure. Furthermore, when SDS was used as a surfactant in the presence of oxalic acid, product mainly consists of nano rod like structures, as shown in Fig. 3b. Moreover, in comparison samples in the presence and absence of oxalic acid as a chelate agent, presence oxalic acid as a chelate agent causes decrease size of final product. Figure 4 shows the FT-IR spectrum of as prepared CCTO. The absorption peak at 3423.76 cm−1 is related to δ (H2O) [21]. All three samples show the main absorption bands at 561, 516, and 437 cm−1. These bands are assigned to the absorption regions for Ti ion, which are associated to ν Ti–O of 653–550 cm−1 and ν Ti–O–Ti of 495–436 cm−1 [22]. The purity of CCTO nanostructures was also confirmed by EDS analysis (Fig. 5). According to Fig. 5, the product is composed of Cu, Ca, Ti, and O element. Moreover, no impurity peaks are observed, which indicates a high level of purity in the product. TEM image was taken to characterize the size and morphology of CaCu3Ti4O12 nanostructures (sample no. 6) with very high spatial resolution (Fig. 6). TEM image of CaCu3Ti4O12 nanostructures (sample no. 6) revealed that the diameter and length of nanorods were 50–100 and 300 nm, respectively.
4 Conclusion
In this work, CaCu3Ti4O12 nanostructures were successfully synthesized by a modified sol–gel method. Besides, the effect of preparation parameters such as type surfactants and chelating agent on the morphology, particle size, and crystal structure of CaCu3Ti4O12 nanostructures were studied by SEM by technique. Cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), polyvinylpyrrolidone (PVP), and polyvinyl alcohol (PVA) were used as the surfactant. SEM results indicate that oxalic acid as chelating agent plays an important role in the morphology and particle size of CaCu3Ti4O12 nanostructures. The lowest temperature for preparation of the pure CaCu3Ti4O12 nanocrystals is about 1040 °C.
References
G. Kianpour, M. Salavati-Niasari, H. Emadi, Superlatt. Microstruct. 58, 120 (2013)
M. Salavati-Niasari, F. Davar, M. Farhadi. J. Sol-Gel. Sci. Technol. 51, 48 (2009)
A. Javidan, S. Rafizadeh, S.M. Hosseinpour-Mashkani, Mater. Sci. Semicond. Process. 27, 468 (2014)
A. Henglein, Chem. Rev. 89, 1861 (1989)
R. Dittmer, E.M. Anton, W. Jo, H. Simons, J.E. Daniels, M. Hoffman, J. Pokorny, I.M. Reaney, J. Rodel, J. Am. Ceram. Soc. 1, 3519 (2012)
H. Zeynali, S.B. Mousavi, S.M. Hosseinpour-Mashkani, Mater. Lett. 144, 65 (2015)
M.A. Subramanian, L. Dong, N. Duan, B.A. Reisner, A.W. Sleight, J. Solid State Chem. 151, 323 (2000)
M.C. Ferrarelli, T.B. Adams, A. Feteira, D.C. Sinclair, A.R. West, Appl. Phys. Lett. 89, 212904 (2006)
C. Masingboon, S. Maensiri, T. Yamwong, P.L. Anderson, S. Seraphin, Appl. Phys. A 91, 87 (2008)
A.P. Ramirez, M.A. Subramanian, M. Gardel, G. Blumberg, D. Li, T. Vogt, S.M. Shapiro, Solid State Commun. 115, 217 (2000)
D.C. Sinclair, T.B. Adams, F.D. Morrison, A.R. West, Appl. Phys. Lett. 80, 2153 (2002)
C.C. Holmes, T. Vogt, S.M. Shapiro, S. Wakimoto, A.P. Ramirez, Science 293, 637 (2001)
S.Y. Chung, I.L.D. Kim, S.J.L. Kang, Nat. Mater. 3, 774 (2004)
D.C. Sinclair, A.R. West, Adv. Mater. 14, 1321 (2002)
T.T. Fang, H.K. Shiau, J. Am. Ceram. Soc. 87, 2072 (2004)
J. Li, M.A. Subramanian, H.D. Rosenfeld, C.Y. Jones, B.H. Toby, A.W. Sleight, Chem. Mater. 16, 5223 (2004)
R.K. Grubbs, E.L. Venturini, P.G. Clem, J.J. Richardson, B.A. Tuttle, G.A. Samara, Phys. Rev. B. 72, 104111 (2005)
D.C. Sinclair, A.R. West, J. Appl. Phys. 109, 106 (2011)
S.M. Hosseinpour-Mashkani, M. Ramezani, M. Vatanparast, Mater. Sci. Semicond. Process. 26, 112 (2014)
S.M. Hosseinpour-Mashkani, M. Ramezani, Mater. Lett. 130, 259 (2014)
M. Salavati-Niasari, S.M. Hosseinpour-Mashkani, F. Mohandes, S. Gholamrezaei, J. Mater. Sci. 26, 2810 (2015)
C. Masingboon, P. Thongbai, S. Maensiri, T. Yamwong, S. Seraphin, Mater. Chem. Phys. 109, 262 (2008)
Acknowledgments
Authors are grateful to council of University of Arak for providing financial support to undertake this work.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseinpour-Mashkani, S.M., Ramezani, M., Sobhani-Nasab, A. et al. Synthesis, characterization, and morphological control of CaCu3Ti4O12 through modify sol–gel method. J Mater Sci: Mater Electron 26, 6086–6091 (2015). https://doi.org/10.1007/s10854-015-3186-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3186-x