Abstract
Nanocarbon materials such as graphene and graphene nanoribbons (GNRs) have been studied for various applications such as electrodes and catalysts. For precise analyses of nanocarbon materials, analyses other than conventionally used analyses such as Raman and infrared spectroscopies and microscopies are essential. C1s X-ray photoelectron spectroscopy (XPS) is one of the general techniques used to analyze the structures of carbon materials. However, XPS has not been commonly utilized to investigate the structures of carbon materials in detail. In this work, the relevance of molecular shapes, molecular sizes, and types of edges on peak positions of C1s XPS spectra were revealed. For example, adjusted peak positions of C1s spectra of rectangular graphene-like structures such as GNRs tended to be higher than those of triangle or hexagonal graphene-like structures at the same atomic ratio of H/C mainly because rectangular structures of GNRs enhanced the conjugation more than those of hexagonal and triangle structures of graphene-related materials. Besides, the structures of graphene-related materials, such as rectangular with either zigzag or armchair edges, triangle, hexagonal, and the other structures, can be estimated without using a microscope by measuring combustion elemental analysis, mass spectrometry, C1s XPS spectra, and the highest occupied molecular orbital and comparing them with the tendencies of H/C ratio and molecular weight versus adjusted peak positions of C1s XPS spectra. Moreover, the reasons for the shift and the broadening of experimental C1s XPS spectra have been revealed to be the charge-up effect caused by the large size of the powder of analyzed compounds.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
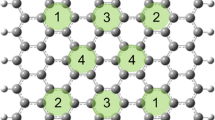
Nanocarbon materials such as graphene [1], carbon nanotubes [2, 3], and graphene nanoribbons (GNRs) [4] with high conductivity, specific surface area, and chemical resistance have been applied for various researches such as electrodes, catalysts, heat dissipation materials, and electromagnetic shielding materials [5,6,7,8,9,10,11,12,13]. The performance of nanocarbon materials for various applications has been known to be influenced by the amount and the types of edges of nanocarbon materials [14,15,16,17,18]. Besides, it is known that zigzag edges are electronically rich and more reactive than armchair edges [18,19,20,21,22]. It is also known that the width of GNRs and the size of polyaromatic hydrocarbons affect the bandgap [22,23,24,25,26,27]. Thus, understanding the shapes such as hexagonal, rectangular, and triangle (Fig. 1 ai), the molecular weight, the types of edges such as zigzag edges, which are similar to Solo, armchair edges, which are similar to Duo, Trio, Quartet/Quatro, and Quintet [28,29,30,31,32,33,34,35,36,37] (Fig. 1 aii) of nanocarbon materials are essential for the research of nanocarbon materials.
Various graphene-related materials with different shapes such as hexagonal [38,39,40,41,42], rectangle (GNRs) [43,44,45,46], triangle [47, 48], circular [40, 42], star-like structures [42], and the other shapes [49,50,51,52] have been intensively studied for the last two decades. Atomic-level observations by scanning tunneling microscope [19, 20, 43, 44, 47] and transmission electron microscope with aberration corrector [53,54,55,56] have been performed to analyze edges and the other defects of nanocarbon materials. However, microscopes can only analyze the small region at the atomic scale and the determination of the bonding states is another challenge [53, 56]. In contrast, some evaluation methods such as infrared-ray (IR) [21, 45, 57,58,59,60,61,62,63], Raman [21, 22, 45, 63,64,65,66,67], nuclear magnetic resonance [45, 68,69,70,71,72], and X-ray photoelectron spectroscopy (XPS) [22, 23, 71,72,73,74,75] have been studied to analyze carbon materials precisely by combining especially with calculation because these methods can analyze the large region and the averaged information of edge structures can be readily obtained.
Among these analytical techniques, XPS is one of the essential chemical state analyses to estimate structures of carbon materials. XPS has been generally utilized to analyze the functional groups of carbon materials [71,72,73,74,75,76,77,78,79], metal complex [80, 81], and some defects such as sp3C [73, 82], point defects [73, 83], Stone-Thrower-Wales defects [73, 83], pentagons [63, 77, 84, 85], and heptagons [71, 85, 86]. However, XPS has not been generally utilized to determine the edge structures and aromaticity except for our recent work [22, 23, 85]. As related research to this work, the aromaticity of coals has been investigated, but only the pi-pi* shape-up peaks were investigated [87]. The broadness of C1s XPS spectra of anthracene-based graphite has been explained to be related to the defect peaks at ca. 285 eV which is shifted from 284.6 eV of sp2C, but the origin of the defect peaks has not been discussed in detail [88]. The C1s XPS spectra of aromatic compounds with different sizes such as coronene, circumpyrene, and circumcoronene have been studied, but the peak positions have been reported to be similar to each other [89] probably because of the calculation without consideration of the highest occupied molecular orbital (HOMO) energy and non-separated C-H and C=C peaks. Thus, the influences of the sizes, shapes, and types of edges of nanocarbon materials on the peak positions of C1s spectra have not been discussed in detail and are still unclear. (a) Characteristic structures of carbon materials (b) Conceptual image of this work.
Our recent work showed that the peak position of graphene-related materials with zigzag edges was higher than that with armchair edges and the peak position of graphene-related materials with zigzag edges was close to that of graphite (284.3 eV) [22]. A similar tendency was also observed in the presence of nitrogen in GNRs [23]. It indicates the possibility of distinguishing types of edges for GNRs by C1s XPS spectra. However, the study of XPS analysis of graphene-related materials with zigzag and armchair edges without hetero atom demonstrated only calculated C1s XPS spectra [22], and not experimental ones except for tetracene, chrysene, and graphite. Besides, XPS spectra for graphene-related materials with edges other than zigzag and armchair edges and shapes other than rectangular shapes have not been investigated in the previous work [22]. Experimental peak positions and the consideration of edges and shapes are the most essential for analyzing various nanocarbon materials in the future.
In this work, effects of 1) molecular shapes of graphene-related materials, such as rectangular, triangle, hexagonal, and others, 2) molecular sizes of graphene-related materials, and 3) types of edges such as zigzag edges, which are similar to Solo, armchair edges, which are similar to Duo, Trio, Quartet/Quatro, and Quintet on peak positions of C1s XPS spectra were investigated in detail by combining experimental and calculated XPS analyses (Fig. 1b).
Experimental
Graphite (HOPG, ZYH, NT-MDT Co.), graphite powder (SP270, Nippon Graphite Industries, Ltd.), and various aromatic compounds such as coronene (> 95.0%, TCI Co., Ltd.), pentacene (> 99%, Sigma-Aldrich Co. LLC.), hexabenzocoronene (> 95%, 1717 CheMall Corp.), dibenzo[b,def]chrysene (> 98.0%, TCI Co., Ltd.), perylene (> 99.5%, Sigma-Aldrich Co. LLC.), quaterrylene (purity not available, Sigma-Aldrich Co. LLC.), and triphenylene (> 98%, Sigma-Aldrich Co. LLC.) were analyzed experimentally using two XPS devices (AXIS ULTRA DLD (Shimadzu Corp.) and JPS-9030 (JEOL Ltd.). The peak positions of C1s XPS spectra were adjusted by the shift of HOMO (Fig. S1). The influence of sizes of powder of analyzing samples on the peak positions and full width at half maximums (FWHMs) were also investigated. The detail is explained in Supplementary Material.
