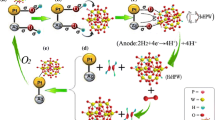
Abstract
In consideration of the increased activity of Pt-based catalysts for methanol oxidation in direct methanol fuel cells, it is necessary to apply an ideal support in the construction of Pt-based catalyst for methanol oxidation. In this study, Ag/g-C3N4 nanosheets (Ag/GCNN) nanocomposites with different Ag contents were synthesized and used as the progressive support to construct Pt/Ag/GCNN catalyst. As a nitrogen-rich polymeric semiconductor, GCNN is easily coordinated with various metals. The introduction of Ag nanoparticles can improve the electrical conductivity of the catalyst and cooperate with Pt for optimizing the performance of electrocatalytic oxidation of methanol. The electrochemical experiment results demonstrated that Pt/Ag/GCNN has higher electrocatalytic activity and stability for methanol oxidation than Pt/GC and Pt/GCNN. The mass activity of Pt/Ag/GCNN-0.6 (18.66 mA mgPt−1) is 7.07, 4.60 and 1.26 times that of Pt/GCNN (4.06 mA mgPt−1), Pt/Ag/GCNN-0.4 (4.06 mA mgPt−1) and Pt/Ag/GCNN-0.8 (14.76 mA mgPt−1) at the forward peak potential. The Jf/Jb ratio of Pt/Ag/GCNN-0.6 is 3.31, which is bigger than that of Pt/GCCN (2.62), Pt/Ag/GCNN-0.4 (2.69), and Pt/Ag/GCNN-0.8 (2.56). A suitable mechanism was proposed to explain that Ag/GCNN supported Pt catalyst can improve the CO toxicity resistance and electrocatalytic performance. Based on the coordination of GCNN, Ag was first modified to form a support, and then the remaining coordination sites were used to support Pt. It provided a new strategy for the design of catalysts for methanol electrooxidation.
Graphical abstract













Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data and code availability
Not applicable.
References
Xu G, Lei Y, Wu K, Lv R, Li J, Sun X, Cai W (2023) Frustrated Lewis pair engineering on ceria to improve methanol oxidation activity of Pt for direct methanol fuel cell applications. Int J Hydrog Energy 48:5953–5960
Hu T, Chen W, Liu Y, Gong L, Jiang Z, Bhalothia D, Maiyalagan T, Jiang Z (2023) Plasma-induced formation of Pt nanoparticles with optimized surface oxidation states for methanol oxidation and oxygen reduction reactions to achieve high-performance DMFCs. Small 19:2304076
Zhao J, Zeng H, Lu Z (2022) Pt nanowires on monolayered graphene oxide for electrocatalytic oxidation of methanol. ACS Appl Nano Mater 5(9):13594–13600
Yuda A, Ashok A, Kumar A (2022) A comprehensive and critical review on recent progress in anode catalyst for methanol oxidation reaction. Catal Rev Sci Eng 64:126–228
Lai SCS, Lebedeva NP, Housmans THM, Koper MTM (2007) Mechanisms of carbon monoxide and methanol oxidation at single-crystal electrodes. Top Catal 46:320–333
Li CZ, Wang ZB, Sui XL, Zhang GuLMDM (2015) Ultrathin graphitic carbon nitride nanosheets and graphene composite material as high performance PtRu catalyst support for methanol electro-oxidation. Carbon 93:105–115
Luo Y, Zhong W, Huang P, Ou H, Fu H, Liu C, Xiao Z, Xu S (2021) Improved electrocatalytic activity of Pt catalyst supported on core–shell CMs@NiO for methanol oxidation. New J Chem 45:12879–12885
Fu X, Wan C, Huang Y, Duan X (2022) Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media. Adv Funct Mater 32(11):2106401
Huang H, Wang X (2014) Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J Mater Chem A 2(18):6266–6291
Zheng Y, Liu J, Liang J, Jaroniec M, Qiao SZ (2012) Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ Sci 5(5):6717–6731
Niu P, Zhang L, Liu G, Cheng HM (2012) Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv Funct Mater 22(22):4763–4770
Xiao P, Zhao Y, Wang T, Zhan Y, Wang H, Li J (2014) Polymeric carbon nitride/mesoporous silica composites as catalyst support for Au and Pt nanoparticles. Chemistry 20(10):2872–2878
Li XH, Antonietti M (2013) Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: functional Mott-Schottky heterojunctions for catalysis. Chem Soc Rev 42(16):6593–6604
