Abstract
Three surfactants, namely, cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), and polyethylene glycol (PEG), were used to prevent particle agglomeration in TiO2 nanoparticle synthesis. The nanoparticles were produced using the sol–gel and ball milling (dry–wet–dry) techniques. Structural and morphological analyses were performed via X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) to explain the effect of the surfactants on TiO2 nanoparticle agglomeration and particle size reduction. XRD results showed that the crystal structures of non-surfactant had pure anatase, whereas those obtained using PEG (sample PEG-TiO2), SDS (sample SDS-TiO2), and CTAB (sample CTAB-TiO2) had a rutile phase. SEM morphological results showed that the use of these surfactants could prevent agglomeration. TEM analysis results showed that PEG-TiO2, SDS-TiO2, and CTAB-TiO2 had smaller particles than those synthesized without surfactants. Particle analysis showed that the particle sizes were in the following order: CTAB-TiO2 < SDS-TiO2 < PEG-TiO2. The use of CTAB resulted in the most significant change. The formation of the rutile phase and agglomeration reduction with the use of CTAB was due to the faster hydrolysis process (compared with the hydrolysis processes with the use of the other surfactants) and a quantum size effect. The details of the hydrolysis mechanism and agglomeration prevention are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) is a metal oxide that is widely used in various fields, including the development of gas sensors [1, 2]. It is also a semiconductor oxide that detects harmful gases, such as NO2, CO2, H2S, and CO [3]. TiO2 is a polymorphic material, with anatase, brookite, and rutile being its primary polymorphs. Rutile is a stable form, whereas anatase and brookite are metastable and readily turn into rutile when heated [4, 5]. The anatase structure has a larger active surface area than the other phases. Its more significant activity impacts the reactivity or sensitivity of TiO2 in applications such as sensors [6, 7]. The anatase structure has advantages, including high electron mobility, a high dielectric constant, a high Fermi energy, and a low capacity to absorb oxygen. Therefore, the anatase phase is more suitable for application in various fields than the other phases [8, 9].
The reduction of TiO2 to the nanoscale can increase the oxygen vacancies on its particle surface and enhance its particle surface area and surface energy. Particles can be converted into nanoparticles in various ways, such as hydrothermal [10, 11], solvothermal [12], and sol–gel [13] methods. The sol–gel approach is a bottom-up synthesis method that uses chemical reactions to form nanoparticles. This was chosen in this study because it has many advantages, including control over the formed nanoparticles’ shape, phase, and size [14]. However, nanometer-sized TiO2 particles agglomerate [15]. The high trend is due to nano-sized materials, high surface energy, and forces between particles (van der Waals and hydrogen bonds) on the particles, so the particles are susceptible to agglomeration [16,17,18]. Agglomeration can be reduced by modifying the sol–gel method by adding surfactants.
Surfactants are the best shape-directing agents in the synthesis of nanomaterials; this advantage is mainly related to the surface adsorption of surface-active molecules on different crystalline planes from the nucleation center, which controls the overall nanoparticle shape [14, 19]. Surfactants play a major role in the formation of nano-emulsions by lowering the interfacial tension and reducing the Laplace pressure (the pressure difference between the inside and outside of the droplet), thereby preventing the micellar envelope from splitting [15]. Therefore, surfactants can prevent particle agglomeration during sol–gel synthesis. By controlling the formation of large groups of particles, surfactants help uniformize the distribution of particles in a solution, which is necessary for proper gel formation. Producing high-quality gels with consistent properties without surfactants is difficult [20]. Surfactants can be anionic, cationic, and non-ionic [21]. The type of surfactant affects the reactivity during hydrolysis [22]. The time of hydrolysis initiation affects the microstructure formed at the end of the synthesis process. This research combines physical and chemical synthesis methods of TiO2 nanoparticles: sol–gel and ball milling. Ball milling is conducted using the dry–wet–dry method at 90% of the critical speed. This process is completed before calcination for ease of particle crushing. The discussion focuses on the different mechanisms of each surfactant, namely, cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), and polyethylene glycol (PEG), during the beginning of the hydrolysis phase and their impact on the microstructure. The quantum-to-phase phenomenon and the surfactant mechanism for preventing particle agglomeration are also explained.