Calculation
Six kinds of GNR-like rectangular structures (Fig. 2a), four triangle structures (Fig. 2b), and seven hexagonal structures with either zigzag or armchair edges (Fig. 2c) were constructed as model structures for calculating XPS spectra. These structures were selected because graphene with different shapes such as rectangle [43,44,45,46], triangle [47, 48], and hexagonal structures [38,39,40,41,42] have been reported.
For the rectangular structures, benzene rings in the vertical direction were numbered from n = 3 to 30 (n = 3, 6, 14, and 30 for zigzag-type structures and n = 3, 6, 14, and 25 for armchair-type structures), and carbon atoms in the horizontal direction were numbered from 3 to 7 for armchair edges and from 2 to 6 for zigzag edges (Fig. 2a). For example, Armchair_3-3 represented the structure containing 3 benzene rings arranged in the horizontal direction with armchair edges and 3 carbon atoms arranged in the vertical direction. As triangle structures, one structure with only Quartet (Triangle_1) and three structures with Quartet and Duo (Triangle_2, Triangle_3, and Triangle_4) were also constructed (Fig. 2b). As hexagonal structures, one structure with only Duo (Hexagon_Z1), three structures with Solo and Duo (Hexagon_Z2, Hexagon_Z3, and Hexagon_Z4), one structure with only Trio (Hexagon_A1), and two structures with Duo and Trio (Hexagon_A2 and Hexagon_A3) were constructed (Fig. 2c). Structures of coronene dimer (Co-Co_2) and oligomers (Co-Co_4 and 8) with Solo and Duo (Fig. 2d) and structures with all Duo (A_Duo_3, A_Duo_6, and A_Duo_14) (Fig. 2e) were calculated. Both Co-Co_1 and A_Duo_1 are coronene molecules, which are the same as Hexagon_Z1. Structures not shown in Fig. 2 are also calculated, but the same definitions are applied to those structures.
The number of C and H atoms in structures and the atomic ratio of H/C (n = ∞) for structures shown in Fig. 2 are summarized in Table 1. As the number of n becomes infinity, the atomic ratio of H/C of e.g., Armchair_3 becomes 67%, whereas those of triangle and hexagonal structures become zero. In other words, the effects of the edges become negligible for triangle and hexagonal structures with large molecular weight because the atomic ratio of H/C becomes zero, whereas the effects of the edges (e.g., Duo for Armchair structures and Solo for Zigzag structures) become constant.
All of the calculations were conducted using B3LYP/6-31g(d) integral=grid=ultrafine of Gaussian 09 [90] in a similar way to our previous work [22, 63, 71,72,73,74,75,76,77,78, 82, 84,85,86] except for the introduction of symmetry to calculate structures with large molecular weight. For the rectangular structures, the triangle structures, the hexagonal structures, D2h, D3h, and D6h symmetries were applied, respectively. For structures of coronene and coronene dimer, and oligomers and structures with all Duo edges, D2h symmetry was applied. The symmetry was applied to calculate large molecules in this work because the accuracy of B3LYP/6-31g(d) is relatively high and the calculation of such large molecules using B3LYP/6-31g(d) without symmetry is difficult, althuogh the other hybrid functionals are possible to be used without symmetry for large molecules [63]. The charge and spin multiplicity of the structures were set as 0 and 1. After the structures were optimized, orbital energies for simulated XPS spectra and Mulliken charges were calculated. For simulating C1s XPS spectra, the calculated orbital energies were used. The HOMO energy was subtracted from each orbital energy to calculate binding energies [22, 82, 84]. The calculated orbital energy was multiplied with 0.79282 and then 58.92 was further added to match the experimental and calculated binding energies (Table S1 and Figs. S2 and S3). Furthermore, modified asymmetric Voigt-type lineshape [91] was applied. The ratios of Lorentzian function to Gaussian function, m, and the asymmetry factor, α, were 0.92 (Fig. S4) and 0, respectively, to compensate for the difference between the calculated and the experimental binding energies. The asymmetry factor (α) was set to 0 for easy comparison, although the experimental value was 0.22 for conductive materials such as graphite (Fig. S4) [73]. Although the asymmetry factor and also the ratios of Lorentzian function to Gaussian function differ depending on the conductivity, the shapes of spectra are not discussed in this work in detail for ease of comparison. The detail of calculation and spectral shape is explained in Supplementary Material.
Results and discussion
Figure 3 and Table S2 show simulated C1s XPS spectra of rectangular structures and the summarized peak positions, respectively. The differences in C1s peak tops among different types of structures with sp2C-H are 0.1 eV or less. The differences in C1s peak tops derived from sp2C-sp2C and sp2C-H are 0.1–0.4 eV. The adjusted peak tops of C1s spectra shifted to higher binding energies as the n values of Armchair_3-n, Armchair_5-n, Armchair_7-n, Zigzag_2-n, Zigzag_4-n, and Zigzag_6-n increased. Besides, as the values of n increased, the peak shifts became smaller and became close to the peak position of graphite (284.3 eV in Fig. S2a and Table S1). Comparing the adjusted peak positions of C1s spectra of Zigzag and Armchair structures with comparable molecular sizes, the binding energies of Zigzag structures were higher than those of Armchair structures. This trend agrees with the trend of graphene-related materials with zigzag and armchair edges [22], which are different structures from the structures used in this work. Unlike the previous work, we also calculated C1s XPS spectra of many graphene-related materials including other edges (Trio, Quartet, triangle, hexagonal, and the others) as explained below.
Simulated C1s XPS spectra of rectangular structures. Simulated total spectra are shown as thin lines. Blue lines show the positions of the peak tops of C1s XPS spectra. Bold lines show the spectra of sp2C-sp2C, indicating carbon atoms bonded with carbon atoms. Sp2C-H (Solo), (Duo), (Trio), (Quartet), and (Quintet) indicate carbon atoms bonded with hydrogen atoms at positions of Solo, Duo, Trio, Quartet, and Quintet, respectively. Sp2C-H (Solo) and (Duo) are shown as dashed and fine dashed lines, respectively. Sp2C-H (Trio), (Quartet), and (Quintet) are shown as dotted lines. a Armchair_3-n, Armchair_5-n, and Armchair_7-n, where the value of n is 3, 6, 14, or 25. b Zigzag_2-n, Zigzag_4-n, and Zigzag_6-n, where the value of n is 3, 6, 14, or 30. (A color version of this figure can be viewed online.)
