Abstract
Two-dimensional (2D) materials based on molybdenum sulfide (MoS2) have shown promising applications in semiconductors, optoelectronics, and catalysis. The variety of applications implies a controlled manipulation of purity, shape, and phase of such materials. This work elaborates on the structural characterization of MoS2 micro-assemblies produced in a chemical vapor deposition (CVD) system with emphasis on the pyramidal structures formed at high temperature and low gas rate, on a silicon dioxide (SiO2) substrate. A precise control of temperature and gas rate in the CVD process prompts the growth of pyramidal and other micron-size arrangements of MoS2 layers. An integrative set of high-resolution and analytical electron microscopy techniques, in conjunction with Raman and X-ray photoelectron spectroscopy (XPS), revealed the structural features of the MoS2 microstructures. Raman and XPS confirmed the presence of MoS2 and some residual oxide phases. Ultra-high-resolution scanning electron microscopy provided direct observation of the distinctive stacking of layers forming the pyramidal microstructures. Cross section samples from selected structures were done using focused ion beam. An extent of transmission electron microscopy and Cs-corrected scanning transmission electron microscopy (Cs-corrected STEM) results is discussed. This approach allowed to understand the growth mechanism of the triangular MoS2 microstructures through spiral grow around a screw dislocation, initiated at the center of the assembly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two-dimensional (2D) transition metal dichalcogenides (TMDs) are a versatile family of nanomaterials with more than forty layered composites. They have the general formula MX2, where M is a transition metal of the groups 4–10 of the periodic table, and X is the respective chalcogen [1,2,3,4]. Molybdenum (IV) disulfide (MoS2) is one in this type of 2D layered materials that come up with a variety of promising applications in nanoelectronics, photonics, sensing, energy storage, and optoelectronics [3, 5,6,7,8]. For instance, MoS2 exhibits weak interlayer Van der Waals interactions that allow it to act as an efficient host for a variety of electron donating atoms, such as lithium or sodium, to produce nano-batteries [9]. In bulk, MoS2 exhibits a semiconductor behavior with an indirect bandgap of 1.29 eV, but it drastically differs when becomes a monolayer, having a transition to a direct bandgap of around 1.8 eV [8, 10]. The displayed properties are directly related to its crystalline structure and particular three-dimensional arrangement. One of the biggest challenges in the synthesis of MoS2 materials is advancing reliable methods to produce specific nanometric-size arrangements and fine tune their properties. Among the different methods employed to modify and control the structural properties of MoS2 are: mechanical synthesis [11], wet chemistry [12], exfoliation [13], and chemical vapor deposition (CVD) [14]. CVD is particularly useful for large-scale production of 2D MoS2, enabling direct growth of materials over-activated solid substrates using gas-phase precursors. An efficient control of the reaction conditions allows the production of uniform thin layers [15], or vertically aligned MoS2 films [16]. Moreover, this method enables the overall shape control of deposited layers, using elemental molybdenum and sulfur as precursors, to produce high-quality single-layer MoS2 thin flakes, with triangular and hexagonal shapes, extended on solid substrates [17, 18]. Shape and structure effect the properties and consequently the applications of multilayered materials; e.g., a preferred pyramidal arrangement of layers exposes a succession of edges over the pyramidal steps which have potential catalytic applications [19,20,21,22]. Hence, the detailed study of morphological and structural features of 2D materials becomes of paramount importance to understand their evolution and properties.
Previous reports described the growth of 2D MoS2 as a screw-dislocation-driven process [23,24,25,26,27,28,29], and the study of layered microstructures has been conducted using atomic force microscopy (AFM) and Raman spectroscopy. Typically, MoS2 exhibits a centrosymmetric 2H-hexagonal phase described by an ABAB stacking of S–Mo–S layers, with coplanar S–Mo–S atoms in the first layer opposite to the second layer. However, other non-centrosymmetric phases have been reported specially when dealing with few layered materials and screw-dislocation pyramidal morphologies [24, 25, 30, 31]. In this work, pyramidal microstructures made of MoS2 were produced in a CVD system. Their structural characteristics were investigated using spectroscopy and electron microscopy techniques, namely Raman, X-ray photoelectron spectroscopy (XPS), ultra-high-resolution scanning electron microscopy (UHR-SEM), focused ion beam (FIB), and transmission electron microscopy (TEM). Emphasis placed on the structural characteristics is aimed to expand the understanding on the growth of MoS2 microstructures.