Experimental method
Three samples of TiO2 nanoparticles were prepared through the sol–gel technique. A titanium isopropoxide (TTIP 99% precursor) was used, and the solvent and surfactant were ethanol (C2H5OH), polyethylene glycol (PEG) with molecular weights of 400, sodium dodecyl sulfate (SDS) 96.9%m and cetyltrimethylammonium bromide (CTAB) 99.76%. The TTIP was purchased from the product of Sigma Aldrich Singapore, and all surfactants were obtained from Merck, India. The first sample, the CTAB-TiO2 nanoparticles, was prepared using CTAB as a surfactant. TiO2 nanoparticle synthesis began by mixing 5 mL of TTIP with 5 mL of ethanol. The solution (sol A) was stirred for 30 min using a magnetic stirrer at a rotational speed of 1500 rpm at a temperature of 27 ˚C. Another solution (sol B) was prepared by dissolving 0.075 g of CTAB into 6 mL of ethanol, adding 3.2 mL of 4.4 M (pH 0.6) HNO3, and stirring the mixture for 15 min using a magnetic stirrer at 1500 rpm at the temperature of 27 ˚C. Next, sol B was added into sol A drops at a stirring speed of 1500 rpm. The resulting mixture was then hydrolyzed at 40 ˚C for four days to produce a yellow solid. The solid was subjected to grinding and then ball milling at 192 rpm. The resulting TiO2 powder was oven-dried to remove the residue from the solution, followed by annealing at 400 °C for 2 h. This process was repeated to synthesize sample 2 (SDS-TiO2) and sample 3 (PEG-TiO2) except that SDS and PEG, respectively, were used in place of CTAB as surfactants.
Characterization
The sintered TiO2 nanoparticle powder was characterized via scanning electron microscopy (SEM) to determine its particle size and morphology. X-ray diffraction (XRD) analysis (X’Pert3 Powder, PANalytical B.V. Company Netherlands) was conducted at 2θ in the range of 10°–90° to determine the phase crystals formed. The phase analysis of the TiO2 nanoparticles was based on ICSD 96–231-0711 for the anatase structure and ICSD 96–900-4143 for the rutile structure. The morphologies and sizes of the TiO2 nanoparticles were observed via field-emission scanning electron microscopy (FESEM; Quanta FEG 650, FEI USA) and transmission electron microscopy (TEM Tecnai G2 20, FEI, USA). The proportions of the anatase and rutile phases were calculated with the Rietveld Method using the Rietica software.
Results
The morphologies of the CTAB-TiO2, SDS-TiO2, PEG-TiO2, and no-surfactant nanoparticles can be seen in the SEM images in Fig. 1. The morphologies of the TiO2 nanoparticles synthesized using the non-ionic surfactant (PEG-TiO2) and the no-surfactant nanoparticles had irregular shapes, large sizes, and considerable aggregation, as shown in Figs. 1b and 1a, respectively. The samples synthesized using the ionic surfactants (CTAB-TiO2 and SDS-TiO2) had the morphology of TiO2 nanoparticles, which were spherical but moderately agglomerated, as shown in Figs. 1c and 1d, respectively.
TEM images (Fig. 2) support the SEM results. The TEM instrument provides a better picture that clearly shows the particles are highly agglomerated. This is evidenced by TiO2-CTAB, SDS, and PEG, in Fig. 2a1, 2b1, and 2c1 and Fig. 2a2, 2b2, and 2c2 (inset: magnification), respectively, experiencing high agglomeration. TiO2 formed looks like small granules that crush each other. These results are well confirmed from the research of Yuenyongsuwan et al. which states that TiO2 nanoparticles have properties easily agglomerated [21]. In addition, the TEM results show that TiO2 has two phases, namely, rutile and anatase. These results are confirmed both from the lines in Fig. 2b3, Fig. 2c3, and Fig. 2d3. which have a slit width of the rutile and anatase A (101) hkl plane respectively 0.301 nm, 0.372 nm, and 0.381 nm. The SAED results also clarify the presence of both anatase and rutile phases with hkl planes for anatase (101), and for rutile (004), (020), (202), (132), and (015).