Figure 4 shows simulated C1s XPS spectra of triangle structures (Fig. 4a), hexagonal structures with zigzag (Fig. 4b) and armchair edges (Fig. 4c), coronene dimer and oligomers (Fig. 4d), and structures with all Duo edges (Fig. 4e). Table S2 shows the summary of the adjusted peak positions. The differences in C1s peak tops of sp2C-H among Duo and Quartet, Solo and Duo, or Duo and Trio in different types of structures such as Hexagon_Z2, Hexagon_Z3, Hexagon_A2, Hexagon_A3, coronene dimer, and oligomers are 0.1 eV or less. Thus, it is challenging to separate the peaks corresponding to different types of edges in each structure. The differences in C1s peak tops derived from sp2C-sp2C and sp2C-H in each structure are 0.1–0.3 eV. As in the case of the rectangular structures shown in Fig. 3, the peak tops of C1s spectra for the triangle, hexagonal, and other structures shifted to higher binding energies as the n value of each structure increased. The degree of shifts became small as the value of n increased. Comparing the binding energies of C1s spectra of structures with equivalent molecular sizes in Fig. 4, the order was Hexagon_Z > Hexagon_A > Triangle, although the difference was small. The dependence of the shapes of graphene-like structures on the peak positions at various molecular weights is discussed later.
Simulated C1s XPS spectra of triangle structures. Blue lines show the positions of the C1s peak tops. Simulated total spectra are shown as thin lines. Bold lines show the spectra of sp2C-sp2C, indicating carbon atoms bonded with carbon atoms. Sp2C-H (Solo), (Duo), (Trio), and (Quartet) indicate carbon atoms bonded with hydrogen atoms at positions of Solo, Duo, Trio, and Quartet, respectively. Sp2C-H (Solo) and (Duo) are shown as dashed and fine dashed lines, respectively. Sp2C-H (Trio) and (Quartet) are shown as dotted lines. a Triangle. b Hexagon_Z. c Hexagon_A. d Co-Co. e A_Duo. (A color version of this figure can be viewed online.)
Comparing the adjusted peak positions of C1s spectra of structures with equivalent molecular sizes, the peak positions of Co-Co 1 and A_Duo 1, which are the same structure (coronene) as Hexagon_Z1 (Fig. 4b), are close to the peak positions of Tiangle_1, and Hexagon_A1, but the degrees of shifts to the high binding energy of Co-Co_4 (Fig. 4d) and A_Duo_14 (Fig. 4e) were smaller than those of especially Hexagon_Z and Hexagon_A as the molecular weight increased because the gaps between HOMO and the lowest unoccupied molecular orbital (HOMO–LUMO) of Co-Co_4 and A_Duo_14 were large even after the increment of the molecular weight, as explained later.
Figure 5 shows the relation between either HOMO or LUMO and the peak top of C1s spectra. For all of the structures (Figs. 2 and S6-S11), the relation between HOMO and the peak top of C1s spectra exhibited linear correlations (Fig. 5a), and the same tendency was obtained for LUMO. It indicates that the adjusted peak tops of C1s depend on the positions of HOMO. As the HOMO–LUMO gaps became smaller, C1s peak tops increased and reached ca. 284.5 eV, which was the upper limit of the peak tops of C1s spectra. Because HOMO directly influences the adjusted peak positions of C1s spectra, it is essential to investigate the dependence of shapes (Fig. 6a), molecular weight (Fig. 6b), and edge structures of carbon materials (Fig. 6ab) on the adjusted peak position of C1s spectra.
Relation between adjusted peak tops of simulated C1s XPS spectra and the orbital energies of HOMO and LUMO. a Relation between the orbital energy of either HOMO or LUMO and adjusted peak tops of C1s XPS spectra. b Relation between the HOMO–LUMO gap and the total Mulliken charge of H/ the number of C. c Relation between the HOMO–LUMO gap and the total Mulliken charge of H/ the number of H. (A color version of this figure can be viewed online)
Relation between adjusted peak tops of C1s XPS spectra and either the atomic ratio of H/C or the molecular weight. a Relation between the percentage of atomic ratios of H/C and adjusted peak tops of C1s spectra. b Relation between molecular weight and peak tops of total C1s spectra. (A color version of this figure can be viewed online)
Figure 5b and c show the relation between the HOMO–LUMO gap and the total Mulliken charge of H/ the number of either C or H. The Mulliken charge was obtained from Figs. S6-S11 and summarized in Table S3 because of the possible trend depending on the types of edges. Negative Mulliken charge becomes higher from -0.37 to -0.12 in the order of carbon of Solo, Duo, Trio, Quartet, and Quintet, and the Mulliken charge approached -0.129 (carbon in benzene) from Solo to Quintet (Fig. S12). It is well known that carbon atoms on Solo become electrically rich as observed under scanning transmission electron microscopy [20] and calculated in our previous works [21]. Thus, obviously Solo is electronically rich but this work could obtain the order of Mulliken charge for all types of edges for hexagonal aromatic ring structures.
From Fig. 5b, it is obvious that two linear correlations, where the increment of “Mulliken charge of H/ the number of C” accompanies the increment of “the HOMO–LUMO gap”, were obtained depending on the shapes of graphene-like structures. Comparing at the same HOMO–LUMO gap, “the Mulliken charge of H/ the number of either C” of rectangular structures was larger than those of triangle and hexagonal structures, indicating that carbon atoms in rectangular structures are electrically richer than triangle and hexagonal structures at the same number of carbon atoms.
In the basal plane of graphene-like structures (Figs. S6-S11), little increase or decrease in the number of electrons on carbon atoms was observed, and the Mulliken charges are about 0.00–0.02, which is equal to 6.00–6.02 electrons. In contrast, the change in the number of electrons on carbon atoms around the edges is large (Figs. S6-S11), affecting the electrons on the C atoms of C-Hs and the C atoms of the adjacent C=C bond to -0.37–0.25 (= 5.63–6.25 electrons). The number of electrons in the H of the C-H at the edge also tends to increase, meaning that Mulliken charge of hydrogen decreases, as the GNR widens or lengthens, but the increase is slight, and in some structures, there is almost no increase. For example, the Mulliken charge of H on DUO in the center of structures increases from 0.131 to 0.135 as the width increases from Armchair_3-3 to Armchair_7-3, but there is almost no change from 0.129 to 0.130 for Zigzag structures as the width increases from Zigzag_2-3 to Zigzag_6-3 (Fig. S7). Because rectangular structures contain more carbon atoms close to edges than the other structures, the Mulliken charge of hydrogen per carbon is larger for rectangular structures (Fig. 5b). Therefore, the differences observed in Fig. 5bc due to the different shapes and in Fig. 6ab, which will be discussed later, are mainly due to the larger proportion of C atoms adjacent to the edges or edges affected by the C-H on edges. However, this alone does not explain the difference between Armchair structures and Co-Co/A_Duo structures, because Co-Co and A_Duo structures also contain many edges.
