Abstract
A microwave-assisted hydrothermal synthesis route to prepare LiFePO4 (LFP) in a very short time and under a low temperature is proposed. Only 10 min at 200 °C was sufficient to produce a high-purity, single-phase LFP, with no need to perform a thermal treatment as a second step that is usual to enhance the structural properties of the material. The obtained LFP was structurally characterized by XRD, HR-TEM and FTIR spectroscopy and morphologically analyzed by SEM. Electrochemical impedance spectroscopy data allowed the estimation of the diffusion coefficient for the lithium ions (DLi+ = 2.0 × 10−14 cm2 s−1) in the LFP structure during the redox reactions related to the charge and discharge processes that occur in a Li-ion battery. Preliminary galvanostatic charge and discharge tests of the prepared LFP as a cathode in a Li-ion cell were carried out in a 1.0 mol L−1 LiPF6 EC/DMC 1:1 (V/V) electrolyte solution, allowing the estimation of the initial specific capacity (126 mA h g−1 at 0.1 C) and coulombic efficiency (94–99%). Hence, the here-reported rapid, clean and facile one-step synthesis of LFP allows the production of a material that has excellent structural properties and a promising electrochemical response, with significant savings in time and energy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decrease of oil reserves worldwide, allied with climate changes due to global warming, has motivated the search for new, renewable and clean sources of energy [1]. This search for alternative energy sources has been driven mainly by the automotive sector, which is continuously expanding and has been one of the main drivers for environmental pollution [2]. Hence, electric vehicles, whose energy source for their engines is typically a lithium-ion battery bank [1,2,3,4], have been produced more and more to replace the traditional automobiles powered by internal combustion engines.
Among the lithium-ion battery components, the cathode material is known to be a key item that determines the performance and cost of the device [4,5,6]. A broad variety of materials has been proposed by many research groups for cathodes in Li-ion batteries. Among these materials, lithiated iron phosphate (LiFePO4), first investigated by Padhi et al. [7], has presented promising results [4, 8]. LiFePO4 is considered a good electrode material because of its high values of theoretical and experimental specific capacities [9, 10], low toxicity and high electrochemical stability [11], besides being benign and produced from low-cost precursors. However, LiFePO4 presents low ionic and electronic conductivities (which lead to a decrease in the experimental specific capacity) as well as coulombic efficiency losses during long-term charge–discharge cycling [12, 13].
New synthesis methodologies have been proposed to enhance the conductivity of LiFePO4, focusing on the decrease in particle size, the production of a carbonaceous coating and the obtention of crystalline and high-purity materials [14,15,16,17,18,19,20,21,22,23,24]. Traditionally LiFePO4 is produced by means of solid-state synthesis, when the solid precursor materials are mixed and submitted to different heating steps at temperatures varying in the range of 350–800 °C, in procedures that may last up to 24 h [25, 26]. Although solid-state synthesis may lead to products that present good structural and electrochemical properties, the associated high temperatures and long heating periods make it energetically expensive. In this context, significant energy savings can be attained by the use of hydrothermal synthesis [27,28,29,30] since the precursors (dissolved and mixed in aqueous solutions) are submitted to lower temperatures (around 200 °C) during 5–18 h [31, 32]. The hydrothermal methodology allows the production of nanostructured and high-purity LiFePO4 that leads to a cathode material with high electrochemical performance [33]. Although the latter presents lower energy consumption compared to solid-state synthesis, both methods involve long heating periods due to the inefficient heat exchange (by heat conduction, irradiation and convection) between the heat source and the reaction system. Therefore, the coupling of the hydrothermal methodology with microwave heating improves the heat-transfer efficiency mainly due to the fact that the heat exchange occurs at the molecular level, as a consequence of the direct interaction between microwave radiation and reactants, leading to a significant reduction of the synthesis time [34,35,36,37,38]. For example, Niu et al. [39] produced high-purity LiFePO4 in a two-step procedure, i.e., microwave-assisted hydrothermal synthesis at 180 °C for 1 h, followed by a thermal treatment (TT) at 700 °C for 2 h in a reducing atmosphere. Gao et al. [40] also used a two-step procedure to produce LiFePO4, but the microwave-assisted hydrothermal synthesis was carried out at 160 °C for 20 min (with addition of ascorbic acid to the precursors as reducing agent) and the obtained product was submitted to TT at 700 °C under a nitrogen atmosphere for 3 h.
