Abstract
The layer of boehmite film with special micro-nanostructure was fabricated on substrate surface through a simple hydrothermal method using common feedstock. Then superhydrophobic surface originated from boehmite film modified with stearic acid was obtained. This paper explores the impact of reaction conditions including reaction time, the content of aluminum ion and precipitant, and the type of precipitant on the specific surface structure and morphology of the boehmite film. The wetting properties of as-prepared boehmite films with various special morphologies after hydrophobic treatment with stearic acid were investigated by measuring the contact angles of water. The boehmite films modified with stearic acid show superhydrophobicity. The water contact angle of superhydrophobic boehmite films is up to 167.1°, and the rolling angle is 2.5°.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, superhydrophobic materials have attracted more and more attention due to their potential applications in many fields, such as self-cleaning [1,2,3,4], oil–water separation [5,6,7], anti-corrosion [8, 9], anti-icing [10, 11] and drag reduction [12]. The conception of superhydrophobicity comes from nature earliest, the most well known of which is “lotus effect” [13]. According to the research [14], the superhydrophobicity of plants in nature was based on surface roughness caused by different microstructures, together with the hydrophobic properties of the epicuticular wax. So superhydrophobic surface can be defined using contact angles as the surface on which water contact angle is higher than 150° and the rolling angle is lower than 10°.
At present, the main structure of superhydrophobic film is composed of a special bottom layer with high surface area and a hydrophobic layer over the top. Inorganic materials such as silica, titania and zinc oxide are widely used as the bottom layers due to their abundant surface structures and the surface control techniques which have been reported frequently so far. Wang et al. [15] coated ZnO nanowire arrays on the glass slide by using seed layer during hydrothermal treatment; then, SiO2/polyelectrolyte shell was deposited on it. After removing polyelectrolyte with calcination and modifying with chemical vapor deposition, ZnO@SiO2 superhydrophobic membranes finally were obtained. Pan et al. [16] prepared superhydrophobic TiO2/PU micro-nanocomposite structure by spraying method using TiO2 and polyurethane modified with silicone as raw materials and ethyl acetate as dispersant. In the above cases, some disadvantages have been found. The preparation process was complex which may lead to high cost, and the organic solvents used in the preparation may bring about environmental problems. Therefore, researchers now are focusing on exploiting simple, low-cost and environment-friendly superhydrophobic films.
Boehmite has attracted more attention because of wide applications in catalyst [17], adsorbent [18] and flame retardant [19]. In addition, boehmite has lower preparation cost and rich morphology. There are numerous reports about fabricating boehmites with various special morphologies. Zhang et al. [20] prepared boehmite nanofibers with high surface area and porous properties by hydrothermal method. The size and morphology of nanofibers can be controlled by adjusting the pH value of the reaction mixture. Chen et al. [21] prepared one-dimensional rod-shaped boehmite through hydrothermal reaction. At the same time, two-dimensional flake-shaped boehmite could be prepared by increasing the PH of the solution to about 10. Zanganeh et al. [22] synthesized 3D boehmite flowers which were self-assembled by petallike boehmite with a length of 800 ~ 1000 nm, a width of 200 ~ 250 nm and a thickness of 20 ~ 50 nm. The specific surface area of these flowerlike boehmites is up to 123 m2g−1, but the process of fabrication is complicated. People believe the prepared boehmite has abundant morphology, high specific surface area and controllable structure, which was in line with the properties of bottom layer of superhydrophobic materials.
In this paper, we present a simple low-cost and environment-friendly fabrication of a superhydrophobic boehmite film. The boehmite film is built up with specific morphology on the substrate surface by one-step hydrothermal method using conventional raw materials; after hydrophobic treatment with stearic acid, the superhydrophobic boehmite film is finally obtained. The resulting surface has excellent superhydrophobicity, and the water contact angle is up to 167.1°, and the rolling angle is 2.5°. The influences of reaction time, the content of aluminum ions and precipitant and the type of precipitant on morphology of boehmite were also investigated.
Materials and methods
Materials
All reagents in the experiment were of analytical grade and used without further purification. Deionized water was used for all the preparation.