Experimental section
Synthesis
MoS2 microstructures were synthesized via CVD, protocol adapted from Chen et al. [24]. The experiment setup is illustrated in Fig. 1. A 5 × 5 mm section of ultra-flat [100] SiO2 wafer was used as a substrate for the preferential growth of MoS2. The SiO2 piece was previously cleaned using an isopropyl alcohol/acetone (1:1 volume) solution. In a typical growth reaction, 0.3 g of molybdenum trioxide (MoO3) powder (99%, Sigma-Aldrich) was placed in an alumina boat located at the center of a quartz tube (2.5 cm in diameter and 60 cm long). The boat position was at the center of the heating zone of the furnace (Lindberg Blue STF55433). Next, 0.6 g of sulfur powder (99% Sigma-Aldrich) was placed in the inlet of the furnace 15 cm away from the center. During a typical procedure, the furnace temperature was set to 900 °C following a linear ramp of 10 °C min−1 under a 20 sccm flow of N2 gas. The system was maintained during 20 min at 900 °C, time in which the sulfur evaporated entirely and reached the center of the reactor to interact with the MoO3 [32, 33]. Finally, the system was cooled down to room temperature under N2 atmosphere.
Characterization
Raman spectroscopy studies were performed with a LabRam HR Evolution from Horiba at ambient conditions with controlled light and a back-scattering configuration at a wavelength of 633 nm laser. For mapping, an acquisition time of 5 s and 10 accumulations were used for each point with a grid of 10 × 10, an objective lens of 100× Vis and grating of 500 nm. X-ray photoelectron spectroscopy (XPS) spectra were obtained in an ESCALAB 250Xi (Thermo Scientific) X-ray photoelectron spectroscope using monochromatic AlKα radiation (hν = 1486.7 eV). Images of the surface morphology of the MoS2 pyramids were acquired with an opto-digital microscope (Olympus DSX500), an ultra-high-resolution field-emission scanning electron microscope (UHR FE-SEM Hitachi 5500), and with a FIB-scanning electron microscope (FIB-SEM, ZEISS Crossbeam 340). A cross section of the pyramidal structure was obtained through FIB microsectioning using Ga ions at 30, 5, and 2 kV. The pyramid was previously coated with a layer of carbon and platinum to protect the sample from gallium ions. Plain-view imaging of the microstructure was performed with a high-resolution transmission electron microscope (HRTEM, JEOL 2010F). Experimental HRTEM images are compared with a simulated 2H.MoS2 structure generated by SimulaTEM software [34]. Atomic resolution imaging was carried out in a Cs-corrected transmission electron microscope (JEOL ARM-200F) operated in scanning mode (STEM) at 200 kV.
Results and discussion
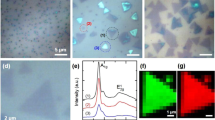
The SiO2 substrate was explored through optical microscopy to determine the structures obtained through the CVD process (Fig. 2). It is possible to observe pyramidal microstructures obtained through the CVD process in the images of bright-field (2a–b) and dark-field (2c–d) mode, such structures are indicated with arrows.
The deposit of MoS2 on the SiO2 substrate as a result of the CVD process was observed with more detail through SEM imaging, Fig. 3. It´s noticed the formation of an irregular film (or “islands”) on the substrate, Fig. 3a. At the edge of this irregular film, it is possible to observe the abundant formation of triangular structures, with some degree of truncation or shape irregularity (Fig. 3b). Some of these microstructures present a perfect triangular shape, such as the one shown in Fig. 3c, with a size of 3.8 µm per side, where the stepped surface is visible.