Furthermore, the calculation of particle size distribution of TEM results is shown in Fig. 3. The figure shows that CTAB-TiO2 has a smaller size compared to SDS-TiO2, PEG 400-TiO2, without surfactant (WS) which is 34.27 nm, 38.77 nm, 41.25 nm, and 48.49 nm.
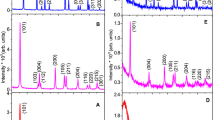
The effect of the surfactant type on the structure and phase composition of the TiO2 nanoparticles was identified through XRD. Figure 4 shows the anatase diffraction peaks at 25.23°, 48.02°, 54.30°, 62.74°, 68.98°, and 76.50° (ICSD 96–231-0711) and the rutile diffraction peaks at 27.40°, 36.04°, 41.22°, 56.59°, 69.78°, and 84.24° (ICSD 96–900-4143). The XRD results were then refined using the Rietvield method with the results presented in Table 1.
In contrast, CTAB-TiO2, PEG-TiO2, and SDS-TiO2 have a rutile phase shown by the peaks in Fig. 4. However, the CTAB-TiO2 sample has a higher percentage of rutile phase which is about 89.01% than the anatase phase which is 10.99%. The values in percentage of each phase of the four samples are shown in Table 1. The formation of the rutile phase was also observed in two other methods, microemulsion and hydrothermal, where CTAB was also used [21]; however, the amount was less than that of the anatase phase. The addition of acetylacetone during the synthesis of TiO2 with CTAB as a surfactant using the sol–gel method as well as the microwave method prevents the formation of the rutile phases. Acetylacetone slowdowns the hydrolysis condensation reactions [23]. Good refinement results are shown from the values of profile factor (Rp), weighted profile factor (Rwp), good of fitness (X2), and Bragg factor (Bragg-R Factor) in Table 1.
Discussion
CTAB is a cationic surfactant with a positively charged head, SDS is anionic with a negatively charged head, and PEG is non-ionic or has no charge [22, 24,25,26]. Figure 5 shows the schematic structure of each surfactant used in the synthesis of TiO2 nanoparticles. The ionic surfactants are polar and able to interact well with both the TTIP precursor and its hydrolyzed product, Ti(OH)4, which will become TiO2 after calcination. PEG is a polymer that does not have a clear head and tail; its long structure can build a randomly folded conformation. The TTIP precursor will be trapped in the folds of the non-ionic polymer chain of PEG with ether groups and produce a non-spherical shape of the final TiO2 particle. The micelle shape of each surfactant is drawn in Table 2.
The morphologies of the TiO2 nanoparticles as SEM and TEM images can be seen in Figs. 1 and 2, respectively. The addition of the ionic surfactants (CTAB and SDS) produced round, agglomerated TiO2 nanoparticles. The use of the nonionic surfactant (PEG) produced irregularly shaped TiO2 nanoparticles. Particle analysis showed that the particle sizes were in the following order: CTAB-TiO2 < SDS-TiO2 < PEG-TiO2. Thus, the use of the different surfactants influenced the particle sizes, shapes, and crystal phases of TiO2. The ionic surfactant micelles (CTAB and SDS) trapped the TTIP precursor and the resulting Ti(OH)4 particles, as shown in Fig. 6a. In this experiment, the surfactant heads, theoretically, pointed inward and formed a ball-like shape because the solvent was organic. Thus, the micelle sheaths prevented particle agglomeration. When the temperature increased, the micelles enlarged along with the increase in the size of the particles inside them [22], as shown in Fig. 6b. The surfactant heads in the micelles interacted with TiO2 ionically. Breaking the surfactant bond with TiO2 would require a large amount of energy because of their strong ionic interaction. When the temperature reached a certain value, the surfactants (organic molecules) decomposed into gases or small molecules that could evaporate, leaving spherical TiO2 nanoparticles, as shown in Fig. 6c. If the calcination process continued, then the small particles formed earlier could agglomerate and coalesce into large particles. This process created agglomerated TiO2 particles with recognizable spherical shapes, as shown in Fig. 6d. The formation of TiO2 from TTIP occurred as follows [27, 28]:
The TiO2 nanoparticles synthesized using the non-ionic surfactant (PEG-TiO2) had unique morphologies because the surfactant itself had a lumpy shape (the precursor followed this shape). The hydrolysis product of TTIP interacted with the ether groups of the non-ionic surfactant clusters, filling and surrounding the PEG surfactant clusters, as depicted in Fig. 7a. Non-ionic surfactants are neutral, so their interaction with TiO2 nanoparticles is weak. During the heating process, this surfactant decomposed and evaporated Fig. 7b. The broken surfactant caused the TiO2 particles formed in and around it to break apart into various shapes, as shown in Fig. 7c. The TiO2 nanoparticles then coalesced and agglomerated in the calcination stage, as shown in Fig. 7d. Thus, the PEG-TiO2 nanoparticles had a larger size due to agglomeration when the TiO2 nanoparticles were not covered in the surfactant (similar to the ionic surfactant micelles). In other words, non-ionic surfactants do not effectively prevent agglomeration. Figures 1a and b show that the size and shape of the PEG-TiO2 nanoparticles did not significantly differ from those of the nanoparticles synthesized without surfactants.