On top of the lower energy consumption and diminutive synthesis time, most works involving microwave-assisted hydrothermal synthesis have been focusing on enhancing the electrochemical performance of LiFePO4 as cathode material for lithium-ion batteries by covering its particles with a thin conducting layer of carbon. Commonly, this carbonaceous layer is obtained by adding organics to the reaction medium and submitting them to TT in a controlled reducing atmosphere; this leads to a final material with an increased conductivity and thus enhanced electrochemical properties. Despite the excellent results attained with such materials, the second step in their synthesis (TT) causes an increase in the energy consumption and reaction time, which weigh negatively when large large-scale applications are considered. Besides, during TT some undesirable compounds and agglomerates of LiFePO4 particles might be formed, which limit the diffusion of Li ions in the olivine structure, leading to a decreased electrochemical performance [14, 41]. In this sense, Yang et al. [42] investigated the synthesis of LiFePO4 by the microwave-assisted hydrothermal method using classic inorganic precursors and without TT afterward, obtaining a material with good electrochemical performance. Nevertheless, there is not much reported on “one-step” synthesis of LiFePO4, thus leaving open opportunities for investigations on the preparation of this pure material, with adequate crystalline structure and good electrochemical properties, without applying a posterior TT.
In this work, we further investigate the one-step methodology to obtain single-phase high-purity LiFePO4 by a rapid microwave-assisted hydrothermal synthesis under a low temperature, with no posterior TT. The as-obtained product was characterized: structurally, by X-ray diffractometry (XRD) and FTIR spectroscopy; structurally and morphologically, by transmission electron microscopy (TEM) and scanning electron microscopy (SEM); electrochemically, by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and charge–discharge tests as a cathode in a Li-ion cell.
Materials and methods
LiFePO4 synthesis
For the LiFePO4 synthesis, the precursor materials were mixed in a Li:Fe:P 3:1:1 mol ratio. First of all, lithium acetate (CH3CO2Li.2H2O 98%, Sigma-Aldrich) was dissolved in deionized water, and phosphoric acid (H3PO4 85%, Synth) was added to this solution, at room temperature. A white precipitate, typical of Li3PO4, was formed and the mixture was stirred for 20 min with a magnetic bar and a N2 flow through it. Iron sulfate (FeSO4.7H2O 99%, Sigma-Aldrich) was then added to the reaction mixture, which was kept under stirring and N2 bubbling for additional 10 min. Then, approximately 20 mL of the resulting greenish and viscous mixture was introduced into a 30-mL borosilicate reaction vessel and heated at 200 °C for 10 min under microwave radiation, in a Monowave 400 (Anton Paar) reactor. The obtained product was washed several times with deionized water (up to neutral pH) and then dried in an oven at 60 °C for 12 h, resulting in a greenish powder as the final reaction product.
It is important to mention that this described procedure was repeated several times in order to obtain an appreciable amount of LiFePO4 (few grams); FTIR spectra of LiFePO4 samples synthesized in the different runs did not show significant variations.
Structural and morphological characterization
XRD spectra were obtained with a Rigaku D/MAX-2000PC diffractometer using a Cu Kα radiation source at 40 kV/150 mA. From the obtained data, acquired in the 5–80° range at a rate of 1° min–1, the crystalline phase was identified by comparison with Joint Committee of Powder Diffraction Standards (JCPDS) data. FTIR spectra were acquired from 400 to 4000 cm–1, using a Thermo Scientific model Nicolet 6700 spectrometer.
SEM images were obtained with a FEI Magellan 400L (2 kV) microscope; TEM and selected-area electron diffraction (SAED) images were acquired with a Supra-35 ZEISS FESEM system in a high-resolution FEI (MET-AR) Tecnai G2 (200 kV) microscope. The SEM and TEM results were analyzed with the ImageJ software [43].
Electrochemical characterization
The working electrodes were prepared as a composite of LiFePO4/carbon black/PVDF at a 70:20:10 mass ratio in cyclopentanone. The resulting composite slurry was applied on Pt and Al current collectors and then casted in a vacuum oven at 120 °C. The Pt and Al electrodes were employed, respectively, for CV and galvanostatic charge–discharge/EIS tests. Assembling of the electrochemical cells was carried out in a Labconco® model 506,000 glove box under an argon (99.999%) atmosphere. Metallic lithium was used as the counter and reference electrodes for the CV tests (0.1 mV s−1) in a 1.0 mol L−1 LiClO4 EC/DMC 1:1 (V/V) electrolyte solution in the potential range of 2.4–4.2 V versus Li/Li+.