The Raman spectrum of the micropyramid are shown in Fig. 4, where the color of each spectrum colors corresponds to the color assigned to each circle shown in the mapped area. Mapping analysis revealed the appearance of characteristic MoS2 signals at 179 (A1g (M) − LA (M)), 230 (LA(M)), 383 (\( E_{2g}^{1} \)), 409 (A1g), 421 (\( B_{2g}^{2} + E_{1u}^{2} ) \), 454 (2LA), and 465 cm−1 (A2u) [35,36,37] corresponding to the spectra of the shown areas. However, there are some differences between the spectrum obtained from the central area of the micropyramid (red spectrum or labeled with the letter d) and the spectrum obtained on the edge labeled with the letter b, black circle. The spectrum obtained from the central area shows a value of the I2LA(M)/IA2u ratio of 0.98, while the I2LA(M)/IA2u ratio for the black spectrum was 1.05, which indicates that the 2LA(M) mode shows a high value of intensity at the edge of the micro pyramid. According to Chakraborty et al. [36], the relative intensity of the 2LA(M) mode respect to the A2u mode decreases as a function of the number of layers, correlating with our results since at the center of the micropyramid the number of layers is greater than in the edge. The relationship between the 2LA(M) and A2u modes for the spectra c and e indicates a 2H-type structure similar to the to the bulk material, while in the a spectrum the presence of MoS2 is null since the mapping was carried out outside the micropyramid. Another vibrational mode observed in the spectrum and taken at the edge of the micropyramid is located at 230 cm−1 (mode LA (M)) taken at the edge of the micropyramid. This mode is identified as first-order mode, and it is attributed to the dispersion of phonons due to defects induced in the structure and the presence of a few atomic layers. Finally, a slight signal was also observed around 300 cm−1, which may be due to the presence of molybdenum oxides remaining from the synthesis [38,39,40].
The XPS analysis of the MoS2 pyramids is displayed in Fig. 5. In the high-resolution spectrum of the S2p region (Fig. 5a), the 2p1/2 and 2p3/2 signals of the MoS2 are observed at 163.1 and 161.8 eV. Also, two signals are observed at 162.4 and 161.3 eV, which can be assigned to non-stoichiometric MoxSy due to the presence of surface defects introduced during the CVD growth [41, 42].The S2s-Mo3d region is displayed in Fig. 5b. The S2s (226.50 eV) and Mo 3d (229.1 and 232.2 eV for 3d5/2 and 3d3/2, respectively) correspond to signals of MoS2 are observed [43,44,45]. Other signals located at 233.3 eV and 236 eV, are observed, assigned to the 3d5/2 and 3d3/2 of the Mo(VI) species [46,47,48]. These results show that the obtained microstructures are almost entirely composed of MoS2; however, some molybdenum oxide and non-stoichiometric MoxSy phases presented.
A more detailed morphological analysis was carried out by SEM tilting the sample up to 54o, which allows the observation of a distinguishable stepped surface of the micropyramids. The pyramid has a triangular base consisting of several layers of MoS2. The pyramids correspond to a truncated trigonal morphology with a high density of surface steps, which can be correlated to the speed of growth in the direction of the [0001] axis. It slightly varies since each step has a different number of layers. The obtained pyramidal morphology is related to screw dislocations occurring during the growth of the nucleus along the vertical axis, and to the triangular-shaped layers in the lateral dimension that form the MoS2 pyramid. A spiral structure of MoS2 pyramids can be clearly seen, and the direction of growth that occurred in a clockwise direction is indicated with white arrows in Fig. 6a. A schematic of the MoS2 pyramid growth with a spiral structure is shown in Fig. 6b. The different steps that are formed during the growth of the pyramid are clearly observed in Fig. 6c. The image shows defects at the edges, which cause deviation from the perfect triangular shape. The combination of morphological features between hexagonal and triangular shapes in the microstructures is due to a complex growth that includes two dislocations that share a common core [29]. In some of the MoS2 pyramids, we observed the formation of small particles at the edges of the layers and some larger particles dispersed throughout the pyramid. These round particles corresponded to nucleation points for the growth of monolayers of MoS2 [14]. These images help to understand the growth mechanism of the triangular crystallites. The growth proceeded along emerging screw dislocation on the surface, as demonstrated by the Burton, Cabrera, and Frank theory (BCF) [49, 50]. This type of growth mechanism has been reported in other nanomaterials such as cobalt, nickel hydroxide, zinc hydroxy sulfate, and other pure metals [51,52,53,54]. The formation of a screw dislocation in MoS2 requires the generation of slipped planes (screw defects) in the first layers of the pyramid; otherwise, the growth will preferentially follow an in-plane growth mode, producing monolayers of MoS2 [25, 55]. Screw dislocations developed under low supersaturation conditions created staggered edges in the bottom layer that act as nucleation sites for the addition of the following precursor atoms, driving the growth of the second layer in the top of the bottom layer. This gradually leads to the continuous growth of spirals, resulting in a pyramidal growth [50]. These conditions of low-saturation conditions were reached in our experiment set up by the low sulfur concentration present at the center of the reactor and by controlling the low drag flow velocity. For most of the reported experiments for the growth of MoS2 using CVD the rate of the carrier gas is usually high, ranging from 100 to 500 sccm [14, 56, 57], in comparison with our process at only 20 sccm.