Boehmite films were synthesized by one-step hydrothermal method. In a typical procedure, Al(NO3)3⋅ 9H2O and CH3COONa were dissolved in 40 ml deionized water, respectively. After stirring, aqueous solutions containing 0.5 wt%, 2.5 wt% and 5 wt% of aluminum ion and aqueous solutions containing 0.2 wt%, 0.6 wt% and 1 wt% of sodium acetate were prepared. Then the two solutions were mixed. The PH value of the mixed solution was about 3.5. The mixed solution was transferred into the autoclave, and a clean substrate slide was then immersed in the solution. The autoclave was sealed and maintained at 190 °C for 12 h and subsequently cooled down for 36 h to room temperature naturally. A layer of white film over substrate surface was obtained, which was washed with deionized water for several times and dried naturally. Other samples were obtained according to the method mentioned above with different reaction conditions.
The above boehmite films were immersed in 10% stearic acid ethanol solution for 60 min at room temperature, then taken out and dried at room temperature. The superhydrophobic boehmite films can be obtained. Actually other all saturated fatty acids such as lauric acid and 12-hydroxystearic acid can be also used for hydrophobic modification of boehmite.
Characterization
The general morphologies of synthesized samples were investigated with FESEM (field emission scanning electron microscope, SU8220, Hitachi, Japan). The samples were coated with 5–10 nm Au layer before the FESEM imagining. Structure and crystal phase of the samples were characterized by XRD (X-ray diffraction, D8 Advanced, Bruker AXS, Germany) using a Cu Kα radiation source. Infrared spectra were recorded with FTIR (Fourier transform infrared spectroscopy, Tensor 27, Bruker, Germany). The contact angles (CAs) and rolling angles were measured on a CA instrument (Contact Angle System OCA40, DataPhysics Co., Germany) at room temperature. Water droplets were placed at three positions for one sample, and the averaged value was adopted as the value of contact angle and rolling angle. The specific surface area and pore size distribution of synthesized samples were performed by BET (Brunauer–Emmett–Teller, Micromeritics TriStar II, Micromeritics, USA) surface area test. The samples were degassed at 150 °C and the vacuum was lower than 103 Pa, the BET method and BJH model were used to calculate the specific surface area and pore size distribution.
Results and discussion
Scanning electron microscopy (SEM) was used to study the structure grown on the surface of glass. The results are shown in Fig. 1. It can be seen that a special hydrothermal method can be used to prepare a boehmite film with a special and regular surface attached to the surface of the substrate. From the figure, we can see a large amount of nanoflakes with the same size are distributed on the surface of glass, and all of them grew outward from the glass surface. X-ray diffraction (XRD) was also used herein to reveal the purity and crystallinity of the prepared boehmite film. Figure 2 shows a typical XRD pattern of the sample obtained from the reaction for 9 h at 190 °C without hydrophobic treatment. All sharp and strong reflection peaks indicate that the resulting species is orthorhombic boehmite (γ-AlOOH, JCPDS card number 21–1307). No peak attributable to impurities is found in Fig. 1, indicating that the prepared γ-AlOOH sample has high purity and crystallinity.
Figure 3 shows an SEM image of a boehmite film on glass after hydrophobic treatment. In Fig. 3a, boehmite nanoflakes are randomly distributed on the glass surface, and some of them are brought together to form a micron-scale structure. It can be seen that after hydrophobic treatment in Fig. 3b, stearic acid just like mucous membranes adheres uniformly to the surface of the boehmite structure, which structure renders the material hydrophobic.
In order to explore the bond between the boehmite film and stearic acid as a modifier, FTIR studies were carried out on stearic acid and superhydrophobic boehmite films. As shown in Fig. 4b, the band at 1065 cm−1 and the shoulder at 1151 cm−1 are assigned to δs Al–O–H and δas Al–O–H vibrations of boehmite, respectively, and the absorption peaks at 734 and 634 cm−1 are assigned to the vibration mode of A–O [23, 24]. Compared to the pure stearic acid (Fig. 4a), the peaks at 2916, 2849 and 1700 cm−1 are significantly weakened and can be assigned to the υs(CH3), υs(CH2) and υs(C=O) [24], respectively, which means that the stearic acid molecules grafted to the boehmite successfully. Therefore, the modifier molecules are attached to the surface of the boehmite film by chemical bonds rather than just physical interactions, which is beneficial to improve the stability of the superhydrophobic membrane.