Vasilchenko D, Zhurenok A, Saraev A, Gerasimov E, Cherepanova S, Tkachev S, Plusnin P, Kozlova E (2022) Highly efficient hydrogen production under visible light over g-C3N4-based photocatalysts with low platinum content. Chem Eng J 445:136721
Song C, Kim S (2015) Preparation and electrochemical characterization of Pt-supported flake-like graphitic carbon nitride on reduced graphene oxide as fuel cell catalysts. J Electrochem Soc 162:F1181–F1190
Zhu M, Zhai C, Sun M, Hu Y, Yan B, Du Y (2017) Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation. Appl Catal B Environ 203:108–115
Meenu PC, Datta SP, Singh SA, Dinda S, Chakraborty C, Roy S (2020) Polyaniline supported g-C3N4 quantum dots surpass benchmark Pt/C: development of morphologically engineered g-C3N4 catalysts towards “metal-free” methanol electro-oxidation. J Power Sources 461:228150–228163
Tian J, Ning R, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2014) Three-dimensional porous supramolecular architecture from ultrathin g-C3N4 nanosheets and reduced graphene oxide: solution self-assembly construction and application as a highly efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl Mater Interfaces 6(2):1011–1017
Huang H, Yang S, Vajtai R, Xin W, Ajayan PM (2014) Pt-decorated 3D architectures built from graphene and graphitic carbon nitride nanosheets as efficient methanol oxidation catalysts. Adv Mater 26:5160–5165
Liang X, Dong F, Tang Z, Wang Q (2022) Surface hydroxy functionalized Pt/g-C3N4-CNS for highly efficient methanol electrocatalytic oxidation. Mol Cata 530:112638
Liang X, Dong F, Tang Z, Wang Q (2022) The Pt/g-C3N4-CNS catalyst via in situ synthesis process with excellent performance for methanol electrocatalytic oxidation reaction. New J Chem 46:3121–3129
Li C, Wang Z, Sui X, Zhang L, Gu D (2016) Graphitic-C3N4 quantum dots modified carbon nanotubes as a novel support material for a low Pt loading fuel cell catalyst. RSC Adv 6:32290
Li CZ, Wang ZB, Sui XL, Zhang LM, Gu DM, Gu S (2014) Graphitic carbon nitride nanosheet coated carbon black as a high performance PtRu catalyst support material for methanol electrooxidation. J Mater Chem A 2:20139–20146
Cheng JX, Hu XL, Zhang JB, Huang HH, Su N (2017) Facile fabrication of Pt/g-C3N4/KB catalyst for methanol oxidation. Chin J Inorg Chem 33(6):993–999
Wang X, Sun M, Guo Y, Hu J, Zhu M (2020) Three dimensional Pt island-on-Au architectures coupled with graphite carbon nitride nanosheets for effective photo-accelerated methanol electro-oxidation. J Colloid Int Sci 558:38–46
Kim M, Hwang S, Yu JS (2007) Novel ordered nanoporous graphitic C3N4 as a support for Pt-Ru anode catalyst in direct methanol fuel cell. J Mater Chem 17:1656–1659
Qian H, Chen S, Fu Y, Wang X (2015) Platinumepalladium bimetallic nanoparticles on graphitic carbon nitride modified carbon black: a highly electroactive and durable catalyst for electrooxidation of alcohols. J Power Sources 300:41–48
Salgado JRC, Fernandes JCS, Rego Do Botelho AM, Ferraria AM, Duarte RG, Ferreira MGS (2011) Pt-Ru nanoparticles supported on functionalized carbon as electrocatalysts for the methanol oxidation. Electrochim Acta 56:8509–8518
Liu H, Ye F, Yao Q, Cao H, Xie J, Lee JY, Yang J (2014) Stellated Ag-Pt bimetallic nanoparticles: an effective platform for catalytic activity tuning. Sci Rep 4:3969
Zhang X, Xie X, Wang H, Zhang J, Pan B, Xie Y (2013) Enhanced photo responsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J Am Chem Soc 135:18–21
Deng YC, Liu J, Huang YB, Ma MM, Liu K, Dou XM, Wang ZJ, Qu SC, Wang ZG (2020) Engineering the photocatalytic behaviors of g-C3N4-based metal-free materials for degradation of a representative antibiotic. Adv Funct Mater 30:2002353
Liao GF, Fang JS, Li Q, Li SH, Xu ZS, Fang BZ (2019) Ag-based nanocomposites: synthesis and applications in catalysis. Nanoscale 11:7062–7096
Zhu B, Xia P, Li Y, Ho W, Yu J (2017) Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl Surf Sci 391:175–183
Nubla K, Radhakrishnan T, Sandhyarani N (2019) A graphitic carbon nitride-titania nanocomposite as a promising catalyst support for electro-oxidation of methanol. New J Chem 43:3273–3279