Dichalcogenide MoS2 present typically a 2H-hexagonal phase with an ABAB stacking. This stacking order can vary depending on the morphology, thickness, growth mechanism, etc. [24, 25, 31, 58]. To confirm the structural arrangement of the produced MoS2 pyramids, HRTEM and atomic-resolution STEM imaging were performed. First, complete structures were recovered by scraping the surface of the SiO2 substrate with a thin blade, then placed into an ethanol solution and resuspended with an ultrasonic bath for 20 min. Afterward, 7µL of the ethanol solution was placed onto a carbon-coated TEM grid, and the solvent was allowed to evaporate at room temperature. A second sample was prepared by FIB cross-sectioning, attaching the fabricated lamella on a copper lift-out grid. Figure 7a (scraped sample) shows an HR-TEM micrograph of a scraped MoS2 pyramid, confirming the crystalline arrangement achieved during the CVD production process. No perceivable defects were found in the analyzed areas. The three interplanar distances were 0.27 nm, with 60° between them. These distances matched with the \( \left( {0\bar{1}10} \right) \), \( \left( {1\bar{1}00} \right) \), and \( \left( {10\bar{1}0} \right) \) planes of the 2H-MoS2 structure, viewed along the \( \left[ {0001} \right] \) direction. The corresponding electron diffraction pattern is shown in Fig. 7b. The pattern shows sharp reflections characteristic of 2H-MoS2. Satellite reflections following the hexagonal symmetry of the pattern are also observed, indicating a rotation of ~ 2.6° of some layers around the [0001] axis due to the spiral growth. Micrographs of the pyramid cross section, Fig. 7c, exhibit an area with a periodicity of layers or steps forming the pyramidal arrangement. The overall imaged area was approximately 1 µm2, and it is composed of about 68 layers. The interlayer distance is 0.6 nm, corresponding to {0001} planes. The electron diffraction pattern of the MoS2 (Fig. 7c, up inset) confirmed the [0001] growth direction. A magnified area of the image indicated with the blue square in Fig. 7a is presented in the left panel in Fig. 7e highlighting the lattice pattern. An HRTEM image of a MoS2 flake with 2H-ABAB structure viewed from the [0001] axis zone was simulated, right panel in Fig. 6e, taking into consideration the experimental acquisition conditions. By tilting the sample around the \( \left[ {10\bar{1}0} \right] \) axis, a second HRTEM image was taken along the \( \left[ {03\bar{3}1} \right] \) axis zone, and its corresponding simulated image is also shown, right panel in Fig. 7f. As noticed, the simulated images are in good agreement with the observed experimental lattice patterns along two different axis zones.
a TEM image of MoS2 pyramid viewed from different zone axis b electron diffraction pattern of MoS2 pyramid viewed from [0001] direction. c Cross section area and d electron diffraction pattern of the pyramid and composed of 68 layers, their evenly space of 0.65 nm which correspond to the (0002) planes of MoS2. e, f Experimental (left) and simulated (right) HRTEM images of a small area of the single pyramid. The lattice pattern is in agreement with a 2H-ABAB layer stacking
Aberration-corrected HAADF-STEM imaging was employed to get a deeper insight into the pyramid´s. Figure 8a shows a MoS2 pyramid coated with a layer of carbon and platinum to protect it before sectioning. The prepared pyramid´s cross section is observed in Fig. 8b. This process enabled to determine that the sample height was of approximately 125 nm close to its center with ~ 200 monolayers of MoS2, offering a considerable amount of chemically active reactive sites for efficient catalytic applications or to act as support for other functional nanostructured materials. The cross section view revealed four steps clearly defined, the height of each step depending on the number of layers of MoS2 conforming them (Fig. 8c). In the analyzed area, each step was formed by 3 to 7 layers, which corresponded to a size of 1.8 and 4.2 nm, respectively (Fig. 8d). A schematic of the pyramid showing the different number of layers in each step is presented in Fig. 8e. The cross-sectional atomic-resolution HAADF-STEM image in Fig. 8f enables direct visualization of the stacking order of the pyramid layers. The brightest atomic columns correspond to projected Mo atomic columns, while the dim ones are exclusively S columns. An atomistic model is inserted in the image for comparison. This arrangement corresponds to an ABAB stacking of MoS2 layers, characteristic of the 2H phase, viewed along the \( \left[ {1\bar{1}00} \right] \) direction. An atomistic model is inserted in the image for comparison.