Figure 5 shows the SEM images of the boehmite grown on the glass at different reaction times (6–12 h). We can see that boehmite on the surface of substrate has a special specific surface and uniform size. In Fig. 5a, there are a lot of nanoflakes aggregated to form broom-like boehmites with sharp shape after hydrothermal reaction for 6 h (Fig. 5b). As shown in Fig. 5c, many nanoflakes grow outward from the glass surface when the reaction proceeded to 9 h. And the nanoflakes are lathy with smooth surface and a little sharp top (Fig. 5d). Figure 5e and f shows that some boehmite nanoflakes are assembled into micron-scale flowerlike structures when the reaction time proceeded to 12 h. And the length of nanoflakes is almost constant, but the width increased doubly.
The boehmite crystal becomes more and more regular in hydrothermal reaction, and the width of nanoflakes becomes wider and wider. Boehmite has different sizes and morphologies on the glass surfaces at different stages of the reaction, which can be described in detail as follows.
In the hydrolysis of aluminum salts, aluminum hydroxide is first formed (see Eq. 1). These amorphous aluminum hydroxide precipitates are unstable, and they are converted to γ-AlOOH crystallites in the next dehydration reaction (see Eq. 2). It is generally believed that the growth process of boehmite crystals is as follows: The first step is the Ostwald ripening process of γ-AlOOH nanoparticles, and the second step is the self-assembly of ripened nanoparticles to form various boehmite structures under the influence of intermolecular forces [25, 26]. During the hydrothermal reaction, small nanoparticles are produced and continue to assemble into larger structures to reduce surface energy. In Fig. 5a, the surface of the nanoflakes is porous because the reaction time is too short to allow the small particles to grow completely into relatively intact crystals, resulting in crystal defects. As the reaction time increases, the Ostwald ripening process is completed, forming nanoflakes with high crystallinity and uniform shape (Fig. 5c). Subsequently, these nanoflakes self-assemble into micron-scale flowerlike structures by intermolecular forces such as hydrogen bonding, van der Waals forces and electrostatic forces (Fig. 5e) [27,28,29] to reduce surface energy.
We have found that aluminum ion content plays an important role in the formation of boehmite films. With the same content of sodium acetate, when the aluminum ion content is 0.5 wt%, the glass surface is covered with a large number of ellipsoid-like particles with the same size, which are composed of flakes, having a uniform distribution and similar orientations (Fig. 6a and b). When the aluminum ion content is increased to 2.5 wt%, the shape of the boehmite is converted into flakes which cross each other and grow outward from the glass surface (Fig. 6c and d). When the aluminum ion content reaches 5 wt%, the flakes appeared denser, and some of the flakes were combined into a micron-scale flowerlike structure (Fig. 6e and f). When the aluminum ion content is low, the hydrothermal reaction solution is near neutral. Due to the low concentration, the boehmite crystallites grown on the surface of the glass have a large growth space, form a thin sheet and self-assemble to form a ellipsoid-like structure under the action of intermolecular forces to reduce the surface energy. When the aluminum ion content is increased to 2.5 wt%, the acidity of the hydrothermal reaction solution increases. In an acidic environment, protons in the reaction solution combine with aluminum hydroxide hydroxyl groups, affecting the crystal growth of boehmite [30]. In an acidic environment, boehmite is more likely to grow into elongated sheets. At the same time, the hydroxyl groups on the surface of the glass act with the hydroxyl groups of the aluminum hydroxide, which cause the crystal structure to grow outward from the glass surface, resulting in the formation of uniformly oriented fibrous sheets [31]. This structure significantly increases the specific surface of the film and provides excellent conditions for the preparation of the hydrophobic structure. When the aluminum ion content is increased to 5 wt%, the surface area of the glass is crowded, and the fibrous sheet grows outward and becomes longer and longer, and some of the sheets form a micron-scale flowerlike structure, which is the results for the reduction in surface energy.
In this paper, acetate and aluminum salts were used to prepare boehmite by hydrolysis reaction. Acetate also has an important influence on the structure of boehmite. Figure 7 shows the SEM images of boehmite films prepared under different sodium acetate contents when aluminum ion content is the same. Figure 7 shows that the surface of the glass substrate is covered with a large number of boehmite crystalline flakes, which are uniform in size and grow from inside to outside over the glass surface. As the sodium acetate content increases, the arrangement of boehmite flakes becomes more and more close. They stick to each other, and the thickness gradually increases. This densely packed structure may affect the specific surface area and thus the hydrophobic properties of the film.
