Abstract
TiO2 has attracted significant interest owning to their excellent photocatalytic properties. However, controlled preparation of TiO2 with satisfactory morphology is still an urgent challenge in this field. In this work, tunable one-dimensional (1D) rod-like and three-dimensional (3D) yolk-like N-doped TiO2 hierarchical architectures were successfully fabricated by one-step solvothermal route. A comparative study on morphological, structural and optical behavior of 1D and 3D TiO2 is conducted by SEM, TEM, BET, XPS, UV–Vis DRS, photoelectrochemical and photodegradation experiments. The resultant N-doped TiO2 with specific surface area of 190.8 m2 g−1 and 166.6 m2 g−1 for rod-like structure and yolk-like structure, respectively, exhibited excellent photocatalytic performance using rhodamine B (RhB), methylene blue (MB) and phenol as the degraded pollutants under visible-light irradiation. Benefiting from the direct electrical path, multiple internal reflections of light and high specific surface area, the rod-like N-doped TiO2 possessed higher photocatalytic efficiency. Specifically, for rod-like N-doped TiO2, the reaction rate constant of the photodegradation for RhB, MB and phenol reached 12.1, 6.9 and 76.0 times, respectively, compared with P25. In comparison with yolk-like N-doped TiO2, the rate constant raised 1.5, 1.3 and 1.3 times. In addition, the formation mechanism of such controllable-morphology structure was also analyzed. This work suggests that the proper hierarchical structure combined with a large specific surface area plays a significant role on photocatalytic performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, TiO2 has attracted tremendous interest in the field of green energy production and environmental remediation due to its abundant reserve, stable physical and chemical properties, non-toxicity and high efficiency [1, 2]. However, considering their drawbacks of unsatisfactory solar energy absorption and rapid electron–hole recombination efficiency, the photocatalytic activity of TiO2 material is very limited. Therefore, a series of strategies, including altering morphology, doping metal/non-metal elements and combining with a second semiconductor, were proposed to improve the light absorption and charge separation ability of TiO2 photocatalytic materials [3,4,5,6,7]. Among various strategies, morphology controlling is a crucial “first step” in term of photocatalytic activities, which can lay a good foundation for subsequent modification work [8]. Moreover, the specific surface area, active sites for adsorption and conversion, charge separation performance and light harvesting are highly dependent on TiO2 morphology [9,10,11]. Therefore, it is significant and meaningful to prepare TiO2 with different morphologies for efficient photocatalysis [12,13,14].
Up to now, zero-dimensional (0D) TiO2 particles, one-dimensional (1D) TiO2 rods or wires, two-dimensional (2D) TiO2 sheets and three-dimensional (3D) TiO2 spheres have been successfully synthesized [15,16,17,18,19]. In many cases, 1D, 2D or 3D TiO2 composed of primary nanobuilding blocks such as 0D nanoparticles [20,21,22]. Compared with traditional 0D particles, such hierarchical structures can lead to unique properties. For example, TiO2 with 1D rod-like hierarchical structure can not only form finite space between internal microrods resembling Fabry–Perot resonator structure and resulting in increasing multiple internal reflections of light, but also provide direct electrical pathway for photogenerated electrons, reducing the probability of interparticle hopping and facilitating carrier transport [23,24,25,26,27]. In addition to 1D materials, 3D hollow-structured TiO2 also has attracted significant attention for the sake of their large specific surface area, excellent multiple reflection and mass transfer capacity [28,29,30].
Indeed, TiO2 with 1D, 2D and 3D hierarchical structures has been studied and highly praised by many different research groups. However, these research works are inclined to highlight the enhanced effect of a certain-dimensional TiO2 [31]. Moreover, numerous works focused on the fabrication of TiO2 with various morphologies by different methods [32,33,34]. Most of them cannot be compared photocatalytic performance directly due to the existence of uncertain complexity and impurity ions in synthesis system [35]. In addition, even in a particular system, most of the researches only present a certain-dimensional morphology control, such as rods, core/shell rods to hollow rods or solid spheres, core/shell spheres to hollow spheres [36,37,38]. There are few reports on tuning among different-dimensional samples, making it difficult for us to directly compare and choose 1D, 2D or 3D TiO2 materials. Therefore, it is highly desired to fabricate catalysts of various dimensions under the same capping agents and process to compare the photocatalytic activity.
Nitrogen doping, as a strategy to improve visible-light-driven range, has become very mature in the field of photocatalysis [39, 40]. However, most of the N-doped TiO2 was prepared by multiple heat treatments for obtaining products with higher crystallinity and better N modification [41]. Such methods not only involve difficult steps and high-energy consumption, but also suffer low specific surface areas due to grain coarsening.