The charge–discharge tests were performed in a 1.0 mol L−1 LiPF6 EC/DMC 1:1 (V/V) electrolyte solution using a Swagelok®-type cell at various current densities, keeping the potential window between 2.4 and 4.5 V versus Li/Li+. Both CV and charge–discharge experiments were carried out using a Metrohm-Autolab PGSTAT204 interface.
The EIS measurements were carried out in a Swagelok®-type cell having as electrolyte a 1.0 mol L–1 LiPF6 EC/DMC 1:1 (V/V) solution, using an Autolab PGSTAT20 interface. For such, a 10 mV peak-to-peak AC signal, from 10 kHz to 10 mHz, was applied at the open-circuit potential (OCP).
All electrochemical measurements were always carried out in triplicate.
Results and discussion
Structural and morphological analyses of the synthesized LiFePO4
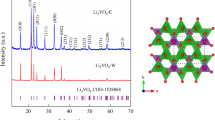
An obtained XRD spectrum, characteristic of the LFP samples, is presented in Fig. 1a. Well-defined peaks can be seen at 17.2°, 20.9°, 25.7°, 30.0°, 32.2°, 35.6° and 52.6°, which were indexed to the diffraction lines (200), (101), (111/201), (211/020), (301), (311) and (222), respectively, of the orthorhombic crystalline phase of pure LiFePO4 (JPCDS 40–1499; see inset in Fig. 1a). A high degree of crystallinity for the synthesized material can be inferred from the high definition of the peaks, whereas the more intense peak at 30.0° (peak 211) is an indication of the particle preferential growth along the {001} and {100} planes, or ac plane in the orthorhombic LiFePO4 crystal (space group Pnma), a fact that favors Li+ diffusion along the shorter plane (or b-axis of the crystal) [44]. Based on this more intense peak, the mean size (Lm) of the LiFePO4 particles was calculated using the Debye–Scherrer equation:
where \(K\) is a constant (\(0.94\)), \(\lambda\) the X-ray wavelength (1.5418 nm) and \(\beta\) the width (in radians) at the half-height of the more intense peak, whose Bragg angle is \(\theta\). The thus obtained nanometric mean size of the synthesized LiFePO4 particles, 41.8 nm, represents a short diffusional path for the lithium ions in the LFP structure and might contribute positively for its electrochemical performance when tested as a cathode material in Li-ion batteries.
Figure 1b shows a typical FTIR spectrum for the as-synthesized LiFePO4. The predominance of the internal vibrational modes of the phosphate group can be seen in the 400–2000 cm−1 region of the spectrum [45]. According to the interactions of the atoms in PO43– with the lithium and iron ions in the crystal, the position and intensity of the FTIR bands may change significantly and lead to information on the crystallinity, purity and composition of the obtained material [45,46,47,48,49].
Thus, the well-defined bands in the region of 400–690 cm−1 are signatures of the vibrational modes \(v_{2}\) and \(v_{4}\) of the phosphate group, whereas the bands in the region of 700–1139 cm–1 refer to the internal vibrational modes \(v_{1}\) and \(v_{3}\) of the phosphate group in the LiFePO4 structure [45]. The lack of bands in the region of 700–900 cm−1 is a strong indication of the high purity of the obtained material, since bands in this region of the spectrum are characteristic of other phosphate complexes such as P2O74−, P3O105− and FePO4, which could be formed as byproducts during the synthesis [47, 48].
The morphology and mean size distribution of the LFP particles are presented in Fig. 2a and b, respectively. The morphology observed for this material is basically globular; its mean particle size (57.4 ± 1.8 nm) was calculated based on the lognormal distribution presented in Fig. 2b. Although this value is higher than the one obtained using the Debye–Scherrer Eq. (41.8 nm), the results indicate a good agreement between the methods. Additionally, as can be inferred from Fig. 2b, the majority of the LFP particles present sizes close to the mean value; this narrow distribution of particle size attained by the herein-proposed synthesis may represent an advantage in comparison with other methods, since no further processes are needed to attain such particle size homogeneity [28]. On the other hand, it is clear from the TEM image (Fig. 2c) that the LFP sample is highly crystalline, with a tendency of crystal growth along one preferential plane. Furthermore, the dotted pattern of the SAED image in Fig. 2d is another evidence for the monocrystalline domain of the synthesized LFP sample, with two main diffraction lines: 211 and 311. In summary, both the TEM and SAED results corroborate the XRD findings presented in Fig. 1a.