a TEM image of the MoS2 pyramid with a white rectangle where the cross section cut was made. b SEM image of cross section obtained using the FIB-SEM. c, d Aberration-corrected STEM imaging showing the position and height of the atomic steps and separation between the MoS2 layers e atomic model of the MoS2 structure showing the steps. f Atomic resolution imaging of the lattice of a MoS2 with the superimposed model of the structure. g Atomic resolution imaging of the layer stacking of MoS2. h Deconvolution of a line profile spectrum of MoS2 (black line) with peak overlaps of Mo (Blue line), and S (purple line) elements, taken from figure (f)
An intercalated stacking of MoS2 originated by the screw dislocation closer to the center of the pyramid is observed in Fig. 8g. It is noted that the ABAB stacking order is retained with very small deviations due to layers deformation or rotation, which can cause the satellite reflections observed in the diffraction pattern in Fig. 6b. The nucleation at the initial stage generates a dislocation center with high spiral activity, which allows maintaining the screw dislocation in the MoS2 layers. A deconvoluted intensity profile of the selected area shown in Fig. 8f highlights the location of heavy atomic columns (Mo) and light atomic columns (S) throughout the line profile (Fig. 8h). Interplanar distances of 0.6 nm and 0.15 nm were measured, corresponding to interlayer Mo-Mo columns and in-plane Mo-Mo columns, respectively. The difference in the intensities of the S columns in the profile is due to a slight deviation of the sample from the exact axis zone. These cross-sectional images present a direct observation of the MoS2 layer intercalation produced by the screw-dislocation-driven growth and confirm the 2H-ABAB stacking order of the produced pyramids in agreement with spectroscopy results [29].
Conclusions
MoS2 micropyramids were produced by using a CVD growth method using at high temperatures and low gas rates. Raman spectroscopy demonstrated the characteristics signals of MoS2 on the SiO2 substrate and highlighted the differences between the vibrational modes at the center and in the edge of pyramids. Signals at 230 and 454 cm−1 attributed to few layers MoS2 were located at the edge of the pyramids suggesting a one-step formation, while at the center, the signal corresponds to the bulk material. XPS confirmed the presence of the MoS2 phase in its majority, however, the presence of the Mo (VI) signals are also observed attributed to the presence of an oxide phase. UHR-SEM imaging revealed the preferential formation of pyramidal structures ranging in size between 1 and 4 µm per side. In combination with FIB-SEM, it was possible to determine that the growth mechanism of these materials is dominated by a spiral growth at screw dislocations, particularly due to the low concentration of the sulfur used during the optimized process. Cross sections of MoS2 pyramids obtained by FIB allowed to reveal the internal arrangement of the materials. In combination with atomic resolution, S/TEM imaging allowed the direct determination of the 2H-ABAB structural stacking along the [0001] direction of the produced pyramids, with horizontal growth along the \( \left[ {10\bar{1}0} \right] \) and \( \left[ {0\bar{1}10} \right] \) directions. The high contrast and resolution of the HAADF signal undoubtedly showed the precise organization of MoS2 into defined atomic steps of S-Mo-S, and the intercalation of layers one by one originated by the screw-dislocation-driven growth.