Herein, we report, for the first time, a novel green solvothermal route for controlled preparation of different-dimensional N-doped TiO2 without annealing. The transforming from 1D rod-like to 3D yolk-like hierarchical architectures could be achieved by simply adjusting the amount of precursor, which can guarantee the comparability of experimental results of photocatalysis. The effect of processing conditions on the morphology is investigated, and the effect of TiO2 dimensionality on the specific surface area, charge separation and photocatalytic performance is also systematically studied. This work provides a guidance for designing and controlling other hierarchical microstructures for photocatalytic degradation.
Experimental section
Material synthesis
The preparation process is illustrated in Fig. 1. In a typical N-doped rod-like TiO2 procedure, 0.43 g of TiOSO4 (Macklin) precursor was dispersed in a mixture solution of 120 mL N propanol and 40 mL deionized water and then stirred at room temperature overnight. Subsequently, the homogenous solution was sonicated for 20 min in an ultrasonic bath. Then, 0.10 g of urea was added into the solution to make sure the mole ratio of urea to TiOSO4 was 0.6. Finally, the as-prepared mixture solution was transferred into a Teflon-lined stainless-steel autoclave at 90 °C for 14 h. The resultant precipitates were filtered, washed thoroughly with water and ethanol for several times and dried at 80 °C for 8 h under electronic oven and denoted as N-TR. The N-doped yolk-like TiO2 was achieved by adding precursor and urea contents at the values of 6.88 g and 1.55 g, respectively, and denoted as N-TYS. Additionally, different precursor contents (0.86 g, 1.72 g and 3.44 g) and reaction time (0.5 h, 2 h, 14 h and 26 h) were also applied in order to investigate the evolution of the morphology. For comparison, the N-free samples were also carried out and denoted as TR and TYS, respectively.
Characterization
The morphology of as-prepared samples was characterized by scanning electron microscopy (SEM, NOVA NanoSEM 430, the Netherlands), and the composition was examined using energy-dispersive spectroscopy (EDS, Zeiss, Oberkochen, Germany) attached to the SEM. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) were performed using FEI Tecnai G2 F20 instrument (FEI, America). The X-ray diffraction (XRD) patterns of the samples were recorded by an X-ray diffractometer (XRD, D/Max-3C, Rigaku Co., Tokyo, Japan) using Cu Kα radiation (λ = 1.5418 Å). The specific surface areas were measured from the Brunauer–Emmett–Teller (BET) plots. XPS (Thermo Scientific K-Alpha) was used to probe the binding energy distributions of the material.
Photoelectrochemical test
Electrochemical measurements including electrochemical impedance spectroscopy (EIS) and photocurrent tests were carried out on a CHI660C electrochemical workstation (Shanghai Chenhua, China) with a conventional standard three-electrode system (Pt plate as the counter electrode, Ag/AgCl as the reference electrode, depositing samples on FTO conductive glasses as working electrode and 0.5 M Na2SO4 aqueous solution as the electrolyte). The photocurrent tests were measured under a 500 W Xe lamp equipped with an ultraviolet cutoff filter (λ > 420 nm) (CHF-XM, Beijing Changtuo Technology Co. Ltd, China) as the visible light. EIS plots were performed in the frequency range from 10−2 to 105 Hz with amplitude of 5 mV under the 500 W Xe lamp.
Photodegradation and active species trapping experiments
The photodegradation activity was estimated by degradation of rhodamine B (RhB), methylene blue (MB) and phenol in an aqueous solution under visible-light irradiation. A 300-W Xenon lamp with a 420 nm cutoff filter (Changtu, China) was used as irradiation source (λ > 420 nm). The distance between the lamp and the sample was 15 cm. In a typical photocatalytic degradation of RhB experiment, 50 mg of the photocatalyst was dispersed in 50 mL of RhB solution (10 mg L−1). The suspension was stirred for 30 min in the dark to reach an adsorption–desorption equilibrium and subsequently be illuminated for different time intervals. Three milliliters of suspension was collected and centrifuged to remove the photocatalyst. The photocatalytic degradation of MB and phenol experiments was similar to that of RhB except the concentration of the dye, where a 5 mg L−1 MB solution and 20 mg L−1 phenol solution were used. The residual RhB, MB and phenol concentration was monitored via a UV–Vis spectrophotometer (UV1901PC) based on the maximum absorption at 554 nm, 664 nm and 270 nm. To investigate the reaction mechanism for photodegradation of RhB, active species trapping experiments were carried out under visible-light irradiation. The active species ·O2−, h+ and ·OH were detected with the addition of benzoquinone (BQ, 2 mmol L−1), disodium ethylenediaminetetraacetate (Na2-EDTA, 5 mmol L−1) and isopropyl alcohol (IPA, 10 mmol L−1), respectively.