Analysis results of the X diffractograms, using Refinement with Ritvield Method, show significant amounts of the rutile phase at 400 ˚C. The rutile phase can already form at a temperature of 500 ˚C [29] or even less than 400 °C [30]. The early rutile phase formation can be caused by several factors, such as a quantum phenomenon (size effect) occurring in the sample [31, 32]. The results of the preceding analysis showed that the CTAB-TiO2 sample had a small particle size. Particle size affects the reduction of the melting point of a material. Nanometer-sized particles move more easily at lower temperatures, so these structural changes occur more quickly. This size effect also influences the absorption of high heat energy; phase change accelerates in the case of larger particles. In addition to the size effect, the acid factor used during synthesis accelerates the formation of crystal nuclei. The addition of acid enhances the availability of H+ ions, thereby accelerating the hydrolysis reaction. Increasing the hydrolysis rate accelerates the formation of crystal nuclei, causing the formation of the base chain leading to the rutile structure [33, 34].
DTA/TGA measurement result of the sample with CTAB shows there are three interesting waves (Fig. 8). At about 290 ˚C, there is a strong exothermic peak accompanied by significant weight loss which could be assigned as residual nitrate decomposition. The waves at about 337 ˚C and 415 ˚C are the two endotherm waves that could be responsible for the formation of the anatase and the rutile phase, respectively. This result emphasizes the possibility of the rutile phase formation at temperature as low as 400 ˚C, with hold time of two hours (Fig. 8).
Conclusion
This research focuses on preventing particle agglomeration by adding a surfactant during the synthesis of TiO2 nanoparticles via the sol–gel and ball milling (dry–wet–dry) methods. The surfactant type controls the nanoparticle microstructure during synthesis. The interaction of the TTIP precursor with the surfactant micelles during synthesis influences the resulting TiO2 nanoparticle morphology and particle size. The ionic surfactants (CTAB and SDS) have a better agglomeration prevention mechanism than the non-ionic surfactant (PEG) and thus produce smaller particle sizes. The addition of CTAB results in the smallest particle size, and the rutile phase of the sample grows at a lower temperature (400 ˚C) than with the use of the other surfactants. This early phase change exerts size effects on the resulting nanoparticles.