In order to explore the effects of different salts on the structure of boehmite, Na2SO3, NaHCO3 and NH4HCO3 were used as hydrolysis precipitants to study the morphology of boehmite film. Figure 8 shows the SEM images of a boehmite films prepared using different precipitants. We can see that when Na2SO3, NaHCO3 and NH4HCO3 with the same content (0.2 wt%) are used as precipitant, the boehmite films are all flowerlike structures. However, the basic units that make up the micron-scale flowers are different. When the precipitant is Na2SO3, the basic unit of the flowerlike structure is needle-shaped crystallites having different lengths (Fig. 8a and b). When the precipitant is NaHCO3, the morphology of the flowerlike structure is similar to that of Na2SO3, but the basic unit size is slightly larger (Fig. 8c and d) and is more regular. Then, when the precipitant becomes NH4HCO3, the flowerlike structure is composed of an elliptical sheet of uniform size and regular structure (Fig. 8e and f). The difference between the precipitants leads to differences in the self-assembled basic unit and the resulting secondary structure, and the reasons remain to be studied. However, this result provides a method for the preparation of boehmite film with high surface area.
In this paper, the surface modification of the boehmite film was carried out to prepare a composite material with hydrophobic property. The hydrophobic properties were investigated by testing the water contact angle. Research reports indicate that the water contact angle depends on the surface tension between solid–gas, solid–liquid and liquid–gas [32]. In fact, solid surfaces are often rough, so surface roughness must be considered when measuring water contact angles on rough solid surfaces. Actually surface roughness has a very important effect on water contact angles [33, 34]. The experimental results show that all the samples have good hydrophobicity, and the structure of the inorganic underlayer has a great influence on the hydrophobic properties of the materials.
The results show that boehmite structures on substrate are composed of crystals with nanosize, and the structure is related to the conditions such as aluminum ion content and reaction time. For example, when aluminum ion content is 0.5 wt%, boehmite is self-assembled from nanosheets with a width of less than 100 nm into an ellipsoid structure with a length of about 1.2 μm (Fig. 6a and b). When aluminum ion content is increased to 5 wt%, the obtained boehmite is self-assembled from nanoflakes with a width of about 300 nm into a flowerlike structure with a diameter of about 3.1 μm (Fig. 6e and f). These structures are formed by the self-assembly of nanoflakes with uniform size under the action of intermolecular forces and hydrogen bond to reduce surface energy.
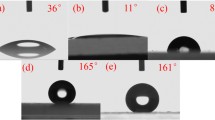
Figure 9 shows the water contact angle test results after hydrophobic treatments of boehmite films prepared at different hydrothermal times. The results show that after modification the water contact angle of non-boehmite glass surface is only 110.7°. When hydrothermal reaction was 6 h, the water contact angle of the modified boehmite film was significantly increased to 156.8°. On further increase in the hydrothermal reaction time to 12 h, the water contact angle reached 167.5° after the hydrophobic treatment. Figure 10 shows the N2 absorption–desorption isotherms of boehmite films synthesized during the two hydrothermal reaction periods. It is obvious that the samples obtained are both typical IV curves with H1 hysteresis loops, which indicate the pore structure of the film samples belongs to ordered mesopores. Among them, the specific surface areas of the obtained boehmite film samples at 6 h and 12 h were 83 m2g−1 and 149 m2g−1, respectively. Combining the above conclusions, it can be seen that the glass surface without growing the boehmite film did not reach the superhydrophobic condition after only the hydrophobic treatment. Conversely, the increase in the hydrothermal reaction time increases the roughness of the boehmite film, the water contact angle also becomes larger to some extent after the hydrophobic treatment and film has the superhydrophobicity. Therefore, it is not difficult to find that the superhydrophobicity can be achieved by the combination of surface roughness and hydrophobic treatment.