Results and discussion
Morphological and structural characteristics
The morphology and structure of the N-TR and N-TYS are elucidated in detail through SEM and TEM and shown in Fig. 2. As shown in Fig. 2a, rod-like shapes with tens of micrometer in length and 0.5–2 μm in diameter are observed. Zooming into the surface of the microrod gives an impression that each rod contains several tiny rods with approximately same length (Fig. 2b). Combined with TEM image (Fig. 2c), these microrods are not completely solid. Meanwhile, considering self-assembled of these tiny rods, the interior of the microrod can form long and narrow channel, leading light into the interior easily. When increasing the precursor concentration, the well-defined yolk-like spheres with a mean diameter of 1 μm can be observed from Fig. 2e. From the enlarged SEM image (Fig. 2f), the external surface of the shell consists of numerous single layer spherical structure, and a small core is found to be embedded in the chamber. The yolk-like structure with shell thickness ~ 100 nm and the diameter of yolk 400–600 nm was clearly demonstrated in TEM image (Fig. 2g). In fact, the microrod, the shell and the core are composed of smaller particles with a diameter of ~ 10 nm (as shown in Fig. S1). As the primary nanobuilding blocks, these nanoparticles self-assemble into unique 1D and 3D structures. Moreover, in Fig. 2d and g, a great many mesopores (size of ~ 5 nm) can be observed in both the structures. The lattice spacing of N-TR and N-TYS is ascertained about 0.35 nm, corresponding to the (1 0 1) plane of the anatase phase. The SAED pattern is also consistent well with the XRD pattern. However, compared with the SAED pattern of N-TR, the N-TYS is not well-defined Debye–Scherrer rings, suggesting that rod-like structure possesses higher crystallinity than yolk/shell structure. In addition, N element of N-TR and N-TYS is sufficiently confirmed by energy-dispersive X-ray spectroscopy (EDX) and depicted in detail in Supporting information (Fig. S2, Text S1). It should be pointed out that the N element barely affects the morphology of TiO2. The SEM images of TR and TYS are displayed in Fig. S3.
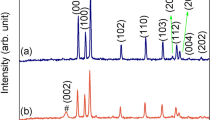
Figure 3a shows the XRD patterns of N-TR and N-TYS. The peaks at 25.24°, 37.88°, 47.81°, 54.14°, 54.92° and 62.53° are observed from the two samples, presenting good agreement with the anatase phase (JCPDS card No. 21-1272). Besides, no other peaks such as N–O and Ti–N were detected in the samples, which may be ascribed to the reason that the content of N was too little to be recorded. The crystallite size is calculated based on the Scherrer formula in Table 1. The Raman spectra of N-TR and N-TYS are also examined to identify the structural properties (Fig. S4). Based on the SEM, TEM, XRD and Raman spectra, it is apparent that these TiO2 hierarchical structures present similar primary nanobuilding blocks and crystal structure but the morphology.
Figure 3b shows the nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves of the N-doped samples. The N2-BET surface areas (SBET) for N-TR and N-TYS are calculated to be 190.82 m2 g−1 and 166.59 m2 g−1, respectively. Although all samples show IV-type isotherm, the hysteresis loop and pore size distribution have significant differences. For rod-shaped TiO2, the observed H4 hysteresis loop suggests the presence of slit-like pores, corresponding to the overlap among rods [42]. Furthermore, the mean pore size distribution is determined as ~ 4.87 nm. The yolk-like structure shows H1 hysteresis loop, indicating the formation of cylindrical pores, and the average pore diameter is about 6.7 nm [42]. The summary of BET surface area, pore diameter and pore volume of the N-TR and N-TYS is depicted in Table 1. We can find that the specific surface area of N-TR is higher than that of N-TYS, but the pore size and volume of N-TR is smaller than that of N-TYS. Apparently, the difference of morphology leads to the different surface areas and porous structures.