References
Avci N, Smet PF, Poelman H, Driessche I. Van, Poelman D (2009) Characterization of TiO2 powders and thin films prepared by non-aqueous sol – gel techniques. J Sol-Gel Sci Technol 52(3):424–431. https://doi.org/10.1007/s10971-009-2028-9
Cano-Casanova L, Amorós-Pérez A, Lillo-Ródenas MÁ, del Román-Martínez MC (2018) Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 11(11):2227. https://doi.org/10.3390/ma11112227
Carter CB, Norton MG (2013) Solid-state phase transformations and reactions. In: ceramic materials: science and engineering. Springer, New York, 457–475. https://doi.org/10.1007/978-1-4614-3523-5_25
Danks AE, Hall SR, Schnepp Z (2016) The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mater Horiz 3(2):91–112. https://doi.org/10.1039/c5mh00260e
Galkina OL, Vinogradov VV, Agafonov AV, Vinogradov AV (2011) Surfactant-assisted sol-gel synthesis of TiO 2 with uniform particle size distribution. Int J Inorg Chem 2011:1–8. https://doi.org/10.1155/2011/108087
Gilbert B, Zhang H, Huang F, Finnegan MP, Waychunas GA, Banfield JF (2003) Special phase transformation and crystal growth pathways observed in nanoparticles. Geochem Trans 4(4):20–27. https://doi.org/10.1039/b309073f
Goto T, Cho SH, Ohtsuki C, Sekino T (2018) Solvothermal synthesis of TiO2-modified hydroxyapatite using water-isopropanol solution. Materials Science Forum 922 MSF:86–91. https://doi.org/10.4028/www.scientific.net/MSF.922.86
Hanaor DAH, Triani G, Sorrell CC (2011) Morphology and photocatalytic activity of highly oriented mixed phase titanium dioxide thin films. Surf Coat Technol 205(12):3658–3664. https://doi.org/10.1016/j.surfcoat.2011.01.007
Iftimie N, Luca D, Lacomi F, Girtan M, Mardare D (2009) Gas sensing materials based on Ti O2 thin films. J Vacuum Sci Technol B: Microelectron Nanometer Struct 27(1):538–541. https://doi.org/10.1116/1.3021050
Langford JI, Wilson AJC (1978) Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Crystallogr 11(2):102–113. https://doi.org/10.1107/s0021889878012844
Kang M, Kim SW, Park HY (2018) Optical properties of TiO2 thin films with crystal structure. J Phys Chem Solids 123(March):266–270. https://doi.org/10.1016/j.jpcs.2018.08.009
Kao LH, Hsu TC, Cheng KK (2010) Novel synthesis of high-surface-area ordered mesoporous TiO2 with anatase framework for photocatalytic applications. J Colloid Interface Sci 341(2):359–365. https://doi.org/10.1016/j.jcis.2009.09.058
Kawashima S, Seo JWT, Corr D, Hersam MC, Shah SP (2014) Dispersion of CaCO3 nanoparticles by sonication and surfactant treatment for application in fly ash-cement systems. Mater Struct/Materiaux et Constructions 47(6):1011–1023. https://doi.org/10.1617/s11527-013-0110-9
Korayem AH, Tourani N, Zakertabrizi M, Sabziparvar AM, Duan WH (2017) A review of dispersion of nanoparticles in cementitious matrices: nanoparticle geometry perspective. Constr Build Mater 153:346–357. https://doi.org/10.1016/j.conbuildmat.2017.06.164
Kumar PS, Pavithra KG, Naushad M (2019) Characterization techniques for nanomaterials. Elsevier Inc, In Nanomaterials for Solar Cell Applications. https://doi.org/10.1016/b978-0-12-813337-8.00004-7
Li Y, Wang HQ, Zhou H, Du D, Geng W, Lin D, Chen X, Zhan H, Zhou Y, Kang J (2017) Tuning the surface morphologies and properties of ZnO films by the design of interfacial layer. Nanoscale Res Lett 12:31–33. https://doi.org/10.1186/s11671-017-2301-8
Liu J, Chen L, Yang H, Zhang Z, Wang Y (2019) Size-dependent solid-solid phase transition process of Ag2S nanoparticles. Progress in Natural Science: Materials International 29(4):397–401. https://doi.org/10.1016/j.pnsc.2019.05.008
Mital GS, Manoj T (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56:1639. https://doi.org/10.1007/s11434-011-4476-1
Miyazawa T, Itaya M, Burdeos GC, Nakagawa K, Miyazawa T (2021) A critical review of the use of surfactant-coated nanoparticles in nanomedicine and food nanotechnology. Int J Nanomed 16:3937–3999. https://doi.org/10.2147/IJN.S298606