Figure 11 shows the water contact angle test results after hydrophobic treatments of boehmite films prepared with different aluminum ion and sodium acetate contents, and different precipitants after hydrophobic treatment. Figure 11a shows that the water contact angle of the film after hydrophobic treatment increases with the increase in aluminum ion content and finally reaches 167.5°. With the increase in the content of aluminum ions, the acidity of the solution increases, and the structure of boehmite changes from ellipsoid to flake (Fig. 6). This structure greatly increases the surface area of the film, and the flake units self-assemble into a micron-sized flowerlike structure to reduce the surface energy. Compared with the separated ellipsoid structure, this flowerlike structure covers the substrate more fully, which leads to better hydrophobicity and higher water contact angle. In Fig. 11b, with the increase in sodium acetate content, the water contact angle of the film after hydrophobic treatment decreases. With the increase in sodium acetate content, the arrangement of flakes becomes more and more compact (Fig. 7). This dense filling structure reduces the surface area and surface roughness, so that the water contact angle of the film after hydrophobic treatment is reduced. Figure 11c shows the water contact angle of materials prepared with different precipitants. When Na2SO3 is used as precipitant, the water contact angle of the film after hydrophobic treatment is only 134.7°. The basic units of boehmite prepared with this precipitant are acicular crystalline structures with different lengths. Compared with other units, the regularity and uniformity of this one are poor (Fig. 8). The self-assembled structure with this unit is not uniform, and the hydrophobic performance is also poor.
From the above description, we believe that the hydrophobicity of the sample has a great relationship with its morphology, and its morphology depends on the formation mechanism of boehmite. At present, there are many studies on the formation mechanism and structural growth control of boehmite. Wu et al. [35] provided a method to control the morphology of boehmite by using the competitive relationship between anions. In this paper, boehmite nanoparticles are gradually assembled into larger micron-sized structures by intermolecular forces and hydrogen bond after Ostwald maturation. We use conventional, inexpensive, low-environmental-hazard raw materials, combined with a simple one-step process to prepare hydrophobic materials. It has the potential to be environmental-friendly novel method. Special surface structures are obtained by controlling the preparation conditions such as temperature, concentration and reaction time. During the preparation process, uniform nanocrystals are first generated; then, they are self-assembled to form larger micron-sized structures to reduce the surface energy. These micron-sized structures have higher surface area and special morphologies, which increased the roughness of the sample surface and helped to achieve superhydrophobicity.
To further explore the superhydrophobicity of the samples, we performed a rolling angle test on the above samples with water contact angle exceeding 150°. The results are listed in Table 1. We can see these samples have excellent superhydrophobicity, especially when the aluminum ion content is 5 wt%, using 0.2 wt% sodium acetate as precipitant, and the hydrothermal reaction time is 12 h, the water contact angle of the sample is up to 167.1°, while the rolling angle is 2.5°. Thus, boehmite nanoflakes grow outward from the glass surface, and some of flakes assemble into micron-scale flowers with uniform size and structure. This special micro-nanostructure not only builds a rough high-specific-surface structure on the glass surface and reduces the surface energy, but also traps air between the droplet and the surface, making the droplet easy to roll off [36, 37]. At this time, the surface is in Cassie–Baxter state. Therefore, in combination with hydrophobic treatment, all samples show higher water contact angle and lower rolling angle.
In order to study the effect of substrate surface on the growth of boehmite structure, we carried out the same hydrothermal reaction experiment on the basis of PTFE (polytetrafluoroethylene) and compared the obtained structure with the glass surface. Figure 12a shows that when the PTFE plate is a substrate, the surface structure is significantly different from the glass surface, and the major difference is the growth direction of boehmite crystallite. The boehmite structure grows mainly on the surface of the glass from the inside to the outside; on the other hand, the growth direction of the boehmite crystallites on the surface of the PTFE is substantially random, which increases the surface roughness to a certain extent. In Fig. 12b, the water contact angle of the boehmite film on PTFE plate after hydrophobic treatment reaches 155.6°, but water droplets still adhere to the surface after the plate is inclined more than 90°. We believe this should be caused by the capillary effect of irregular rough porous structure. It reduces the amount of air trapped between water droplet and surface, increases the wet contact area of the water droplet and makes the surface with higher adhesion and friction [36, 37]. At this point, the surface of the PTFE is in Wenzel state. The hydroxyl groups on the surface of the glass substrate have important guiding effects on the growth of the boehmite structure. The growth mode from inside to outside is advantageous for constructing a high-specific-surface structure. The water contact angle on PTFE plates is not as large as on glass substrates, because there is rarely hydroxyl group on the surface of PTFE.
To study the sustained hydrophobic properties of hydrophobic materials, the hydrophobic samples were immersed in water at room temperature for a period of time and then taken out for hydrophobic performance testing. Figure 13 shows the changes in contact angle and rolling angle of the sample soaked in water for different times after hydrophobic treatment. Obviously, with the increase in soaking time, the water contact angle of the sample changes, showing a slight downward trend on the whole. However, after soaking for 336 h, the water contact angle of the sample can still reach up to 150°, and the rolling angle is lower than 10°, indicating that the hydrophobic material has certain water resistance.