To investigate the influence of morphology on chemical state of elements, X-ray photoemission spectroscopy (XPS) is recorded and shown in Fig. 4. The survey scan spectrum shows that N-TR and N-TYS contain Ti, O, C and N elements, indicating that the N atom is successfully doped into the samples (Fig. 4a). After correcting with C 1s peak (284.8 eV), all the peaks of N-TR shift to higher binding energies, indicating the difference of morphology could slightly affect the chemical environment of elements (Fig. 4b–d). Moreover, the high-resolution N 1s peak (Fig. 4c) is carefully analyzed. By fitting the curves, four peaks can be observed. For N-TYS, the peaks at 398.9 eV, 399.7 eV, 400.8 eV and 401.8 eV correspond to the Pydinic N, substitutional N for O in the form of O–Ti–N bonding and the surface adsorption of N2 and NOx species, respectively. The result indicates that nitrogen atoms are mainly incorporated into substitutional sites of the lattice [41, 43, 44]. When changing the precursor content to obtain N-TR, the area of Pydinic N peak significantly increases, indicating that the rod-like structure can not only introduce substitutional nitrogen but also easily form carbon and nitrogen bonding. In addition, the C 1s peak can also be analyzed (Fig. 4d). The main peak located at 284.8 eV is assigned to the C–C bonds, and the other two peaks can be fitted to C–N and O–C=O bonding [43, 45]. An apparent increase in relative intensity of C–N bonds in N-TR can be observed, which once again proved that nitrogen is easier to be introduced to bond with carbon in the microrods than yolk-like spheres. Based on the XPS analysis, the atomic ratio of nitrogen element is 3.24% in N-TR and 2.57% in N-TYS. Considering the molar ratio of TiOSO4·2H2O to urea is the same and area of other N 1s peaks is similar, the above difference mainly ascribed to the bonding of carbon and nitrogen. The difference of morphology leads to different specific surface areas and mesopores structures; this may be the reason for different nitrogen incorporation conditions.
In the present case, the N-doped 1D rod-like and 3D yolk-like TiO2 hierarchical structures are successfully synthesized by only adjusting the precursor concentration. Therefore, it is necessary to explore the formation process of the two unique structures in detail. The experiments of intermediate precursor contents (0.86 g, 1.72 g, 3.44 g) are carried out and shown in Fig. 5a–c. Meanwhile, the experiments of different solvothermal reaction times (0.5 h, 2 h, 14 h, 26 h) are also illustrated and discussed in Fig. S5 and Text S2. As precursor content increases from 0.43 to 1.72 g, the morphology of the resultant TiO2 samples can be tuned from microrods to irregular aggregation of rods and spheres, which can be described in detail as a lot of messy particles appear among the microrods, and then, these microrods shorten and become thicker within the spheres growing out of the rods (Fig. 5a and b). On further increasing the concentration content to 3.44 g and 6.88 g, the rod-like structure disappears and the yolk-like structure eventually forms (Fig. 5c). Though the evolution of the morphology depends on the precursor concentration, further sufficient evidence is still needed to explain the formation mechanism of the two unique hierarchical structures. Therefore, the SEM image of TiOSO4·2H2O precursor is investigated in Fig. 5d. Interestingly, TiOSO4·2H2O also exhibits 1D rod-like morphology. Compared with N-TR, the TiOSO4·2H2O rods have smoother surface and larger aspect ratio. In addition, at low dose of precursor, the precursor solution is milky white (Fig. 5f). From the SEM image of precursor solution of N-TR (Fig. 5e), the rod-like structure is highly similar to TiOSO4·2H2O. When the precursor concentration is higher than supersaturation, the solution will hydrolyze to form a white translucent suspension instead of the previous milky white suspension (Fig. 5f). Moreover, a gelatinous precipitate at the bottom of the beaker was observed. Therefore, this state should be hydrogels.
According to all above results, a possible formation mechanism of the microrods and yolk-like spheres is illustrated in Fig. 6. At the initial stage, the hydrolysis and alcoholysis reaction of TiOSO4·2H2O produces nascent TiO2 nuclei. At low amounts of TiOSO4·2H2O, TiO2 nuclei follows the in situ growth mechanism [23, 46, 47]. The seeds in situ growth to form tiny rods follow the morphology of TiOSO4·2H2O. As time goes on, these tiny rods assemble more closely and gradually become shorter and thicker, eventually self-assembled into rod-like hierarchical structure. At high dose of TiOSO4·2H2O, the precursor solution will undergo a sol process to form active monomers via hydrolysis and alcoholysis reaction. These active monomers gradually evolved into numerous TiO2 nanoparticles with uniform size in a chemical reaction. Due to the high surface energy, these TiO2 nanoparticles spontaneously aggregate into the spherical solid structural TiO2 [48, 49]. Thereafter, the Ostwald ripening process occurred. The nanoparticles located in the inner core have higher curvature compared with those in the outer shell. Thus, they have higher surface energies and faster dissolving rate than the shell. As a result, the nanoparticles of the inner surface will be dissolved and re-deposited on the outer surface. With the reaction time prolonged, the interior core further dissolved, and the cavity between core and shell was expanded, finally forming the well-defined yolk-like TiO2 hierarchical structure [29, 50,51,52]. Other transitional morphologies are formed by the combination of these two mechanisms.