There are two main differences among armchair, Co-Co, and A_Duo structures. The first difference is the presence of zigzag edges at both ends of the GNRs for armchair structures (Table 2) and the presence of zigzag-like edges [57] for Co-Co structures (Table 3). In Co-Co and A_Duo, the HOMO–LUMO gap is wider due to the absence of zigzag edges at both ends, and the trend is different from armchair structures as shown in HOMO orbitals in Tables 2 and 3. By comparing zigzag-like edges on Co-Co structures and armchair edges on A_Duo, HOMO–LUMO gaps of Co-Co structures with zigzag-like edges were smaller than those of armchair edges on A_Duo. The second difference is the ease of conjugation. As the aromatic ring increases, C=C on DUO tends to be more doubly bonded than 1.5-bonded according to Clar's law [92]. As C=C on Duo becomes short, being close to double bond, C–C bond between one of the Cs on Duo and the C bonded to the C on Duo becomes long, resulting in possible less conjugation.
In fact, as shown in Fig. 7a, in Armchair_3, where the benzene rings are bonded to each other to form linear chain structures, even though the molecular weight of Armchair_3 becomes larger (up to 1904), the length of C=C on Duo is almost the same and approximately 1.39 Å. In contrast, the lengths of C=C on Duo in the structures of Co-Co and A_Duo are also the same but much shorter (1.37 Å) (Fig. 7a). As shown in Fig. 7c, the length of the C=C bond on Duo in the structure of Armchair_3-3 drawn by GaussView's software is approximately 1.39 Å and the C=C bond is drawn as 1.5 bond rather than a double bond, whereas that of the C=C bond on Duo in the structure of A_Duo_3 is as short as 1.37 Å and the C=C bond is drawn as double bond. From the above and other results, it was found that the lengths of C=C on Duo are particularly long in the Armchair structures, while those in other structures (Co-Co, A_Duo, Triangle, and Hexagon_A) are short. The relation between the length of bonding and binding energy of N1s orbital has been reported [93]. Therefore, the bond length of C=C is also considered to be one of the important factors for the shift of C1s XPS spectra.
In addition to the change in the length of C=C on Duo, the bond length between the C on Duo and C next to Duo is also crucial (Fig. 7bc). The long bond length between C on Duo and C next to Duo and the shortening of C=C on Duo probably cause the part of Duo to be less related to conjugation, resulting in a wider band gap. In addition, a steric hindrance is present between two C-Hs on Duo [59, 60], and this steric hindrance may also have affected the C=C length explained above, but in the case of the same Armchair with different lengths in width (especially the less rotatable Armchair_5 and Armchair_7), the difference is difficult to explain by the steric hindrance. Thus, the difference is considered to come from the change in the conjugate state. These changes in the length of Duo and C=C surrounding Duo are important not only in the XPS discussion but also in other studies because they affect the peak positions of D' band, which is related to the quadrant stretching vibration of C=C derived from the defect structure in Raman and IR spectroscopic analysis [94].
In the triangle and hexagonal structures, the Solo edge is prevented from extending longer by the presence of Quartet, Trio, and Duo. Thus, the electrons on carbon atoms in the Solo in triangle and hexagonal structures are less likely to be conjugated than rectangular structures with zigzag edges even when the molecular weight increases, and the triangle and hexagonal structures are expected to have wider band gaps than Armchair_Z.
Comparing the lengths of C=Cs on Duo in Fig. 7ab with those between C on Solo and C next to Solo (Fig. 8a) as well as those of C=C next to Solo (Fig. 8b), it is found that the lengths of C=C between C on Solo and C next to Solo (Fig. 8a) as well as the C=C next to Solo (Fig. 8b) are longer than those of the C=C of Duo (Fig. 7a) and the C=C between the carbon atom on Duo and the neighboring bonded carbon atom (Fig. 7b), respectively. In Zigzag_2 (Fig. 8b), as the molecular weight increases, the C=C next to Solo becomes much longer, much closer to the single bond, which makes it easier to conjugate at Solo and the next C on the edges of GNRs, resulting in a narrower HOMO–LUMO gap even at small molecular weights. Although not directly related to this XPS study, the longer C=C next to Solo correlates with the smaller force constant in Raman and IR analyses [95], which leads to a lower shift in the G band for GNR with zigzag edges, although it has not been explained in our previous work [22].
Figure 5c shows that as “the Mulliken charge of H/ the number of H” increases, “the HOMO–LUMO gap” decreases, which is the opposite trend of Fig. 5b. As the molecular weight becomes large, the averaged Mulliken charge on hydrogen, which is written as “total Mulliken charge on hydrogen/ the number of hydrogen”, increased (Figs. 5c and S6-S11). The HOMO–LUMO gaps of Triangle and Hexagon_A structures were larger than those of the rectangular structures at the same “Total Mulliken charge of H/ the number of H”, while those of Hexagon_Z and Co–Co were similar to those of rectangular structures. Among rectangular structures, the HOMO–LUMO gaps of structures with zigzag edges are smaller than those of structures with armchair edges at the same “Total Mulliken charge of H/the number of H”. In a similar way to Zigzag structures, the plots of Hexagon_Z were positioned in the area of rectangular structures, probably because zigzag edges in Hexagon_Z tend to lower the HOMO–LUMO gap. Because of the higher correlation of Fig. 5b than c, Fig. 5b can be utilized to analyze the shapes of graphene-related materials.
Figure 6 shows the dependence of shapes (Fig. 6a), molecular weight (Fig. 6b), and the edge structures of graphene-like materials (Fig. 6ab) on the adjusted peak tops of C1s spectra. The percentage of atomic ratios of H/C and adjusted peak tops of C1s spectra showed a high linear correlation except for some structures that reached the maximum peak position of C1s spectra and saturated at ca. 284.5 eV (Fig. 6a).
Interestingly, rectangular structures showed a different trend from the other structures such as triangle, hexagonal, Co-Co, and A_Duo structures in Fig. 6ab. It is mainly because the HOMO–LUMO gaps of rectangular structures tend to become much smaller than those of the other structures (Fig. 5a). Thus, the adjusted peak positions of C1s spectra of rectangular structures such as GNRs tended to be higher than those of the other structures at the same atomic ratio of H/C (Fig. 6a). One of the reasons is that rectangular structures of GNRs enhanced conjugation. At less than 40% of the percentage of the atomic ratio of H/C, peak tops of C1s spectra of Armchair_7, Zigzag_4 and Zigzag_6 saturated at 284.3–284.5 eV. The orbital energies of HOMO of the corresponding structures saturated with the values at -3.8 eV- -3.6 eV, whose values depend on structures, and HOMO–LUMO gaps were 0.3 eV or less (Fig. 5a). Thus, peak tops of C1s saturate, as the atomic ratios of H/C become smaller (Fig. 6a) and the molecular weights become larger (Fig. 6b). As the widths of armchair and zigzag GNRs increase, peak tops of C1s spectra shifted higher (HOMO energy becomes higher and HOMO–LUMO gap becomes smaller (Fig. 5)). The tendency is consistent with the well-known reported electronic states [24, 96]. From the results explained above, this work could show the correlation between electronic states and C1s XPS spectra.