Electrochemical characterization
The electrochemical activity of the obtained LFP was initially evaluated by CV at a slow sweep rate (0.1 mV s−1) in the potential range of 2.4–4.2 V versus Li/Li+ in a 1.0 mol L−1 LiClO4 EC/DMC 1:1 (V/V) electrolyte solution. As illustrated in Fig. 3, the typical obtained voltammogram presents well-defined oxidation and reduction peaks at 3.5 and 3.4 V, respectively, corresponding to the Fe3+/Fe2+ redox reactions of a LiFePO4 cathode that occur during the charging and discharging processes in a lithium-ion cell.
The charging process occurs simultaneously with the oxidation of Fe2+ and extraction of Li+ from the olivine structure (Eq. 2), while Fe3+ is reduced and Li+ inserted in the material structure during the discharging process (Eq. 3).
The potential value between the oxidation and reduction peaks is 3.45 V versus Li/Li+, which approximately corresponds to the OCP of the working electrode; the two phases LiFePO4 and FePO4, characteristic of the charging and discharging processes, coexist around this value of the electrode potential [50, 51]. The peak-potential separation for the redox process is about 100 mV, indicating that the prepared electrode presents good reversibility. Besides that, the absence of additional voltammetric peaks in Fig. 3 is another evidence that other Fe compounds (byproducts or impurities) were not formed during the LFP synthesis and that the electrolyte is electrochemically stable in the considered potential range.
The obtained EIS data were analyzed to access information on processes that occur in the phases and at the LiFePO4/electrolyte interface. Typical EIS data obtained for the here-synthesized LiFePO4 are presented as complex plane (or Nyquist) and Bode plots in Fig. 4a and 4b, respectively. The semicircle in the Nyquist plot can be attributed to a parallel RC circuit relaxing at the highest frequencies in the analyzed system. The linear region at lower frequencies can be attributed to diffusional or capacitive processes occurring in the phases or at the interfaces. Therefore, an equivalent electric circuit (EEC) model (see inset in Fig. 4a) was proposed to simulate and interpret the obtained impedance data, with the following elements: the ohmic resistance (RΩ) related to the ionic resistance of the electrolyte; the charge-transfer resistance (Rct) at the LFP/electrolyte interface; the resistance associated with a solid electrolyte interface (RSEI) formed on the electrode [52]; non-ideal capacitive processes (CPE1 and CPE2) related to changes in the thickness, roughness and porosity of the electrode material [53]—CPE1 was attributed to the double-layer charge at the LiFePO4/electrolyte interface and CPE2 to the spatial capacitance defined by the LFP layer; the Warburg impedance (W) related to Li-ion diffusion in the electroactive-material channels.
a Typical complex plane and b Bode plots for the LiFePO4 (LFP) sample obtained by microwave-assisted hydrothermal synthesis at 200 °C for 10 min. The equivalent circuit whose response (red lines) fitted the experimental EIS data is shown as an inset in a. Electrolyte solution: 1.0 mol L−1 LiPF6 in EC/DMC 1:1 (V/V)
The good fitting of the continuous lines in Fig. 4a and b to the EIS data indicates a good agreement between the response of the proposed EEC and the experimental data; this is confirmed by the low Chi-square value of 3.7 × 10−5 for this fitting. The fitted values for the different EEC elements are listed in Table 1.
The analysis of the impedance data in the low-frequency region allowed estimating the diffusion coefficient of Li ions in the LFP structure. Indeed, a linear relationship between the real impedance values and those of the inverse of the square root of the angular frequency was obtained (Fig. 5); the Li-ion diffusion coefficient was calculated from the slope (\(\sigma_{{\text{w}}}\)) of this line, using the following equation [54, 55]:
where \(D_{{{\text{Li}}^{ + } }}\) is the Li-ion diffusion coefficient (cm2 s−1), R the universal gas constant (8.314 J K−1 mol−1), T the thermodynamic temperature (298 K), A the electrode geometric area (0.785 cm2), F the Faraday constant (96,485 C mol–1), C the concentration of Li+ (0.0228 mol cm−3, assuming a mass density of 3.6 g cm−3 and a molar mass of 157.78 g mol−1 for LiFePO4) and n the number of electrons transferred in the redox reaction of the Fe3+/Fe2+ redox couple.