Photocatalytic properties and mechanism
In this part, in addition to N-TR and N-TYS, a series of photocatalytic characterizations of undoped samples were also investigated under the same condition. Although the theme of this work is to discuss the influence of different-dimensional TiO2 hierarchical architectures on photocatalytic performance, it is essential to know the role of nitrogen introduction in our experiment. It is generally accepted that N doping has the potential of narrowing the band gap via elevating the valence band, which further increases the visible-light response region. To investigate the influence of 1D and 3D N-doped TiO2 on the optical absorption performance, UV–Vis diffuse reflectance spectra of TR, TYS, N-TR and N-TYS were recorded by UV–Vis spectrophotometer. Compared with the TR and TYS, absorption edges of N-doped samples are slightly red-shifted and their visible-light harvesting abilities increase, which can be observed clearly in Fig. 7a. For the four samples, the band gaps are estimated from plots of (F(R)*hν)1/2 versus hν. As shown in Fig. 7b, the band gaps Eg estimated from the intercept of the tangents of the plots are determined to be 3.15 eV for both N-doped samples, while N-free samples are 3.20 eV [53]. This result confirms that the wider visible-light response region corresponds to narrower band gap. Interestingly, morphology and elemental chemical state variation have a little influence on the band gap, which eliminate the effects of different visible-light response regions on photocatalytic activity.
The photoelectrochemical properties of the four TiO2 samples were also investigated. The electrochemical impedance spectrum (EIS) was measured to investigate charge transport behaviors, and the results are shown in Fig. 8a. Whether N doping or not, the arc radius of the 1D rod-like structure is smaller than 3D yolk-like structure, suggesting that microrods have the lower resistance, higher charge mobility and separation efficiency. In addition, the transient photocurrent response curves were measured to investigate the ability of separating photogenerated electrons and holes in Fig. 8b. The photocurrent response shows a similar trend that observed from the electrochemical impedance spectrum, where the rod-like structure exhibits higher photocurrent density than yolk-like structure. In addition, the N-doped TiO2 possesses higher charge separation efficiency due to the increased visible-light absorption region. In summary, different-dimensional TiO2 hierarchical architectures have great impact on charge separation and transport. In this case, 1D rod-like structure is more benefit to charge separation and transport than 3D yolk-like structure, which is expected to enhance photocatalytic performance. The difference may be attributed to the fact that one-dimensional structure could provide a fast charge transport pathway, which could facilitate the electron transfer and suppress electron–hole pair recombination.
The final goal for the fabrication of unique 1D and 3D hierarchical architectures with N doping is to improve its photocatalytic property in the visible-light range. In this work, we carried out the photocatalytic degradation of RhB, MB and phenol to evaluate the photodegradation performance of TR, TYS, N-TR and N-TYS under visible-light irradiation [54]. For comparison, the degradation efficiency of P25, an excellent commercial TiO2 photocatalyst, was also investigated under the same condition. The results are shown in Fig. 9. During a 30-minute dark adsorption reaction, we found an interesting phenomenon: the adsorption of dyes for core–shell structural TiO2 is better than the rod structural TiO2. Although the specific surface area of the core–shell structure is slightly smaller, the pore size and pore volume are larger, which may be the reason for its better adsorption performance. But this amount of adsorption is negligible for degradation. Whether doping or not, the rod-like structure exhibits much higher RhB, MB and phenol photodegradation efficiency than yolk-like structure (Fig. 9a, c and e). In addition, P25 exhibits the lowest photodegradation activity. In order to compare the difference conveniently, the degradation kinetic model was also studied. Figure 9b, d and f shows that the relationship between time and ln (C0/C) is almost a straight line, which proves that the process of degradation fits the pseudo-first-order reaction [55]. The photodegradation of RhB rate constant (k) values of P25, TYS, TR, N-TYS and N-TR is determined to be 0.0339, 0.0922, 0.1138, 0.2816 and 0.4088 min−1, respectively. The photodegradation of MB rate constant values is determined to be 0.0068, 0.0112, 0.0177, 0.0376 and 0.04723 min−1, and the photodegradation of phenol rate constant values is determined to be 0.0001, 0.0017, 0.0022, 0.0058 and 0.0076 min−1. Based on the above data, we can find that the degradation of RhB is the easiest, while phenol is relatively difficult. However, the performance law between the samples is unchanged, that is, the photodegradation rate: N-TR > N-TYS > TR > TYS > P25. To exclude the effect of dye self-degradation phenomenon, we also carried out the blank experiments. Fig. S6 shows that RhB and phenol photodegradation are negligible without TiO2 under visible-light illumination, while MB photodegradation is relatively serious.
In addition, the photodegradation performance of other samples with different intermediate amounts of precursor for the photodegradation of RhB was also investigated and is shown in Fig. S7. As precursor content increases from 0.43 to 6.88 g, the degradation rate decreases first and then rise. This result indicates that in the photocatalytic field, the advantages of 1D and 3D hierarchical structures are obvious, and irregular transition morphology is not conducive to the improvement of photocatalytic performance (Fig. S7c).
