Abstract
The effect of grain size and phase compositions on piezoelectric coefficient of BaTi0.98Hf0.02O3 ceramics prepared at a series of sintering temperatures (1320, 1350, 1370, and 1400 °C) was studied. The results showed that the grain size of the ceramics is 0.9, 21.3, 21.6, and 37.2 μm, respectively, and the corresponding phase compositions are the tetragonal–orthogonal, tetragonal–orthogonal–rhombohedral, tetragonal–orthogonal, and tetragonal–orthogonal–rhombohedral, while the piezoelectric coefficient is 475, 352, 258, and 327 pC/N, i.e., it decreases first and then increases as the grain size goes up. The phase compositions and grain size of the ceramics are interrelated, and they co-affect the piezoelectric coefficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developing the new materials with high piezoelectric coefficient (\( d_{33} \)) is one of the important fields of materials science. At present, the high \( d_{33} \) at room temperature mainly exists in PbTiO3-based perovskite-type ferroelectrics, and they are widely used in pressure sensors, actuators, and ultrasonic imaging, etc. [1,2,3]. However, considering the Pb-based materials are harmful to human and environment, the exploration of lead-free piezoelectrics is one of the research priorities in this field [4,5,6,7].
Nearly 20 years, the lead-free ceramics with high \( d_{33} \) is developing rapidly. Based on compositions, it can be divided into three systems, such as K1−xNaxNbO3, Bi1/2Na1/2TiO3 and BaTiO3 (BT)-based ceramics [8]. BT ceramic is the earliest discovered piezoelectrics whose \( d_{33} \) is about 190 pC/N [1, 9]. At present, the higher \( d_{33} \) of 700 ± 30 pC/N at room temperature has been achieved in 0.82Ba(Ti0.89Sn0.11)O3–0.18(Ba0.7Ca0.3)TiO3 ceramic [10].
The mechanism of high \( d_{33} \) for BT-based ceramics is mainly studied as follows: (1) Relationship between high \( d_{33} \) and multiphase coexistence in a specific system such as Ren et al. [11] reported the \( d_{33} \) of BaZr0.2Ti0.8O3–0.5Ba0.7Ca0.3TiO3 ceramic is 620 pC/N, which originates from the coexistence of rhombohedral (R) and tetragonal (T) phases due to the compositions being near the morphotropic phase boundary (MPB). Das et al. [12] found that 0.5BaZr0.2Ti0.8O3–0.5Ba0.7Ca0.3TiO3–0.8 wt%CeO2 ceramic has high \( d_{33} \) value (673 pC/N), while the R–T phases also coexist [13,14,15,16,17]. In a word, the multiphase coexistence in specific ceramic around MPB is favorable to the improvement in \( d_{33} \), but the effect of the phase ratios on \( d_{33} \) is not clear; and (2) relationship between high \( d_{33} \) and grain size (\( g \)) in a specific system. For example, with decreasing \( g \), \( d_{33} \) increases in BT ceramics found by Hoshina et al. [18], but reduces in the Ba0.90Ca0.10Ti0.90Sn0.10O3–xY2O3 one, and it increases first and then decreases in Ba1−xCaxTi0.90Sn0.10O3–0.08Dy2O3 obtained by Chen et al. [19,20,21].
In addition, the domain structures also have some influence on the \( d_{33} \). For instance, Li et al. [22] have reported “blurred” grain boundaries may reduce the internal stress and lead to the formation of continuity of domains across the boundaries, resulting in the \( d_{33} \) of BaZr0.2Ti0.8O3–0.5Ba0.7Ca0.3TiO3 ceramic up to 650 pC/N. Besides, Das et al. [23] studied the domain widths of some BaZr0.2Ti0.8O3–0.5Ba0.7Ca0.3TiO3 ceramics with larger \( d_{33} \) and found that the largest \( d_{33} \) corresponds to the widest 90° domains. Li et al. [24] found 0.5BaZr0.2Ti0.8O3–0.5Ba0.7Ca0.3TiO3–xZnO can achieve the highest domain wall density at x = 0.08, at the same time, \( d_{33} \) (603 pC/N) is the maximum.