References
Wang QH, Kalantar-Zadeh K, Kis A et al (2012) Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol 7:699–712. https://doi.org/10.1038/nnano.2012.193
Saldanha PL, Brescia R, Prato M et al (2014) Generalized one-pot synthesis of copper sulfide, selenide-sulfide, and telluride-sulfide nanoparticles. Chem Mater 26:1442–1449. https://doi.org/10.1021/cm4035598
Haque F, Daeneke T, Kalantar-zadeh K, Ou JZ (2018) Two-dimensional transition metal oxide and chalcogenide-based photocatalysts. Nano-Micro Lett 10:1–27. https://doi.org/10.1007/s40820-017-0176-y
McGarrigle EM, Myers EL, Olla O et al (2007) Chalcogenides as organocatalysts. Chem Rev 107:5841–5883
Dai X, Du K, Li Z et al (2015) Enhanced hydrogen evolution reaction on few-layer MoS2 nanosheets-coated functionalized carbon nanotubes. Int J Hydrog Energy 40:8877–8888
Hinnemann B, Moses PG, Bonde J et al (2005) Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc 127:5308–5309. https://doi.org/10.1021/ja0504690
Rowley-Neale SJ, Brownson DAC, Smith GC et al (2015) 2D nanosheet molybdenum disulphide (MoS2) modified electrodes explored towards the hydrogen evolution reaction. Nanoscale 7:18152–18168. https://doi.org/10.1039/C5NR05164A
Ganatra R, Zhang Q (2014) Few-layer MoS2: a promising layered semiconductor. ACS Nano 8:4074–4099. https://doi.org/10.1021/nn405938z
Ramakrishna Matte HSS, Gomathi A, Manna AK et al (2010) MoS2 and WS2 analogues of graphene. Angew Chem Int Ed 49:4059–4062. https://doi.org/10.1002/anie.201000009
Mak KF, Lee C, Hone J et al (2010) Atomically thin MoS2: a new direct-gap semiconductor. Phys Rev Lett 105:2–5. https://doi.org/10.1103/PhysRevLett.105.136805
Novoselov KS, Jiang D, Schedin F et al (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci USA 102:10451–10453. https://doi.org/10.1073/pnas.0502848102
O’Neill A, Khan U, Coleman JN (2012) Preparation of high concentration dispersions of exfoliated MoS2 with increased flake size. Chem Mater 24:2414–2421. https://doi.org/10.1021/cm301515z
Eda G, Yamaguchi H, Voiry D et al (2011) Photoluminescence from chemically exfoliated MoS2. Nano Lett 11:5111–5116. https://doi.org/10.1021/nl201874w
Najmaei S, Liu Z, Zhou W et al (2013) Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat Mater 12:754–759. https://doi.org/10.1038/nmat3673
Lee Y, Lee J, Bark H et al (2014) Synthesis of wafer-scale uniform molybdenum disulfide films with control over the layer number using a gas phase sulfur precursor. Nanoscale 6:2821–2826. https://doi.org/10.1039/c3nr05993f
Kong D, Wang H, Cha JJ et al (2013) Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett 13:1341–1347. https://doi.org/10.1021/nl400258t
Kim D, Sun D, Lu W et al (2011) Toward the growth of an aligned single-layer MoS2 film. Langmuir 27:11650–11653. https://doi.org/10.1021/la201878f
Wu S, Huang C, Aivazian G et al (2013) Vapor-solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. ACS Nano 7:2768–2772. https://doi.org/10.1021/nn4002038
Dong H, Liu C, Ye H et al (2015) Three-dimensional nitrogen-doped graphene supported molybdenum disulfide nanoparticles as an advanced catalyst for hydrogen evolution reaction. Sci Rep 5:2–11. https://doi.org/10.1038/srep17542
Daage M (1994) Structure-function relations in molybdenum sulfide catalysts: the “rim-edge” model. J Catal 149:414–427
Tye CT, Smith KJ (2006) Catalytic activity of exfoliated MoS2 in hydrodesulfurization, hydrodenitrogenation and hydrogenation reactions. Top Catal 37:129–135. https://doi.org/10.1007/s11244-006-0014-9
Xie J, Zhang J, Li S et al (2013) Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J Am Chem Soc 135:17881–17888. https://doi.org/10.1021/ja408329q
Sarma PV, Patil PD, Barman PK et al (2015) Controllable growth of few-layer spiral WS2. RSC Adv 6:376–382. https://doi.org/10.1039/c5ra23020a