Based on the above experimental results, several significant conclusions can be summarized. Firstly, it is clearly observed that photocatalytic activity of TR, TYS, N-TR, N-TYS is higher than that of P25, especially N-TR is 12 times than P25 for the photodegradation of RhB. It is generally accepted that P25 belongs to 0D nanoparticles with an average particle size of 25 nm. Interestingly, in our work, 1D microrods and 3D yolk-like spheres as hierarchical architectures are composed of 0D nanoparticles. The result confirms that unique superstructures have benefitted impact on photocatalytic capabilities. Moreover, the XRD pattern of N-TR hardly changes after degradation, indicating the stability of as-prepared samples (Fig. S8). Secondly, 1D rod-like structure exhibits higher photodegradation efficiency than 3D yolk-like structure, which ascribes to the reason that rod structure possesses unique properties such as relatively high specific area (190.8 m2 g−1, Table 1), relatively better crystallinity, direct electrical pathway and higher light utilization rate [24, 25]. Although the hierarchical yolk-like structure endows the N-TYS with sufficient interior space to capture photons, the narrow space of N-TR, which is formed among internal nanorods, makes it easier to increase multiple internal reflections of light [23]. In addition, 1D structure also benefits for charge separation and transport. Finally, the N-doped samples exhibit higher photodegradation efficiency than bare TiO2 because of the synergetic effects between enhanced visible-light harvesting capabilities and higher electron separation efficiency.
To further elucidate their intrinsic mechanism for photocatalytic degradation of RhB, the effect of active species of N-TR was investigated via radical trapping experiments. As shown in Fig. 10, after adding BQ and Na2-EDTA, the degradation process of RhB is obviously suppressed, while IPA has a little effect on the degradation, indicating that the active species ·O2− and h+ play significant role in photocatalytic reaction but ·OH.
According to the above mentioned, we propose the mechanism for the visible-light photocatalytic reaction over N-doped TiO2 (Fig. 11). N doping can narrow the band gap and cause visible-light response, accounting for the visible-light absorption of N-doped samples. Under visible-light irradiation, N-TR and N-TYS can be excited and produce electrons and holes pairs. Subsequently, the electrons which transmitted to the conduction band react with O2 to produce active species ·O2−. The redox properties of ·O2− and h+ lead to rapid photocatalytic degradation of RhB.
Conclusions
In summary, the tunable 1D rod-like and 3D yolk-like N-doped TiO2 hierarchical architectures were successfully fabricated by one-step solvothermal route via changing the precursor concentration. The morphologies, one-dimension and three-dimension are caused by in situ growth and Ostwald ripening, respectively. The unique N-doped rod-like and yolk-like hierarchical structures significantly increase the specific surface area and light utilization rate; meanwhile, nitrogen doping expands the visible-light response range, both of which improve the photocatalytic performance compared with commercial P25. Furthermore, through the systematic comparison, N-doped 1D rod-like structure with the unique hierarchical structure and larger specific surface area exhibits the excellent performance in terms of photodegradation, charge separation ability and electron–hole recombination efficiency, indicating that the proper TiO2 dimension with a large specific surface area is greatly promising technique for enhancement of photocatalysis. This work can provide a guidance for researchers in the field of morphology control.
References
Natarajan S, Bajaj HC, Tayade RJ (2018) Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J Environ Sci-China 65:201–222
Nakata K, Ochiai T, Murakami T, Fujishima A (2012) Photoenergy conversion with TiO2 photocatalysis: new materials and recent applications. Electrochim Acta 84:103–111
Liang HJ, Jia ZC, Zhang HC, Wang XB, Wang JJ (2017) Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B. Appl Surf Sci 422:1–10
Wang P, Jia CC, Li J, Yang P (2019) Ti3+-doped TiO2(B)/anatase spheres prepared using thioglycolic acid towards super photocatalysis performance. J Alloy Compd 780:660–670
Liu Q, Ding DY, Ning CQ, Wang XW (2015) Black Ni-doped TiO2 photoanodes for high-efficiency photoelectrochemical water-splitting. Int J Hydrogen Energy 40:2107–2114