Conclusion
In summary, this paper uses a simple precipitation–hydrothermal method, using cheap raw materials to prepare a boehmite structure with special morphology and high surface area, combined with hydrophobic modification of organic fatty acids, to obtain the superhydrophobic composite. The experimental results show that the hydrothermal reaction time, the content of aluminum ions and precipitant and the type of precipitant are the main factors controlling the structure of boehmite. The high-surface-area structure grown on the surface of the solid substrate is formed by self-assembly of boehmite crystallites, which greatly enhances the roughness of the surface of the substrate and plays a key role in the construction of the hydrophobic material. As a cheap and environmental-friendly functional material, the boehmite structure provides an opportunity for large-scale applications of hydrophobic surfaces.
References
Zhang XT, Liu SQ, Salim A, Seeger S (2019) Hierarchical structured multifunctional self-cleaning material with durable superhydrophobicity and photocatalytic functionalities. Small. https://doi.org/10.1002/smll.201901822
Yu NL, Xiao XY, Ye ZH, Pan GM (2018) Facile preparation of durable superhydrophobic coating with self-cleaning property. Surf Coat Tech 347:199–208
Fu YC, Jiang JX, Zhang QH, Zhan XL, Chen FQ (2017) Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning and antibacterial performances. J Mater Chem A 5:275–284
Syafiq A, Vengadaesvaran B, Rahim A, Pandey AK, Bushroa AR, Ramesh K, Ramesh S (2019) Transparent self-cleaning coating of modified polydimethylsilox-ane (PDMS) for real outdoor application. Prog Org Coat 131:232–239
Tang X, Si Y, Ge JL, Ding B, Liu LF, Zheng G, Luo WJ, Yu JY (2013) In situ polymerized superhydrophobic and superoleophilic nanofibrous membranes for gravity driven oil-water separation. Nanoscale 5:11657–11664
Gu J, Xiao P, Chen J, Liu F, Huang YJ, Li GY, Zhang JW, Chen T (2014) Robust preparation of superhydrophobic polymer/carbon nanotube hybrid membranes for highly effective removal of oils and separation of water-in-oil emulsions. J Mater Chem A 2:15268–15272
Cao Y, Zhang X, Tao L, Li K, Xue ZX, Feng L, Wei Y (2013) Mussel-inspired chemistry and michael addition reaction for efficient oil/water separation. ACS Appl Mater Inter 5:4438–4442
Xiong JW, Sarkar DK, Chen XG (2017) Superhydrophobic honeycomb-like cobalt stearate thin films on aluminum with excellent anti-corrosion properties. Appl Surf Sci 407:361–370
Zhao WG, Zhu RJ, Jiang JY, Wang ZM (2019) Environmentally-friendly superhydrophobic surface based on Al2O3@KH560@SiO2 electrokinetic nanoparticle for long-term anti-corrosion in sea water. Appl Surf Sci 484:307–316
Sarshar MA, Song D, Swarctz C, Lee J, Choi CH (2018) Anti-icing or deicing: icephobicities of superhydrophobic surfaces with hierarchical structures. Langmuir 34:13821–13827
Liu Y, Li XY, Jin JF, Liu J, Yan YY, Han ZW, Ren LQ (2017) Anti-icing property of bio-inspired micro-structure superhydrophobic surfaces and heat transfer model. Appl Surf Sci 400:498–505
Wang Y, Liu XW, Zhang HF, Zhou ZP (2015) Superhydrophobic surfaces created by a one-step solution-immersion process and their drag-reduction effect on water. RSC Adv 5:18909–18914
Zhang JH, Gao XF, Jiang L (2007) Application of superhydrophobic edge effects in solving the liquid outflow phenomenon. Langmuir 23:3230–3235
Neinhuis C, Barthlott W (1997) Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann Bot-London 79:667–677
Wang LL, Zhang XT, Yang F, Li B, Liu YC (2009) Bioinspired preparation of ultrathin SiO2 shell on ZnO nanowire array for ultraviolet-durable superhydrophobicity. Langmuir 25:13619–13624