Chen L, Liu B, Abbas AN et al (2014) Screw-dislocation-driven growth of two-dimensional few-layer and pyramid-like WSe2 by sulfur-assisted chemical vapor deposition. ACS Nano 8:11543–11551. https://doi.org/10.1021/nn504775f
Zhang L, Liu K, Wong AB et al (2014) Three-dimensional spirals of atomic layered MoS2. Nano Lett 14:6418–6423. https://doi.org/10.1021/nl502961e
Nie Y, Barton AT, Addou R et al (2018) Dislocation driven spiral and non-spiral growth in layered chalcogenides. Nanoscale 10:15023–15034. https://doi.org/10.1039/c8nr02280a
Guo Y, Fu X, Peng Z (2018) Controllable synthesis of MoS2 nanostructures from monolayer flakes, few-layer pyramids to multilayer blocks by catalyst-assisted thermal evaporation. J Mater Sci 53:8098–8107. https://doi.org/10.1007/s10853-018-2103-0
Zheng J, Yan X, Lu Z et al (2017) High-mobility multilayered MoS2 flakes with low contact resistance grown by chemical vapor deposition. Adv Mater 29:2–7. https://doi.org/10.1002/adma.201604540
Shearer MJ, Samad L, Zhang Y et al (2017) Complex and noncentrosymmetric stacking of layered metal dichalcogenide materials created by screw dislocations. J Am Chem Soc 139:3496–3504. https://doi.org/10.1021/jacs.6b12559
Macchione MA, Mendoza-Cruz R, Bazan-Diaz L et al (2020) Electron microscopy study of the carbon-induced 2H-3R-1T phase transition of MoS2. New J Chem 44:1190–1193. https://doi.org/10.1039/c9nj03850g
Cortés N, Rosales L, Orellana PA et al (2018) Stacking change in MoS2 bilayers induced by interstitial Mo impurities. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-20289-1
Lee Y-H, Zhang X-Q, Zhang W et al (2012) Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv Mater 24:2320–2325. https://doi.org/10.1002/adma.201104798
Li X-L, Ge J-P, Li Y-D (2004) Atmospheric pressure chemical vapor deposition: an alternative route to large-scale MoS2 and WS2 inorganic fullerene-like nanostructures and nanoflowers. Chem - A Eur J 10:6163–6171. https://doi.org/10.1002/chem.200400451
Gómez-Rodríguez A, Beltrán-del-Río LM, Herrera-Becerra R (2010) SimulaTEM: multislice simulations for general objects. Ultramicroscopy 110:95–104. https://doi.org/10.1016/j.ultramic.2009.09.010
Fan JH, Gao P, Zhang AM et al (2014) Resonance Raman scattering in bulk 2H-MX2 (M = Mo, W; X = S, Se) and monolayer MoS2. J Appl Phys. https://doi.org/10.1063/1.4862859
Chakraborty B, Matte HSSR, Sood AK, Rao CNR (2013) Layer-dependent resonant Raman scattering of a few layer MoS2. J Raman Spectrosc 44:92–96. https://doi.org/10.1002/jrs.4147
Zheng W, Zhu Y, Li F, Huang F (2018) Raman spectroscopy regulation in van der Waals crystals. Photonics Res 6:1101. https://doi.org/10.1364/prj.6.001101
Cross JS, Schrader GL (1995) Low pressure chemical vapor deposition of molybdenum oxides from molybdenum hexacarbonyl and oxygen. Thin Solid Films 259:5–13. https://doi.org/10.1016/0040-6090(94)06427-X
Dieterle M, Mestl G (2002) Raman spectroscopy of molybdenum oxides: Part II. Resonance Raman spectroscopic characterization of the molybdenum oxides Mo4O11 and MoO2. Phys Chem Chem Phys 4:822–826. https://doi.org/10.1039/b107046k
Moura JVB, Silveira JV, da Silva Filho JG et al (2018) Temperature-induced phase transition in h-MoO3: stability loss mechanism uncovered by Raman spectroscopy and DFT calculations. Vib Spectrosc 98:98–104. https://doi.org/10.1016/j.vibspec.2018.07.008
Kondekar NP, Boebinger MG, Woods EV, McDowell MT (2017) In situ XPS investigation of transformations at crystallographically oriented MoS2 interfaces. ACS Appl Mater Interfaces 9:32394–32404. https://doi.org/10.1021/acsami.7b10230
Syari’ati A, Kumar S, Zahid A et al (2019) Photoemission spectroscopy study of structural defects in molybdenum disulfide (MoS2) grown by chemical vapor deposition (CVD). Chem Commun 55:10384–10387. https://doi.org/10.1039/c9cc01577a