Darbandi M, Dickerson JH (2016) Nanoscale engineering of TiO2 nanoparticles: evolution of the shape, phase, morphology, and facet orientation. Mater Lett 180:212–218
Sarkar B, Singhal N, Goyal R, Bordoloi A, Konathala LNS, Kumar U, Bal R (2016) Morphology-controlled synthesis of TiO2 nanostructures for environmental application. Catal Commun 74:43–48
He XY, Zhang CL (2019) Recent advances in structure design for enhancing photocatalysis. J Mater Sci 54:8831–8851. https://doi.org/10.1007/s10853-019-03417-8
Zha RH, Nadimicherla R, Guo X (2015) Morphology engineering of nanostructured TiO--2 particles. RSC Adv 5:6481–6488
Verbruggen SW (2015) TiO2 photocatalysis for the degradation of pollutants in gas phase: from morphological design to plasmonic enhancement. J Photochem Photobiol C 24:64–82
Dufour F, Pigeot-Remy S, Durupthy O et al (2015) Morphological control of TiO2 anatase nanoparticles: What is the good surface property to obtain efficient photocatalysts? Appl Catal B-Environ 174:350–360
Sambandam B, Surenjan A, Philip L, Pradeep T (2015) Rapid synthesis of C-TiO2: tuning the shape from spherical to rice grain morphology for visible light photocatalytic application. ACS Sustain Chem Eng 3:1321–1329
Chen Y, Li WZ, Wang JY, Gan YL, Liu L, Ju MT (2016) Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl Catal B-Environ 191:94–105
Lv J, Gao HZ, Wang HG et al (2015) Controlled deposition and enhanced visible light photocatalytic performance of Pt-modified TiO-2 nanotube arrays. Appl Surf Sci 351:225–231
Yu X, Zhao ZH, Zhang J, Guo WB, Li LL, Liu H, Wang ZL (2017) One-step synthesis of ultrathin nanobelts-assembled urchin-like anatase TiO2 nanostructures for highly efficient photocatalysis. CrystEngComm 19:129–136
Chen W, Wang Y, Liu S et al (2018) Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl Surf Sci 445:527–534
Wang H, Wu D, Wu W et al (2019) Preparation of TiO2 microspheres with tunable pore and chamber size for fast gaseous diffusion in photoreduction of CO2 under simulated sunlight. J Colloid Interface Sci 539:194–202
Anwer S, Bharath G, Iqbal S et al (2018) Synthesis of edge-site selectively deposited Au nanocrystals on TiO2 nanosheets: an efficient heterogeneous catalyst with enhanced visible-light photoactivity. Electrochim Acta 283:1095–1104
Sharma PK, Cortes M, Hamilton JWJ, Han YS, Byrne JA, Nolan M (2019) Surface modification of TiO2 with copper clusters for band gap narrowing. Catal Today 321:9–17
Colomer MT, del Campo A (2019) Preparation of nanostructured TiO2 films with high catalytic activity and their 3D spatial distribution of anatase and rutile phases. J Mater Sci 54:9414–9425. https://doi.org/10.1007/s10853-019-03550-4
Devan RS, Patil RA, Lin JH, Ma YR (2012) One-dimensional metal-oxide nanostructures: recent developments in synthesis, characterization, and applications. Adv Funct Mater 22:3326–3370
Leng M, Chen Y, Xue JM (2014) Synthesis of TiO2 nanosheets via an exfoliation route assisted by a surfactant. Nanoscale 6:8531–8534
Mohan Kumar K, Godavarthi S, Karthik TVK et al (2016) Green synthesis of S-doped rod shaped anatase TiO2 microstructures. Mater Lett 183:211–214
Zhu K, Vinzant TB, Neale NR, Frank AJ (2007) Removing structural disorder from oriented TiO2 nanotube arrays: reducing the dimensionality of transport and recombination in dye-sensitized solar cells. Nano Lett 7:3739–3746
Li SS, Chang CP, Lin CC et al (2011) Interplay of three-dimensional morphologies and photocarrier dynamics of polymer/TiO2 bulk heterojunction solar cells. J Am Chem Soc 133:11614–11620
Zhang ZH, Wu HJ (2014) Multiple band light trapping in ultraviolet, visible and near infrared regions with TiO2 based photonic materials. Chem Commun 50:14179–14182
Lei BX, Liao JY, Zhang R, Wang J, Su CY, Kuang DB (2010) Ordered crystalline TiO2 nanotube arrays on transparent FTO glass for efficient dye-sensitized solar cells. J Phys Chem C 114:15228–15233
Gao M, Zhu L, Ong WL, Wang J, Ho GW (2015) Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal Sci Technol 5:4703–4726
Lou XW, Wang Y, Yuan C, Lee JY, Archer LA (2006) Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv Mater 18:2325–2329
Chen G, Cheng H, Zhang W, Yang Z, Qiu M, Zhu X, Chen M (2015) Template-free synthesis of single-/double-walled TiO2 nanovesicles: potential photocatalysts for engineering application. AIChE J 61:1478–1482
Yang J, Wen ZH, Shen XX, Dai J, Li Y, Li YJ (2018) A comparative study on the photocatalytic behavior of graphene-TiO2 nanostructures: effect of TiO2 dimensionality on interfacial charge transfer. Chem Eng J 334:907–921