Pan HB, Wang CD, Liu JX, Yu HB (2015) Preparation and properties of titanium oxide/polyurethane superhydrophobic coating. Polym Mater Sci Eng 31:62–65
Reshma P, Vikneshvaran S, Velmathi S (2018) Boehmite-an efficient and recyclable acid-base bifunctional catalyst for aldol condensation reaction. J Nanosci Nanotechnol 18(6):4270–4275
Meng FC, Rong G, Zhang XL, Huang WJ (2014) Facile hydrothermal synthesis of hierarchically structured γ-AlOOH for fast Congo red removal. Mater Lett 129:114–117
Huang PH, Chang SJ, Li CC, Chen CA (2017) Boehmite-based microcapsules as flame-retardants for lithium–ion batteries. Electrochim Acta 228:597–603
Zhang J, Shi FJ, Lin J et al (2008) Nanoparticles assembly of boehmite nanofibers without a surfactant. Mater Res Bull 43:1709–1715
Chen XY, Lee SW (2007) pH-dependent formation of boehmite (γ-AlOOH) nanorods and nanoflakes. Chem Phys Lett 438:279–284
Zanganeh S, Kajbafvala A, Zanganeh N, Mohajerani MS, Lak A, Bayati MR, Zargar HR, Sadrnezhaad SK (2010) Self-assembly of boehmite nanopetals to form 3D high surface area nanoarchitectures. Appl Phys A 99:317–321
Ji W, Wang Z, Ma J, Gong J (2016) Hydrothermal synthesis of boehmite on alumina membranes for superhydrophobic surfaces. Surf Eng 32:102–107
Liu LJ, Zhao JS, Zhang Y, Zhao F, Zhang YB (2011) Fabrication of superhydrophobic surface by hierarchical growth of lotus-leaf-like boehmite on aluminum foil. J Colloid Interface Sci 358:277–283
Bell TE, González-Carballo JM, Tooze RP, Torrente-Murciano L (2017) γ-Al2O3 nanorods with tuneable dimensions-a mechanistic understanding of their hydrothermal synthesis. RSC Adv 7:22369–22377
Wang X, Shi G, Shi FN et al (2016) Synthesis of hierarchical cobalt dendrites based on nanoflake self-assembly and their microwave absorption properties. Rsc Adv 6:40844–40853
Xu B, Wang J, Yu HB, Gao H (2011) Large-scale synthesis of hierarchical flowerlike boehmite architectures. J Environ Sci 23:S49–S52
Tang Z, Liang J, Li X, Li JF, Guo HL, Liu YQ, Liu CG (2013) Synthesis of flower-like boehmite (γ-AlOOH) via a one-step ionic liquid-assisted hydrothermal route. J Solid State Chem 202:305–314
Liu SL, Zeng W, Chen T (2017) Synthesis of hierarchical flower-like NiO and the influence of surfactant. Phys E 85:13–18
Bokhimi X, Toledo-Antonio JA, Guzmán-Castillo ML, Hernández-Beltrán F (2001) Relationship between crystallite size and bond lengths in boehmite. J Solid State Chem 159:32–40
Jiao WQ, Wu XZ, Xue T et al (2016) Morphological-controlling growth of nanosized boehmite with enhanced aspect ratios in organic additive-free cationic-anionic double hydrolysis method. Cryst Growth Des 16:5166–5173
Young T (1805) An essay on the cohesion of fluids. Philos T R Soc B 95:65–87
Wenzel R (1936) Resistance of solid surface to wetting by water. Ind Eng Chem 28:988–994
Herminghaus S (2000) Roughness-induced non-wetting. Europhys Lett 52:165
Wu XY, Zhang BQ, Hu ZS (2013) Morphology-controlled hydrothermal synthesis of boehmite via an anions competition method. Powder Technol 239:272–276
Quéré D, Lafuma A, Bico J (2003) Slippy and sticky microtextured solids. Nanotech 14:1109–1112
Huang XJ, Kim DH, Im M, Lee JH, Yoon JB, Choi YK (2009) ‘‘Lock-and-key’’ geometry effect of patterned surfaces: wettability and switching of adhesive force. Small 5:90–94
Acknowledgements
This research was funded by the project of Production, Education & Research of Guangdong Province (20090916). The authors are grateful for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, R., Jiang, Q. & Chen, J. The superhydrophobic surface constructed with boehmite micro-nanostructure. J Mater Sci 55, 5795–5807 (2020). https://doi.org/10.1007/s10853-020-04416-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04416-w