For all structures, adjusted peak tops of C1s spectra increase with the increment of molecular weight (Fig. 6b). As the molecular weight becomes large, the atomic ratios of H/C become constants (Table 1) and the orbital energies of HOMO and the peak positions of C1s become unchanged (Table S2). HOMO energies of rectangular structures tend to approach the Fermi level at a relatively small molecular weight (ca. 1,000), reaching the peak position of C1s XPS spectrum to the binding energy of graphite (284.3 eV) or even higher. The experimental binding energy for graphite is 284.3 eV (Table S1), but in Figs. 5a and 6b, the maximum value of the peak positions exceeds 284.3 eV. This is because the HOMO of graphene-like structures used to calculate the scaling factor is farther from the Fermi level than those of structures that exceed 284.3 eV.
Unlike other rectangular structures, Armchair_3 saturated the adjusted peak top of C1s spectra at 283.1 eV (Fig. 6b). One of the reasons is the lower limit of the atomic ratio of H/C for Armchair_3 (Table 1), causing a large HOMO–LUMO gap (Table S2). However, the adjusted peak positions of C1s spectra for triangle and the hexagonal structures increased without saturation up to the molecular weight of ca. 2,700 (2,640 for Triangle_4, 2,630 for Hexagon_Z4, and 2,706 for Hexagon_A3). It is expected that the adjusted peak tops of triangle and hexagonal structures keep increasing up to the peak position of graphite because triangle and hexagonal structures reach graphene as the molecular weight increases (Table 1).
Using the relation in Fig. 6a, the shape of the graphene-related materials can be estimated by measuring the sample using combustion elemental analysis and XPS. For example, the H/C can be obtained from the experimental measurement of combustion elemental analysis, and the peak positions of C1s spectra and the HOMO positions can be determined from the experimental measurement of XPS (Fig. S1 and S2). Thus, it is possible to distinguish the structures among rectangular and triangle/hexagonal. Besides, if the molecular shape is known and the peak positions of C1s and HOMO can be obtained by calculation, the molecular weight can be estimated from Fig. 6b. For example, if the molecular shape is Zigzag_2 and the peak top of measured C1s XPS spectra is 283.2 eV, which is not plotted in Fig. 6, the molecular weight is estimated to be 228 (C18H12). Thus, the structure is presumed to be Zigzag_2-4. Using Fig. 6b, the molecular shape can be narrowed down to some extent among various molecular shapes by obtaining the molecular weight experimentally from the mass spectrometry and the experimental peak positions of C1s spectra and HOMO. In other words, by utilizing combustion elemental analysis, mass spectrometry, C1s, and HOMO, it is possible to estimate the structure of graphene-related materials.
Table 2 shows HOMO and the next HOMO (NHOMO) of rectangular structures with either armchair (Table 2a) or zigzag edges (Table 2b). Electron densities of HOMO reveal a more detailed relationship between the shape/types of edges/the molecular sizes and the orbital energy of HOMO, which directly relates to adjusted peak positions of C1s spectra. By increasing the width of armchair structures, zigzag edges appeared on both sides of Armchair_7 and the zigzag edges became HOMO of Armchair_7-6 and 7-14. It has been reported that carbon atoms on zigzag edges become electrically rich as observed under scanning transmission electron microscopy [20] and calculated in our previous works [21]. Rectangular structures with zigzag edges have higher conjugation at the HOMO level than those with armchair edges, generating conductive path on zigzag edges and causing a smaller HOMO–LUMO gap.
Table 3 shows HOMO and NHOMO of triangle, hexagonal, and other structures. For triangle structures in Table 3a and hexagonal structures in Table 3b, both HOMO and NHOMO are shown because the two orbital energies were close to each other, whereas the energies of HOMO and NHOMO of other structures are a little far from each other (Tables 2 and 3). As the molecular weight of hexagonal structures becomes large (Table 3b), HOMO for Hexagon_Z are localized on zigzag edges, while those for Hexagon_A are delocalized in the basal plane as for triangle structures (Table 3a) because both Hexagon_A and triangle structures contain armchair edges. A similar tendency was observed in previous work [97]. Armchair_7 (Table 2a), Co-Co (Table 3ci), and A_Duo (Table 3cii) have the same width except for the concaved shape of Co-Co and A_Duo. The peak positions of C1s spectra for Co-Co are closer to those for A_Duo than those for Armchair_7 (Fig. 6b and Table S2). As the molecular size of Co-Co and A_Duo became larger, HOMOs were localized in the middle of the molecule (Table 3) because Co-Co and A_Duo contain no zigzag edges on both sides, unlike Armchar_7.
Using the results of this work, peak tops of C1s spectra of graphene-related materials can be predicted from the molecular shape and molecular size, if the molecular structures of graphene-related materials had regularity. The molecular shape and molecular size can also be estimated from experimental peak tops of C1s spectra if molecular structures of the graphene-related materials had regularity. Especially, by combining this technique with other techniques such as infrared spectroscopy [57] and Raman spectroscopy [22], the estimation of structures becomes much more reliable. However, it has to keep in mind that the peak position of carbon materials with low molecular weight may be influenced by the sizes of power of analyzed samples due to a charge-up effect (Fig. S13). Samples in the form of fine powder tend to show the smaller FWHM (Fig. S13c) and the lower peak position (Fig. S13ab) than those of coarse powder. The spectral shapes are also influenced by the analytical conditions such as pass energy (Fig. S14) and the conductivity of samples [82]. Therefore, the appropriate analysis conditions have to be applied in order to utilize the method in this work.
Conclusions
The influence of shapes and molecular weight of graphene-like structures on adjusted peak positions of C1s XPS spectra was investigated by calculating C1s XPS spectroscopy in addition to the experimental C1s XPS spectra of the model compounds with various edge structures and we suggested the estimation methods of molecular structures of graphene-related materials from C1s XPS spectra. This work revealed that the adjusted peak positions of C1s XPS spectra of rectangular structures such as GNRs tended to be higher than those of triangle, hexagonal, and the other structures at the same atomic ratio of H/C mainly because rectangular structures of GNRs enhanced the conjugation more than those of hexagonal and triangle structures of graphene-related materials and HOMO becomes close to Fermi level. Besides, as the molecular weight increased, the HOMO energy approached the Fermi level, causing the shift of the adjusted peak position of the C1s XPS spectrum of graphene-related materials close to that of graphene, although some structures showed much low binding energy of C1s spectra even though the molecular weight increased. Since H/C, molecular weight, and the difference between C1s XPS and HOMO can be experimentally obtained by combustion elemental analysis, mass spectrometry, and XPS, the relationship obtained in this work between the adjusted peak position of C1s XPS spectra and either the H/C ratio or molecular weight can be utilized to estimate the shapes and types of edges of graphene-related materials.