Aoi Y, Kobayashi S, Kamijo E, Deki S (2005) Fabrication of three-dimensional ordered macroporous titanium oxide by the liquid-phase deposition method using colloidal template. J Mater Sci 40:5561–5563. https://doi.org/10.2472/jsms.53.1313
Paramasivam I, Jha H, Liu N, Schmuki P (2012) A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 8:3073–3103
Dong F, Zhao WR, Wu ZB (2008) Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confinement effect. Nanotechnology 19:365607–365616
Chen K, Fan QH, Chen CL, Chen ZS, Alsaedi A, Hayat T (2019) Insights into the crystal size and morphology of photocatalysts. J Colloid Interface Sci 538:638–647
Li HX, Bian ZF, Zhu J et al (2007) Mesoporous titania spheres with tunable chamber stucture and enhanced photocatalytic activity. J Am Chem Soc 129:8406–8407
Zeng L, Song WL, Xie CS (2014) Fabrication of TiO2 rod in tube nanostructure with enhanced photocatalytic activity: investigation of the effect of the states of the precursor on morphology. RSC Adv 4:36708–36712
Yang XY, Peng HL, Zou ZM et al (2018) Diethylenediamine-assisted template-free synthesis of a hierarchical TiO2 sphere-in-sphere with enhanced photocatalytic performance. Dalton T 47:16502–16508
Ye T, Chen W, Xu H, Geng NN, Cai Y (2018) Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J Mater Sci 53:613–625. https://doi.org/10.1007/s10853-017-1509-4
Yang GD, Jiang Z, Shi HH, Xiao TC, Yan ZF (2010) Preparation of highly visible-light active N-doped TiO2 photocatalyst. J Mater Chem 20:5301–5309
Li X, Liu P, Mao Y, Xing M, Zhang J (2015) Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl Catal B-Environ 164:352–359
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem Mater 13:3169–3183
Liu C, Zhang L, Liu R et al (2016) Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J Alloy Compd 656:24–32
Lin Z, Waller G, Liu Y, Liu M, Wong C-P (2012) Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv Energy Mater 2:884–888
Chen CS, Liu XY, Long H, Ding F, Liu QC, Chen XA (2019) Preparation and photocatalytic performance of graphene Oxide/WO3 quantum Dots/TiO2@SiO2 microspheres. Vacuum 164:66–71
Tian G, Chen Y, Bao H-L et al (2012) Controlled synthesis of thorny anatase TiO2 tubes for construction of Ag-AgBr/TiO2 composites as highly efficient simulated solar-light photocatalyst. J Mater Chem 22:2081–2088
Tian GH, Chen YJ, Zhou W, Pan K, Tian CG, Huang XR, Fu HG (2011) 3D hierarchical flower-like TiO2 nanostructure: morphology control and its photocatalytic property. CrystEngComm 13:2994–3000
Zhang Y, Wang CW, Hou HS, Zou GQ, Ji XB (2017) Nitrogen doped/carbon tuning yolk-like TiO2 and its remarkable impact on sodium storage performances. Adv Energy Mater 7:1600173–1600184
Bao Y, Kang QL, Ma JZ, Liu C (2017) Monodisperse hollow TiO2 spheres for thermal insulation materials: template-free synthesis, characterization and properties. Ceram Int 43:8596–8602
Hua Chun Z (2007) Ostwald ripening: a synthetic approach for hollow nanomaterials. Curr Nanosci 3:177–181
Nguyen DT, Kim K-S (2016) Self-development of hollow TiO2 nanoparticles by chemical conversion coupled with Ostwald ripening. Chem Eng J 286:266–271
Yang HG, Zeng HC (2004) Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J Phys Chem B 108:3492–3495
Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini MC, Giamello E (2007) N-doped TiO2: theory and experiment. Chem Phys 339:44–56
Prakash K, Karuthapandian S, Senthilkumar S (2019) Zeolite nanorods decorated g-C3N4 nanosheets: a novel platform for the photodegradation of hazardous water contaminants. Mater Chem Phys 221:34–56
Chen CS, Cao SY, Long H et al (2015) Highly efficient photocatalytic performance of graphene oxide/TiO2-Bi2O3 hybrid coating for organic dyes and NO gas. J Mater Sci-Mater Electron 26:3385–3391
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51772102, 51972114, 51902107); the National Natural Science Foundation of Guangdong Province (2019A1515011002, 2019A1515011992); the Guangdong YangFan Innovative & Entrepreneurial Research Team Program (2016YT03C327); Project funded by China Postdoctoral Science Foundation (2018M643074, 2019T120728); and Fundamental Research Funds for the Central Universities (2019MS002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No author has financial or other contractual agreements that might cause conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, B., Chen, W., Ma, J. et al. Fabrication of tunable 1D rod-like and 3D yolk-like TiO2 hierarchical architectures for efficient photocatalysis. J Mater Sci 55, 3760–3773 (2020). https://doi.org/10.1007/s10853-019-04247-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04247-4