A DLi+ value of 2.0 × 10−14 cm2 s−1 was obtained for the here-synthesized LFP sample, in agreement with other values reported for high-performance LiFePO4 electrodes [12, 44, 56, 57]. This result may be related to the particle preferential growth along the {001} and {100} planes, which favors Li+ diffusion along the shorter {010} plane. Li-ion diffusion takes place only along the b-axis during charge and discharge, and the charge transfer takes place mainly on the ac plane. A decrease in the diffusion length along the {010} plane makes the insertion/extraction process faster and, consequently, improves the electrochemical performance of the electrode [58, 59]. The proportion between the {001} and {100} planes can be estimated from the peak intensity ratio I(020)/I(200) in the X-ray diffractograms of LiFePO4 [60] and was proposed to give an idea about the preferential direction of the particle growth. In the present work, the ratio I(020)/I(200) that is shown in Fig. 1 points toward the preferential growth along the ac plane.
Galvanostatic charge and discharge tests
Preliminary charge and discharge tests were carried out to assess the performance of the here-synthesized LiFePO4. Figure 6a shows typical charge and discharge profiles obtained at the 0.1 C rate in the potential range of 2.4–4.5 V versus Li/Li+ for the cell:
a Typical charge and discharge profiles at the 0.1 C rate and b specific capacity (Cs) as a function of different charge–discharge rates (indicated in the figure) for the LiFePO4 sample obtained by microwave-assisted hydrothermal synthesis at 200 °C for 10 min. Electrolyte solution: 1.0 mol L−1 LiPF6 in EC/DMC 1:1 (V/V)
The chronopotentiometric profiles obtained for the LFP electrode present a neat plateau around 3.45 V, related to the redox processes represented by Eqs. 2 and 3; its initial specific capacity was calculated as 126 mA h g−1 at a discharge rate of 0.1 C. Besides, from the potential gap values between the charge and discharge plateaus (70 mV), one can infer a high reversibility for this electrode prepared by the microwave-assisted hydrothermal method.
The LFP electrode was submitted to several charge–discharge rates (0.1, 0.2, 0.25, 0.33 and 0.5 C) to assess its rate capability in comparison with the 0.1 C rate. The respective results are presented in Fig. 6b. When the charge–discharge rate was doubled to 0.2 C, the charge retention from the initial value (126 mA h g−1) was of 86%, while it decreased to 62% when the charge–discharge rate was increased fivefold (to 0.5 C). After these successive charge–discharge cycles, the electrode was submitted again to the lowest charge–discharge rate (0.1 C), when 95% of its initial charge was recovered, attesting once again the excellent properties of the here-prepared material.
Finally, it is noteworthy that the electrochemical performance of the obtained LFP electrode is comparable to that of others whose LFP was similarly synthesized (MWH methodology) and then submitted (two-step process) [39, 40, 61,62,63] or not (one-step process) [42] to a TT to obtain better structural properties or to increase its conductivity by generating a carbon coating on its particles (Table 2). Analyzing this table, it is clear that the rapid, clean and facile one-step synthesis here reported leads to the production of a competitive LFP with excellent structural characteristics and good electrochemical properties at shorter times and under a lower temperature. These results may open the possibility of applying the thus obtained LFP as a cathode material of lithium-ion batteries without the addition of a carbonaceous material and the further heat treatment that increases the synthesis time and final cost of the material, despite the eventual increase in the values of specific capacity after this two-step process [40, 61,62,63].
Conclusions
A rapid one-step procedure based on a microwave-assisted hydrothermal reaction was successfully employed to prepare LiFePO4 (LFP) under a low temperature (10 min at 200 °C). High-purity and single-phase LFP with good electrochemical properties was obtained, without submitting it to a posterior thermal treatment as is usual in other hydrothermal synthesis routes to enhance the properties of the material. The diffusion coefficient of lithium ions in the LFP structure during the charge and discharge processes was estimated as 2.0 × 10−14 cm2 s–1. Preliminary charge and discharge tests of the here-prepared LiFePO4 as a cathode in a Li-ion cell allowed the estimation of its initial specific capacity as 126 mA h g−1 at the 0.1 C rate. Thus, the rapid, clean and facile one-step synthesis of LFP here reported allows the production of a material that has excellent structural and promising electrochemical properties, with significant savings in time and energy.