References
Geim AK, Novoselov KS (2017) The rise of graphene. Nat Mater 6:183–191
Radushkevich LV, Lukyanovich VM (1952) The structure of carbon forming in thermal decomposition of carbon monoxide on an iron catalyst. Sov J Phys Chem 26:88–95
Ijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature 363:603–605
Yang X, Dou X, Rouhanipour A, Zhi L, Räder HJ, Müllen K (2008) Two-dimensional graphene nanoribbons. J Am Chem Soc 130:4216–4217
Choi W, Lahiri I, Seelaboyina R, Kang YS (2010) Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci 35:52–71
Agudosi ES, Abdullah EC, Numan A, Mubarak NM, Khalid M, Omar N (2020) A review of the graphene synthesis routes and its applications in electrochemical energy storage. Crit Rev Solid State Mater Sci 45:339–377
Zhang Y, Heo YJ, Son YR, In I, An KH, Kim BJ, Park SJ (2019) Recent advanced thermal interfacial materials: a review of conducting mechanisms and parameters of carbon materials. Carbon 142:445–460
González M, Pozuelo J, Baselga J (2018) Electromagnetic shielding materials in GHz range. Chem Rec 18:1000–1009
Wu N, Hu Q, Wei R, Mai X, Naik N, Pan D, Guo Z, Shi Z (2021) Review on the electromagnetic interference shielding properties of carbon based materials and their novel composites: Recent progress, challenges and prospects. Carbon 176:88–105
Li X, Yu J, Wageh S, Al-Ghamdi AA, Xie J (2016) Graphene in photocatalysis: a review. Small 12:6640–6696
Amollo TA, Mola GT, Kirui MSK, Nyamori VO (2018) Graphene for thermoelectric applications: prospects and challenges. Crit Rev Solid State Mater Sci 43:133–157
Julkapli NM, Bagheri S (2015) Graphene supported heterogeneous catalysts: An overview. Int J Hydrog Energy 40:948–979
Mohan VB, Lau K, Hui D, Bhattacharyya D (2018) Graphene-based materials and their composites: a review on production, applications and product limitations. Compos B: Eng 142:200–220
Tao L, Wang Q, Dou S, Ma Z, Huo J, Wang S, Dai L (2016) Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem Commun 52:2764–2767
Ikeda T, Hou Z, Chai GL, Terakura K (2014) Possible oxygen reduction reactions for graphene edges from first principles. J Phys Chem C 118:17616–17625
Pak AJ, Paek E, Hwang GS (2014) Impact of graphene edges on enhancing the performance of electrochemical double layer capacitors. J Phys Chem C 118:21770–21777
Zhan C, Zhang Y, Cummings PT, Jiang D (2017) Computational insight into the capacitive performance of graphene edge planes. Carbon 116:278–285
Afshari F, Ghaffarian M (2017) Electronic properties of zigzag and armchair graphene nanoribbons in the external electric and magnetic fields. Physica E 89:86–92
Kobayashi Y, Fukui K, Enoki T, Kusakabe K, Kaburagi Y (2005) Observation of zigzag and armchair edges of graphite using scanning tunneling microscopy and spectroscopy. Phys Rev B 71:193406
Kobayashi Y, Fukui K, Enoki T, Kusakabe K (2006) Edge state on hydrogen-terminated graphite edges investigated by scanning tunneling microscopy. Phys Rev B 73:125415
Yamada Y, Kawai M, Yorimitsu H, Otsuka S, Takanashi M, Sato S (2018) Carbon materials with zigzag and armchair edges. ACS Appl Mater Interfaces 10:40710–40739
Kim J, Lee N, Min YH, Noh S, Kim N, Jung S, Joo M, Yamada Y (2018) Distinguishing zigzag and armchair edges on graphene nanoribbons by X-ray photoelectron and Raman spectroscopies. ACS Omega 3:17789–17796
Yamada Y, Tanaka H, Kubo S, Sato S (2021) Unveiling bonding states and roles of edges in nitrogen-doped graphene nanoribbon by X-ray photoelectron spectroscopy. Carbon 185:342–367
Barone V, Hod O, Scuseria GE (2006) Electronic structure and stability of semiconducting graphene nanoribbons. Nano Lett 6:2748–2754
Yamaguchi J, Hayashi H, Jippo H, Shiotari A, Ohtomo M, Sakakura M, Hieda N, Aratani N, Ohfuchi M, Sugimoto Y, Yamada H, Sato S (2020) Small bandgap in atomically precise 17-atom-wide armchair-edged graphene nanoribbons. Commun Mater 1:36
Stein SE, Brown RL (1987) pi.-Electron properties of large condensed polyaromatic hydrocarbons. J Am Chem Soc 109:3721–3729
Yano Y, Mitoma N, Ito H, Itami K (2020) A quest for structurally uniform graphene nanoribbons: synthesis, properties, and applications. J Org Chem 85:4–33
Geoenewege MP (1956) Some regularities in the infra-red spectra of polynuclear aromatic compounds in the C-H wagging region. Spectrochim Acta 11:579–575
Aihara J (1987) Photochemical stability of polycyclic aromatic hydrocarbons in the interstellar medium. Bull Chem Soc Jpn 60:3143–3147
Hony S, Kerckhoven C, Peeters E, Tielens AGGM, Hudgins DM, Allamandola LJ (2001) The CH out-of-plane bending modes of PAH molecules in astrophysical environments. Astron Astrophys 370:1030–1043
Hu A, Duley WW (2008) Surface enhanced Raman spectroscopic characterization of molecular structures in diamond-like carbon films. Chem Phys Lett 450:375–378
Sadjadi S, Zhang Y, Kwok S (2015) On the origin of the 11.3 micron unidentified infrared emission feature. Astrophys J 807(1):95
Pauzat F, Talbi D, Ellinger Y (1997) The PAH hypothesis: a computational experiment on the combined effects of ionization and dehydrogenation on the IR signatures. Astron Astrophys 319:318–330
Centrone A, Brambilla L, Renouard T, Gherghel L, Mathis C, Müllen K, Zerbi G (2005) Structure of new carbonaceous materials: The role of vibrational spectroscopy. Carbon 43:1593–1609
Bauschlicher CW Jr, Peeters E, Allamandola LJ (2009) The infrared spectra of very large irregular polycyclic aromatic hydrocarbons (PAHs): observational probes of astronomical PAH geometry, size and charge. Astrophys J 697:311–327
Tommasini M, Lucotti A, Alfè M, Ciajolo A, Zerbi G (2016) Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy. Spectrochim Acta A 152:134–148
Chang Q, Gao R, Li H, Yu G, Wang F (2018) Effect of CO2 on the characteristics of soot derived from coal rapid pyrolysis. Combust Flame 197:328–339