Qi F, Li Y, Wang Y, Wang Y, Liu S, Zhao X (2016) Ag-Doped g-C3N4 film electrode: fabrication, characterization and photoelectrocatalysis property. RSC Adv 6:81378
Liang X, Dong F, Tang Z, Wang Q (2021) The significant promotion of g-C3N4 on Pt/CNS catalyst for the electrocatalytic oxidation of methanol. Int J Hydrog Energ 46:39645–39657
Xiong B, Zhou Y, Zhao Y, Wang J, Chen X, O’Hayre R, Shao Z (2013) The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 52:181–192
Seger B, Kamat PV (2009) Electrocatalytically active graphene-platinum nanocomposites: role of 2-D carbon support in PEM fuel cells. J Phys Chem C 113:7990–7995
Chen X, Wu G, Chen J, Xie Z, Wang X (2011) Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J Am Chem Soc 133:3693–3695
Meenu PC, Roy S (2023) Unveiling the mechanistic significance of reducibility and lattice oxygen evolution in the Ce1−x−yZrxNiyO2−δ catalyst for methanol electro-oxidation. ACS Appl Energy Mater 6:11212–11225
Meenu PC, Roy S (2023) Electro-oxidation reaction of methanol over reducible Ce1−x−yNixSryO2−δ: a mechanistic probe of participation of lattice oxygen. ACS Appl Mater Interfaces 15:36154–36166
Aboutalebi SH, Chidembo AT, Salari M, Konstantinov K, Wexler D, Liu HK, Dou SX (2011) Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ Sci 4:1855–1865
Lu Q, Zhao X, Luque R, Eid K (2023) Structure-activity relationship of tri-metallic Pt-based nanocatalysts for methanol oxidation reaction. Coord Chem Rev 493:215280
Macias-Ferrer D, Melo-Banda JA, Silva-Rodrigo R, Lam-Maldonado M, Páramo-García U, Verde-Gómez JY, Del-Ángel-Vicente P (2020) Highly dispersed PtCo nanoparticles on micro/nano-structured pyrolytic carbon from refined sugar for methanol electro-oxidation in acid media. Catal Today 349:159–167
Haldorai Y, Arreaga-Salas D, Rak CS, Huh YS, Han YK, Voit W (2016) Platinized titanium nitride/graphene ternary hybrids for direct methanol fuel cells and titanium nitride/graphene composites for high performance supercapacitors. Electrochim Acta 220:465–474
Wang G, Kuang S, Zhang J, Hou S, Nian S (2016) Graphitic Carbon nitride/mutiwalled Carbon nanotubes composite as Pt-free counter electrode for high-effiency dye-senstized solar cells. Electrochim Acta 187:243–248
Yang X, Wang Q, Qing S, Gao Z, Tong X, Yang N (2021) Modulating electronic structure of an Au-nanorod-core–PdPt-alloy-shell catalyst for efficient alcohol electro-oxidation. Adv Energy Mater 11:2100812
Zhong W, Liu Y, Zhang D (2012) theoretical study of methanol oxidation on the PtAu(111) bimetallic surface: CO pathway vs non-CO pathway. J Phys Chem C 116:2994–3000
Acknowledgements
This work was financially supported by financially supported by National Natural Science Foundation of China (22272070; 22066017), Science and Technology Project of Huzhou (2023GZ59), Zhejiang Province Natural Science Foundation of China (LY24E020007), Natural Science Foundation of Jiangxi Province (20224BAB214023; 20232BAB204010), National College Students Innovation entrepreneurship training Program (202313287012) and Huzhou College (RK65011).
Author information
Authors and Affiliations
Contributions
MX contributed to Methodology. YL contributed to conceptualization, writing-original and revised draft, funding acquisition. LZ contributed to formal analysis. PH contributed to funding acquisition. SX contributed to visualization, writing–review & editing, funding acquisition. YL contributed to writing-review & editing. YW contributed to investigation. XL contributed to data curation. YX contributred to resources, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Handling Editor: Andréa de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Luo, Y., Zeng, L. et al. Ag/g-C3N4 nanosheets as a progressive support of Pt catalyst for improved electrocatalytic oxidation of methanol. J Mater Sci 59, 3573–3584 (2024). https://doi.org/10.1007/s10853-024-09469-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09469-9