References
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29
Tie SF, Tan CW (2013) A review of energy sources and energy management system in electric vehicles. Renew Sust Energ Rev 20:82–102
Weiss M, Patel MK, Junginger M, Perujo A, Bonnel P, Grootveld G (2012) On the electrification of road transport: learning rates and price forecasts for hybrid-electric and battery-electric vehicles. Energ Policy 48:374–393
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18:252–264
Goodenough JB, Kim Y (2010) Challenges for rechargeable li batteries. Chem Mater 22:587–603
Manthiram A (2011) Materials challenges and opportunities of lithium ion batteries. J Phys Chem Lett 2:176–184
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Kurzweil P (2015) Lithium battery energy storage: state of the art including lithium-air and lithium-sulfur systems. In: Moseley PT, Garche J (eds) Electrochemical Energy Storage for Renewable Sources and Grid Balancing. Elsevier, Amsterdam, pp 269–307
Chen J, Vacchio MJ, Wang S, Chernova N, Zavalij PY, Whittingham MS (2008) The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ionics 178:1676–1693
Huang Z, Luo P, Wang D (2017) Preparation and characterization of core-shell structured LiFePO4/C composite using a novel carbon source for lithium-ion battery cathode. J Phys Chem Solids 102:115–120
Qiao YQ, Feng WL, Li J, Shen TD (2017) Ultralong cycling stability of carbon-nanotube/LiFePO4 nanocomposites as electrode materials for lithium-ion batteries. Electrochim Acta 232:323–331
Prosini PP, Lisi M, Zane D, Pasquali M (2002) Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ionics 148:45–51
Zaghib K, Guerfi A, Hovington P, Vijh A, Trudeau M, Mauger A, Goodenough JB, Julien CM (2013) Review and analysis of nanostructured olivine-based lithium recheargeable batteries: status and trends. J Power Sources 232:357–369
Zhang L, Xiang H, Zhu X, Yang W, Wang H (2012) Synthesis of LiFePO4/C composite as a cathode material for lithium-ion battery by a novel two-step method. J Mater Sci 47:3076–3081. https://doi.org/10.1007/s10853-011-6139-7
Qiu Y, Geng Y, Yu J, Zuo X (2014) High-capacity cathode for lithium-ion battery from LiFePO4/(C + Fe2P) composite nanofibers by electrospinning. J Mater Sci 49:504–509. https://doi.org/10.1007/s10853-013-7727-5
Yang Z, Dai Y, Wang S, Yu J (2016) How to make lithium iron phosphate better: a review exploring classical modification approaches in-depth and proposing future optimization methods. J Mater Chem A 4:18210–18222
Smecellato PC, Davoglio RA, Biaggio SR, Bocchi N, Rocha-Filho RC (2017) Alternative route for LiFePO4 synthesis: Carbothermal reduction combined with microwave-assisted solid-state reaction. Mater Res Bull 86:209–214
Zhao C, Wang L, Wu H, Chen J, Gao M (2018) Ultrafast fabrication of LiFePO4 with high capacity and superior rate cycling performance for lithium ion batteries. Mater Res Bul 97:195–200
Hu G, Xie X, Cao Y, Xu L, Du K, Wang W, Peng Z (2019) Ultrasonic-assisted synthesis of LiFePO4/C composite for lithium-ion batteries using iron powder as the reactant. J Alloy Compd 773:1165–1171
Xie X, Hu G, Cao Y, Du K, Gan Z, Xu L, Wang Y, Peng Z (2019) Rheological phase synthesis of Fe2P2O7/C composites as the precursor to fabricate high performance LiFePO4/C composites for lithium-ion batteries. Ceram Inter 45:12331–12336
Rajoba SJ, Jadhav LD, Kalubarme RS, Yadav SN (2019) Influence of synthesis parameters on the physicochemical and electrochemical properties of LiFePO4 for Li-ion battery. J Alloy Compd 774:841–847
Zhou W, Liu C, Wen Z, Xu J, Han T, Li G, Huang D, Liang X, Lan Z, Ning H, Huang H, Guo J (2019) Effects of defect chemistry and kinetic behavior on electrochemical properties for hydrothermal synthesis of LiFePO4/C cathode materials. Mater Chem Phys 227:56–63