Wu B, Geng D, Guo Y, Huang L, Xue Y, Zheng J, Chen J, Yu G, Liu Y, Jiang L, Hu W (2011) Equiangular hexagon-shape-controlled synthesis of graphene on copper surface. Adv Mater 23:3522–3525
Mohsin A, Liu L, Liu P, Deng W, Ivanov IN, Li G, Dyck EO, Duscher G, Dunlap RJ, Xiao K, Gu G (2013) Synthesis of millimeter-size hexagon-shaped graphene single crystals on resolidified copper. ACS Nano 7:8924–8931
Ma T, Ren W, Zhang X, Liu Z, Gao Y, Yin L, Ma X, Ding F, Cheng H (2013) Edge-controlled growth and kinetics of single-crystal graphene domains by chemical vapor deposition. Proc Natl Acad Sci USA 110:20386–20391
Yu Q, Jauregui LA, Wu W, Colby R, Tian J, Su Z, Cao H, Liu Z, Pandey D, Wei D, Chung TF, Peng P, Guisinger NP, Stach EA, Bao J, Pei S, Chen YP (2011) Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat Mater 10:443–449
Wu B, Geng D, Xu Z, Guo Y, Huang L, Xue Y, Chen J, Yu G, Liu Y (2013) Self-organized graphene crystal patterns. NPG Asia Mater 5:e36
Simonov KA, Vinogradov NA, Vinogradov AS, Generalov AV, Zagrebina EM, Mårtensson N, Cafolla AA, Carpy T, Cunniffe JP, Preobrajenski AB (2014) Effect of substrate chemistry on the bottom-up fabrication of graphene nanoribbons: combined core-level spectroscopy and STM study. J Phys Chem C 118:12532–12540
Kimouche A, Ervasti MM, Drost R, Halonen S, Harju A, Joensuu PM, Sainio J, Peter L (2015) Ultra-narrow metallic armchair graphene nanoribbons. Nat Commun 6:10177
Jordan RS, Li YL, Lin C, McCurdy RD, Lin JB, Brosmer JL, Marsh KL, Khan SI, Houk KN, Kaner RB, Rubin Y (2017) Synthesis of N = 8 Armchair Graphene Nanoribbons from Four Distinct Polydiacetylenes. J Am Chem Soc 139:15878–15890
Cai J, Ruffieux P, Jaafar R, Bieri M, Braun T, Blankenburg S, Muoth M, Seitsonen AP, Saleh M, Feng X, Müllen K, Fasel R (2010) Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466:470–473
Chen X, Liu S, Liu L, Liu X, Liu X, Wang L (2012) Growth of triangle-shape graphene on Cu(111) surface. Appl Phys Lett 100:163106
Wei E, Jin J, Wang Z, Lu Y, Wang L (2017) Spatially resolving and energy splitting of edge state in zigzag edged triangle graphene quantum dots on Cu(111) surface. Phys E Low Dimens Syst Nanostruct 89:10–14
Bronner C, Marangoni T, Rizzo DJ, Durr RA, Jørgensen JH, Fischer FR, Crommie MF (2017) Iodine versus bromine functionalization for bottom-up graphene nanoribbon growth: role of diffusion. J Phys Chem C 121:18490–18495
Chen Z, Zhang W, Palma CA, Rizzini AL, Liu B, Abbas A, Richter N, Martini L, Wang X, Cavani N, Lu H, Mishra N, Coletti C, Berger R, Klappenberger F, Kläui M, Candini A, Affronte M, Zhou C, Renzi VD, Pennino U, Barth JV, Räder HJ, Narita A, Feng X, Müllen K (2016) Synthesis of graphene nanoribbons by ambient-pressure chemical vapor deposition and device integration. J Am Chem Soc 138:15488–15496
Yang W, Lucotti A, Tommasini M, Chalifoux WA (2016) Bottom-up synthesis of soluble and narrow graphene nanoribbons using alkyne benzannulations. J Am Chem Soc 138:9137–9144
Narita A, Verzhbitskiy IA, Frederickx W, Mali KS, Jensen SA, Hansen MR, Bonn M, Feyter S, Casiraghi C, Feng X, Müllen K (2014) Bottom-up synthesis of liquid-phase-processable graphene nanoribbons with near-infrared absorption. ACS Nano 8:11622–11630
Suenaga K, Koshino M (2010) Atom-by-atom spectroscopy at graphene edge. Nature 468:1088–1090
Girit CO, Meyer JC, Erni R, Rossell MD, Kisielowski C, Yang L, Park CH, Crommie MF, Cohen ML, Louie SG, Zettl A (2009) Graphene at the edge: stability and dynamics. Science 323:1705–1708
Kim K, Coh S, Kisielowski C, Crommie MF, Louie SG, Cohen ML, Zettl A (2013) Atomically perfect torn graphene edges and their reversible reconstruction. Nat Commun 4:2723
Yamada Y, Murota K, Fujita R, Kim J, Watanabe A, Nakamura M, Sato S, Hata K, Peter E, Ciston J, Song C, Kim K, Regan W, Gannett W, Zettl A (2014) Subnanometer vacancy defects introduced on graphene by oxygen gas. J Am Chem Soc 136:2232–2235
Sasaki T, Yamada Y, Sato S (2018) Quantitative analysis of zigzag and armchair edges on carbon materials with and without pentagons using infrared spectroscopy. Anal Chem 90:10724–10731
Fuente E, Menéndez JA, Díez MA, Suárez D, Montes-Morán MA (2003) Infrared spectroscopy of carbon materials: a quantum chemical study of model compounds. J Phys Chem B 107:6350–6359
Yamada Y, Masaki S, Sato S (2020) Brominated positions on graphene nanoribbon analyzed by infrared spectroscopy. J Mater Sci 55:10522–10542. https://doi.org/10.1007/s10853-020-04786-1
Kanazawa S, Yamada Y, Sato S (2021) Infrared spectroscopy of graphene nanoribbons and aromatic compounds with sp3C-H (methyl or methylene groups). J Mater Sci 56:12285–12314. https://doi.org/10.1007/s10853-021-06001-1
Yamada Y, Gohda S, Abe K, Togo T, Shimano N, Sasaki T, Tanaka H, Ono H, Ohba T, Kubo S, Ohkubo T, Sato S (2017) Carbon materials with controlled edge structures. Carbon 122:694–701
Tommasini M, Lucotti A, Alfè M, Ciajolo A, Zerbi G (2016) Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 152:134–148
Diana N, Yamada Y, Gohda S, Ono H, Kubo S, Sato S (2021) Carbon materials with high pentagon density. J Mater Sci 56:2912–2943. https://doi.org/10.1007/s10853-020-05392-x
Eckmann A, Felten A, Mishchenko A, Britnell L, Krupke R, Novoselov KS, Casiraghi C (2012) Probing the nature of defects in graphene by raman spectroscopy. Nano Lett 12:3925–3930
Smith MW, Dallmeyer I, Johnson TJ, Brauer CS, McEwen JS, Espinal JF, Garcia-Perez M (2016) Structural analysis of char by Raman spectroscopy: improving band assignments through computational calculations from first principles. Carbon 100:678–692
Enoki T, Fujii S, Takai K (2012) Zigzag and armchair edges in graphene. Carbon 50:3141–3145
Kitao T, MacLean MWA, Nakata K, Takayanagi M, Nagaoka M, Uemura T (2020) Scalable and precise synthesis of armchair-edge graphene nanoribbon in metal-organic framework. J Am Chem Soc 142:5509–5514