Monshi A, Foroughi MR, Monshi MR (2012) Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J Nano Sci Eng 02(03):154–160. https://doi.org/10.4236/wjnse.2012.23020
Yuenyongsuwan J, Nithiyakorn N, Sabkird P, O’Rear EA, Pongprayoon T (2018) Surfactant effect on phase-controlled synthesis and photocatalyst property of TiO2 nanoparticles. Mater Chem Phys 214:330–336. https://doi.org/10.1016/j.matchemphys.2018.04.111
Morsy SMI (2014) Role of surfactants in nanotechnology and their applications. Int J Curr Microbiol App Sci 3(5):237–260
Payormhorm J, Chuangchote S, Laosiripojana N (2017) CTAB-assisted sol-microwave method for fast synthesis of mesoporous TiO2 photocatalysts for photocatalytic conversion of glucose to value-added sugars. Mater Res Bull 95:546–555. https://doi.org/10.1016/j.materresbull.2017.08.016
Qu Y, Wang W, Jing L, Song S, Shi X, Xue L, Fu H (2010) Surface modification of nanocrystalline anatase with CTAB in the acidic condition and its effects on photocatalytic activity and preferential growth of TiO 2. Appl Surf Sci 257(1):151–156. https://doi.org/10.1016/j.apsusc.2010.06.054
Sargam Y, Wang K, Tsyrenova A, Liu F, Jiang S (2021) Effects of anionic and nonionic surfactants on the dispersion and stability of nanoSiO2 in aqueous and cement pore solutions. Cement Concrete Res 144(August 2020):106417. https://doi.org/10.1016/j.cemconres.2021.106417
Singh LP, Bhattacharyya SK, Mishra G, Ahalawat S (2011) Functional role of cationic surfactant to control the nano size of silica powder. Appl Nanosci (Switzerland) 1(3):117–122. https://doi.org/10.1007/s13204-011-0016-1
Mahshid S, Askari M, Ghamsari MS (2007) Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J Mater Process Technol 189(1–3):296–300. https://doi.org/10.1016/j.jmatprotec.2007.01
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Shuaib DT, Ajala AO, Mohammed AK (2020) Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: a review. Applied Water Science, Springer 11(2):102–113. https://doi.org/10.1007/s13201-020-1138-y
Ramalingam S (2019) Synthesis of nanosized titanium dioxide (Tio2) by sol-gel method. Int J Innov Technol Exploring Eng 9(2S2):732–735. https://doi.org/10.35940/ijitee.b1174.1292s219
Reyes-Coronado D, Rodríguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, De Coss R, Oskam G (2008) Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 19(14):145605. https://doi.org/10.1088/0957-4484/19/14/145605
Li Y, Fan Y, Chen Y (2002) A novel method for preparation of nanocrystalline rutile TiO2 powders by liquid hydrolysis of TiCl4. J Mater Chem 12(5):1387–1390. https://doi.org/10.1039/b200018
Rsheed AA, Aldawood S, Aldossary OM (2021) The size and shape effects on the melting point of nanoparticles based on the Lennard-Jones potential function. Nanomateriasl J 11(11):2916. https://doi.org/10.3390/nano11112916
Tryba B, Tygielska M, Colbeau-Justin C, Kusiak-Nejman E, Kapica-Kozar J, Wróbel R, Guskos N (2016) Influence of pH of sol-gel solution on phase composition and photocatalytic activity of TiO 2 under UV and visible light. Mater Res Bull 84:152–161. https://doi.org/10.1016/j.materresbull.2016.07.035
Simonsen ME, Søgaard EG (2009) Sol–gel reactions of titanium alkoxides and water: influence of pH and alkoxy group on cluster formation and properties of the resulting products. J Sol-Gel Sci Technol 53(3):485–497. https://doi.org/10.1007/s10971-009-2121-0
Funding
The authors would like to thank Brawijaya University for the financial support in the HPU research scheme 2023 with contract no. 612.14/UN10.C20/2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rachmaniar, S., Nugraha, D.A., Santjojo, D.J.D.H. et al. Prevention of particle agglomeration in sol–gel synthesis of TiO2 nanoparticles via addition of surfactant. J Nanopart Res 26, 45 (2024). https://doi.org/10.1007/s11051-024-05943-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05943-2