Sun J, Li Z, Ren X, Wang L, Liang G (2019) High volumetric energy density of LiFePO4/C microspheres based on xylitol-polyvinyl alcohol complex carbon sources. J Alloy Compd 773:788–795
Omidi AH, Babaei A, Ataie A (2020) Low temperature synthesis of nanostructured LiFePO4/C cathode material for lithium ion batteries. Mater Res Bull 125:110807
Jugović D, Uskoković D (2009) A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J Power Sources 190:538–544
Calderón CA, Thomas JE, Lener G, Barraco DE, Visintin A (2017) Electrochemical comparison of LiFePO4 synthesized by a solid-state method using either microwave heating or a tube furnace. J Appl Electrochem 47:1179–1188
Bao L, Xu G, Sun X, Zeng H, Zhao R, Yang X, Shen G, Han G, Zhou S (2017) Mono-dispersed LiFePO4@C core-shell [001] nanorods for a high power Li-ion battery cathode. J Alloy Compd 708:685–693
Milev A, George L, Khan S, Selvam P, Kamali Kannangara GS (2016) Li-ion kinetics in LiFePO4/carbon nanocomposite prepared by a two-step process: the role of phase composition. Electrochim Acta 209:565–573
Bolloju S, Rohan R, Wu S-T, Yen H-X, Dwivedi GD, Lin YA, Lee JT (2016) A green and facile approach for hydrothermal synthesis of LiFePO4 using iron metal directly. Electrochim Acta 220:164–168
Kumar S, Chand P, Joshi A, Singh V (2020) Modeling of electrical behavior of LiFePO4 cathode materials for lithium ion batteries. Mater Today-Proc 28:337–341
Wang Y, Zhang D, Chang C, Deng L, Huang K (2014) Controllable growth of LiFePO4 microplates of (010) and (001) lattice planes for Li ion batteries: a case of the growth manner on the Li ion diffusion coefficient and electrochemical performance. Mater Chem Phys 148:933–939
Julien CM, Mauger A, Zaghib K (2011) Surface effects on electrochemical properties of nano-sized LiFePO4. J Mater Chem 21:9955–9968
Satyavani TVSL, Srinivas Kumar A, Subba Rao PSV (2016) Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: a review. Eng Sci Technol Inter J 19:178–188
Tompsett Geoffrey A, Conner William C, Yngvesson KS (2006) Microwave synthesis of nanoporous materials. Chem Phys Chem 7(2):296–319
Meng L-Y, Wang B, Ma M-G, Lin K-L (2016) The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater Today Chem 1–2:63–83
Liu S, Yan P, Li H, Zhang X, Sun W (2020) One-step microwave synthesis of micro/nanoscale LiFePO4/graphene cathode with high performance for lithium-ion batteries. Front Chem 8:104. https://doi.org/10.3389/fchem.2020.00104
Ruiz-Jorge F, Benítez A, Fernández-García S, Sánchez-Oneto J, Portela JR (2020) Effect of fast heating and cooling in the hydrothermal synthesis on LiFePO4 microparticles. Ind Eng Chem Res 59:9318–9327
Su L, Jha SK, Phuah XL, Xu J, Nakamura N, Wang H, Okasinski JS, Reeja-Jayan B (2020) Engineering lithium-ion battery cathodes for high-voltage applications using electromagnetic excitation. J Mater Sci 55:12177–12190. https://doi.org/10.1007/s10853-020-04871-5
Niu B, Qi E-l, Wang J-Q (2011) A simple and facile preparation of LiFePO4 by a one-step microwave hydrothermal method. J Inorg Organomet P 21:906–912
Gao C, Zhou J, Liu G, Wang L (2017) Microwave-assisted synthesis and surface decoration of LiFePO4 hexagonal nanoplates for lithium-ion batteries with excellent electrochemical performance. J Mater Sci 52:1590–1602. https://doi.org/10.1007/s10853-016-0453-z
Yuan L-X, Wang Z-H, Zhang W-X, Hu X-L, Chen J-T, Huang Y-H, Goodenough JB (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energ Environ Sci 4:269–284
Yang G, Ji H, Miao X, Hong A, Yan Y (2011) Crystal growth behavior of LiFePO4 in microwave-assisted hydrothermal condition: from nanoparticle to nanosheet. J Nanosci Nanotechno 11(6):4781–4792
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Churikov A, Ivanishchev A, Ivanishcheva I, Sycheva V, Khasanova N, Antipov E (2010) Determination of lithium diffusion coefficient in LiFePO4 electrode by galvanostatic and potentiostatic intermittent titration techniques. Electrochim Acta 55:2939–2950