Wang QF, Yanzhang RP, Ren XN et al (2016) Two-dimensional molybdenum disulfide and tungsten disulfide interleaved nanowalls constructed on silk cocoon-derived N-doped carbon fibers for hydrogen evolution reaction. Int J Hydrog Energy 41:21870–21882. https://doi.org/10.1016/j.ijhydene.2016.07.257
Iranmahboob J, Gardner SD, Toghiani H, Hill DO (2004) XPS study of molybdenum sulfide catalyst exposed to CO and H2. J Colloid Interface Sci 270:123–126. https://doi.org/10.1016/j.jcis.2003.11.013
Wang W, Li L, Tan S et al (2016) Preparation of NiS2//MoS2 catalysts by two-step hydrothermal method and their enhanced activity for hydrodeoxygenation of p-cresol. Fuel 179:1–9. https://doi.org/10.1016/j.fuel.2016.03.068
Lee YJ, Barrera D, Luo K, Hsu JWP (2012) In situ chemical oxidation of ultrasmall MoOx nanoparticles in suspensions. J Nanotechnol 2012:3–8. https://doi.org/10.1155/2012/195761
Rajagopal S, Nataraj D, Khyzhun OY et al (2011) Systematic synthesis and analysis of change in morphology, electronic structure and photoluminescence properties of pyrazine intercalated MoO3 hybrid nanostructures. CrystEngComm 13:2358–2368. https://doi.org/10.1039/C0CE00303D
Huang L, Xu H, Zhang R et al (2013) Synthesis and characterization of g-C3N4/MoO3 photocatalyst with improved visible-light photoactivity. Appl Surf Sci 283:25–32. https://doi.org/10.1016/j.apsusc.2013.05.106
Jin S, Bierman MJ, Morin SA (2010) A new twist on nanowire formation: screw-dislocation-driven growth of nanowires and nanotubes. J Phys Chem Lett 1:1472–1480. https://doi.org/10.1021/jz100288z
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Formkontrolle bei der Synthese von Metallnanokristallen: einfache Chemie, komplexe Physik? Angew Chemie 121:62–108. https://doi.org/10.1002/ange.200802248
Dong F, Wu L, Sun Y et al (2011) Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J Mater Chem 21:15171. https://doi.org/10.1039/c1jm12844b
Wang H, Robinson JT, Diankov G, Dai H (2010) Nanocrystal growth on graphene with various degrees of oxidation. Am Chem Soc 132:3270–3271
Morin SA, Forticaux A, Bierman MJ, Jin S (2011) Screw dislocation-driven growth of two-dimensional nanoplates. Nano Lett 11:4449–4455. https://doi.org/10.1021/nl202689m
Viswanath B, Kundu P, Mukherjee B, Ravishankar N (2008) Predicting the growth of two-dimensional nanostructures. Nanotechnology 19:195603. https://doi.org/10.1088/0957-4484/19/19/195603
Sarma PV, Patil PD, Barman PK et al (2016) Controllable growth of few-layer spiral WS2. RSC Adv 6:376–382. https://doi.org/10.1039/C5RA23020A
Gutiérrez HR, Perea-López N, Elías AL et al (2013) Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett 13:3447–3454. https://doi.org/10.1021/nl3026357
van der Zande AM, Huang PY, Chenet DA et al (2013) Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat Mater 12:554–561. https://doi.org/10.1038/nmat3633
Kan M, Wang JY, Li XW et al (2014) Structures and phase transition of a MoS2 monolayer. J Phys Chem C 118:1515–1522. https://doi.org/10.1021/jp4076355
Acknowledgements
The authors thanks CONACyT-Mexico for the support. To Alejandro Arizpe Zapata and Luis Gerardo Silva Vidaurri for technical support at CIMAV Monterrey to perform Raman and XPS measurements and E. Arzt for support through the INM. Enrique Samaniego-Benitez thanks Cátedras Research Program of CONACyT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samaniego-Benitez, J.E., Mendoza-Cruz, R., Bazán-Díaz, L. et al. Synthesis and structural characterization of MoS2 micropyramids. J Mater Sci 55, 12203–12213 (2020). https://doi.org/10.1007/s10853-020-04878-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04878-y