Mazur AS, Vovk MA, Tolstoy PM (2020) Solid-state 13C NMR of carbon nanostructures (milled graphite, graphene, carbon nanotubes, nanodiamonds, fullerenes) in 2000–2019: a mini-review. Fuller Nanotub Carbon Nanostruct 28:202–213
de Souza FAL, Ambrozio AR, Souza ES, Cipriano DF, Scopel WL, Freitas JCC (2016) NMR spectral parameters in graphene, graphite, and related materials: ab initio calculations and experimental results. J Phys Chem C 120:27707–27716
He H, Riedl T, Lerf A, Klinowski J (1996) Solid-state NMR studies of the structure of graphite oxide. J Phys Chem 100:19954–19958
Kato T, Yamada Y, Nishikawa Y, Ishikawa H, Sato S (2021) Carbonization mechanisms of polyimide: methodology to analyze carbon materials with nitrogen, oxygen, pentagons, and heptagons. Carbon 178:58–80
Gohda S, Yamada Y, Murata M, Saito M, Kanazawa S, Ono H, Sato S (2020) Bottom-up synthesis of highly soluble carbon materials. J Mater Sci 55:11808–11828. https://doi.org/10.1007/s10853-020-04813-1
Yamada Y, Yasuda H, Murota K, Nakamura M, Sodesawa T, Sato S (2013) Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J Mater Sci 48:8171–8198. https://doi.org/10.1007/s10853-013-7630-0
Yamada Y, Kim J, Matsuo S, Sato S (2014) Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy. Carbon 70:59–74
Kato T, Yamada Y, Nishikawa Y, Otomo T, Sato H, Sato S (2021) Origins of peaks of graphitic nitrogen in N1s X-ray photoelectron spectra of carbon materials: quaternary or tertiary nitrogen? J Mater Sci 56:15798–15811. https://doi.org/10.1007/s10853-021-06283-5
Senda T, Yamada Y, Morimoto M, Nono N, Sogabe T, Kubo S, Sato S (2019) Analyses of oxidation process for isotropic pitch-based carbon fiber using model compounds. Carbon 142:311–326
Kim J, Yamada Y, Suzuki Y, Ciston J, Sato S (2014) Pyrolysis of epoxidized fullerenes analyzed by spectroscopies. J Phys Chem C 118:7076–7084
Kanazawa S, Yamada Y, Gohda S, Sato S (2021) Bottom-up synthesis of oxygen-containing carbon materials using a lewis acid catalyst. J Mater Sci 56:15698–15717. https://doi.org/10.1007/s10853-021-06284-4
Susi T, Pichler T, Ayala P (2015) X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J Nanotechnol 6:177–192
Yamada Y, Suzuki Y, Yasuda H, Uchizawa S, Hirose-Takai K, Sato Y, Suenaga K, Sato S (2014) Functionalized graphene sheets coordinating metal cations. Carbon 75:81–94
Yamada Y, Miyauchi M, Kim J, Takai KH, Sato Y, Suenaga K, Ohba T, Sodesawa T, Sato S (2011) Exfoliated graphene ligands stabilizing copper cations. Carbon 49:3375–3378
Fujimoto A, Yamada Y, Koinuma M, Sato S (2016) Origins of sp3C peaks in C1s X-ray photoelectron spectra of carbon materials. Anal Chem 88:6110–6114
Susi T, Kaukonen M, Havu P, Ljungberg MP, Ayala P, Kauppinen EI (2014) Core level binding energies of functionalized and defective graphene. Beilstein J Nanotechnol 5:121–132
Kim J, Yamada Y, Kawai M, Tanabe T, Sato S (2015) Spectral change of simulated X-ray photoelectron spectroscopy from graphene to fullerene. J Mater Sci 50:6739–6747. https://doi.org/10.1007/s10853-015-9229-0
Kim J, Lee N, Choi DY, Kim DY, Kawai R, Yamada Y (2021) Pentagons and heptagons on edges of graphene nanoflakes analyzed by X-ray photoelectron and Raman spectroscopies. J Phys Chem Lett 12:9955–9962
Kim J, Han JW, Yamada Y (2021) Heptagons in the basal plane of graphene nanoflakes analyzed by simulated X-ray photoelectron spectroscopy. ACS Omega 6:2389–2395
Kelemen SR, Rose KD, Kwiatek PJ (1993) Carbon aromaticity based on XPS II to II∗ signal intensity. Appl Surf Sci 64:167–173
Estrade-Szwarckopf E (2004) XPS photoemission in carbonaceous materials: a defect peak beside the graphitic asymmetric peak. Carbon 42:1713–1721
Smith M, Scudiero L, Espinal J, McEwen JS, Garcia-Perez M (2016) Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon 110:155–171
Gaussian 09, Revision A.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2009) Gaussian 09, revision E.01. Gaussian Inc, Wallingford, CT
Kojima I, Fukumoto N, Kurahashi M (1986) Analysis of X-ray photoelectron spectrum with asymmetric Gaussian-Lorentzian mixed function. Bunseki Kagaku 35:T96-T100. [written in Japanese]
Solà M (2013) Forty years of Clar’s aromatic π-sextet rule. Front Chem 1:1–8
Wu JB, Lin YF, Wang J, Chang PJ, Tasi CP, Lu CC, Chiu HT, Yang YW (2003) Correlation between N 1s XPS binding energy and bond distance in metal amido, imido, and nitrido complexes. Inorg Chem 42:4516–4518
Yamada Y, Tanaka H, Tanaka Y, Kubo S, Taguchi T, Sato S (2022) Toward strategical bottom-up synthesis of carbon materials with exceptionally high pyridinic-nitrogen content: development of screening techniques. Carbon 198:411-434. https://doi.org/10.1016/j.carbon.2022.06.069
Anno T, Ito M, Shimada R, Sado A, Mizushima W (1957) Relations among bond order, force constant and bond length for the C-C and the C-N bond in conjugated molecules. Bull Chem Soc Jpn 30:638–647
Han MY, Özyilmaz B, Zhang Y, Kim P (2007) Energy band-gap engineering of graphene nanoribbons. Phys Rev Lett 98:206805
Zarenia M, Chaves A, Farias GA, Peeters FM (2011) Energy levels of triangular and hexagonal graphene quantum dots: a comparative study between the tight-binding and Dirac equation approach. Phys Rev B 84:245403
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP21K04773.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Conflict of interest the authors declare that they do not have any conflict of interest
Additional information
Handling Editor: Gregory Rutledge.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, K., Kim, J., Kubo, S. et al. Effects of molecular shapes, molecular weight, and types of edges on peak positions of C1s X-ray photoelectron spectra of graphene-related materials and model compounds. J Mater Sci 57, 15789–15808 (2022). https://doi.org/10.1007/s10853-022-07599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07599-6