Burba CM, Frech R (2004) Raman and FTIR Spectroscopic Study of LixFePO4 (0 ≤ x ≤ 1). J Electrochem Soc 151:A1032–A1038
Ravet N, Gauthier M, Zaghib K, Goodenough J, Mauger A, Gendron F, Julien CM (2007) Mechanism of the Fe3+ reduction at low temperature for LiFePO4 synthesis from a polymeric additive. Chem Mater 19:2595–2602
Salah AA, Jozwiak P, Zaghib K, Garbarczyk J, Gendron F, Mauger A, Julien CM (2006) FTIR features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim Acta A Mol Biomol Spectrosc 65:1007–1013
Zaghib K, Julien C (2005) Structure and electrochemistry of FePO4.2H2O hydrate. J Power Sources 142:279–284
Paques-Ledent MT, Tarte P (1974) Vibrational studies of olivine-type compounds—II Orthophosphates,-arsenates and-vanadates AIBIIXVO4. Spectrochim Acta A Mol Spectrosc 30:673–689
Mei R, Song X, Yang Y, An Z, Zhang J (2014) Plate-like LiFePO 4 crystallite with preferential growth of (010) lattice plane for high performance Li-ion batteries. RSC Adv 4:5746–5752
Lin J-H, Chen J-S (2012) Synthesis and electrochemical characterization of LiFePO4/C composites prepared by the microemulsion method. Electrochim Acta 62:461–467
Xi Y, Liu Y, Qin Z, Jin S, Zhang D, Zhang R, Jin M (2018) Ultralong cycling stability of cotton fabric/LiFePO4 composites as electrode materials for lithium-ion batteries. J Alloy Compd 737:693–698
Davoglio RA, Cabello G, Marco JF, Biaggio SR (2018) Synthesis and characterization of α-MnO2 nanoneedles for electrochemical supercapacitors. Electrochim Acta 261:428–435
Taylor S, Gileadi E (1995) Physical interpretation of the Warburg impedance. Corrosion 51:664–671
Shi M, Chen Z, Sun J (1999) Determination of chloride diffusivity in concrete by AC impedance spectroscopy. Cem Concr Res 29:1111–1115
Li L, Tang X, Liu H, Qu Y, Lu Z (2010) Morphological solution for enhancement of electrochemical kinetic performance of LiFePO4. Electrochim Acta 56:995–999
Zhang Q, Huang S-Z, Jin J, Liu J, Li Y, Wang H-E, Chem L-H, Wang B-J, Su B-L (2016) Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability. Sci Rep UK 6:1–12
Guo H, Liu Y, Xi Y, Xu C, LQ, (2016) Investigation on high performance LiFePO4 nanoplates with the 010 face prominent for lithium battery cathode materials. Solid State Ion 298:44–50
Zhao Y, Peng L, Liu B, Yu G (2014) Single-crystalline LiFePO4 nanosheets for high-rate Li-ion batteries. Nano Lett 14:2849–2853
Dokko K, Koizumi S, Nakano H, Kanamura K (2007) Particle morphology, crystal orientation, and electrochemical reactivity of LiFePO4 synthesized by the hydrothermal method at 443 K. J Mater Chem 17:4803–4810
Murugan AV, Muraliganth T, Manthiram A (2008) Comparison of microwave assisted solvothermal and hydrothermal syntheses of LiFePO4/C nanocomposite cathodes for lithium ion batteries. J Phys Chem C 112:14665–14671
Chen R, Wu Y, Kong XY (2014) Monodisperse porous LiFePO4/C microspheres derived by microwave-assisted hydrothermal process combined with carbothermal reduction for high power lithium-ion batteries. J Power Sources 258:246–252
Zhang L, Liang H (2013) Rapid synthesis of LiFePO4 nanoparticles by microwave-assisted hydrothermal method. Russ J Electrochem 49:492–495
Acknowledgements
Financial support and/or scholarships from the Brazilian funding agencies CNPq—National Council for Scientific and Technological Development (process nos. 148138/2016-0, 311970/2017-6 and 309900/2019-0), CAPES—Coordination for the Improvement of Higher Education Personnel (finance code 001) and FAPESP—São Paulo Research Foundation are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bezerra, C.A.G., Davoglio, R.A., Biaggio, S.R. et al. High-purity LiFePO4 prepared by a rapid one-step microwave-assisted hydrothermal synthesis. J Mater Sci 56, 10018–10029 (2021). https://doi.org/10.1007/s10853-021-05914-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-05914-1