BaTi1−xHfxO3 and BaTi1−xZrxO3 ceramics have similar phase diagrams [25,26,27] and piezoelectric properties [9, 28, 29]. However, there is still no BaTi1−xHfxO3-based ceramic whose \( d_{33} \) exceeds 600 pC/N [28,29,30,31,32,33,34,35,36,37,38]. Therefore, it is necessary to analyze and study the influence factors to \( d_{33} \) in BaTi1−xHfxO3 ceramics. Up to now, as far as the authors know, there is no study on the co-effect of multiphase coexistence and \( g \) on the high \( d_{33} \) in a specific system. Moreover, it has been found that BaTi1−xHfxO3 ceramics may be MPB near x = 0.04 [25, 31], and could have larger \( d_{33} \). In this paper, BaTi0.98Hf0.02O3 (BTH) ceramics were selected as a candidate system and prepared by solid-state reaction at a series of sintering temperatures (\( T_{s} \)). The influence of phase compositions and \( g \) on \( d_{33} \) was studied by measuring and analyzing the room temperature \( d_{33} \), hysteresis loop, phase compositions, \( g \), and temperature-dependent complex dielectric constant of the ceramics.
Experimental procedure
BTH ceramics were prepared by the conventional solid-state reaction technique with various \( T_{\text{s}} \). High purity (99.99%) powders of BaCO3, TiO2, and HfO2 were used as starting materials. The ingredients were weighed in stoichiometric proportions and wet-mixed with deionized water. After 16 h mixing and then drying, the powder was calcined at 1100 °C for 2 h in air, and BaCO3, TiO2, and HfO2 reacted to form BTH powders. The powders with 2.5 wt% binders were compacted into disk-shaped pellets with a diameter of 13.0 mm and thickness of 1.0–2.0 mm at 10 MPa pressure, followed by burning the binder. BTH pallets were sintered at \( T_{\text{s}} = 1320 \), 1350, 1370, and 1400 °C for 4 h in air.
The room temperature phase compositions and g of BTH ceramics were tested by DX-2600 X-ray diffractometer and KYKY2800B scanning electron microscope (SEM).
The sintered specimens were coated with silver paint on the upper and bottom surfaces and fired at 850 °C for 15 min for electrical measurements. The room temperature hysteresis loops of specimens were performed using the TF Analyzer 2000E at 1 Hz, and the coercive field (\( E_{\text{c}} \)) and remnant polarization (\( P_{\text{r}} \)) were obtained. The poling process was performed under a static electric field of 2.5Ec at room temperature for 20 min in a silicone oil bath, and after 24 h, \( d_{33} \) was measured by Piezotest PM300 at room temperature, 300 Hz, and 0.5 N. The complex dielectric constant (\( \varepsilon^{*} = \varepsilon^{\prime } - i\varepsilon^{\prime \prime } \)) of the specimens was carried out in the temperature range of 100–430 K with heating rate 2 K/min and frequency range of 100 Hz–10 kHz.
Results and discussion
Figure 1a shows \( d_{33} \) of BTH ceramics at room temperature sintered at various \( T_{\text{s}} \). It can be seen that the overall trend of \( d_{33} \) reduces with increasing \( T_{\text{s}} \), and specifically, \( d_{33} \) are 475, 327, 352, and 258 pC/N corresponding to \( T_{\text{s}} = 1320 \), 1350, 1370, and 1400 °C. The hysteresis loops at room temperature of BTH ceramics are given in Fig. 1b. All ceramics exhibit the ferroelectric behaviors, which suggest that they are in the ferroelectric phase at room temperature, and the results agree precisely with the characteristic of BaTi1−xHfxO3 phase diagram [25]. 2\( E_{\text{c}} \) and 2\( P_{\text{r}} \) are read from Fig. 1b and plotted in Fig. 1c, d. The variation trend of 2\( E_{\text{c}} \) is the same as that of \( d_{33} \), but 2\( P_{\text{r}} \) decreases at first and then increases.
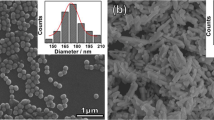
Figure 2 illustrates SEM images (Fig. 2a–d) and the corresponding statistical distribution of grain size (Fig. 2e–h) of BTH ceramics for various \( T_{\text{s}} \), we can find that \( g \) are 0.9, 37.2, 21.3, and 21.6 μm for \( T_{\text{s}} = 1320 \), 1350, 1370, and 1400 °C as shown in Fig. 1e. Compared with 1320 °C, the samples sintered at other \( T_{\text{s}} \) have wide distribution of g. Obviously, the variation trend of g is opposite to that of \( d_{33} \), but it is not inverse linear by comparing Fig. 1a, e, and it is different from that of pure BT ceramics [18, 39]. \( d_{33} \) is maximum when g = 0.9 μm, which is larger than the reported values of BaTi1−xHfxO3 [28, 38], BaTi1−xZrxO3 [40, 41], BaTi1−xSnxO3 [42] ceramics at room temperature, but similar to that value obtained by two-step sintering methods [18, 43]. Because the fine-grained ceramics have a high density of 90° domain walls that is easily moved by an external ac field, high \( d_{33} \) can be obtained [18, 43,44,45].
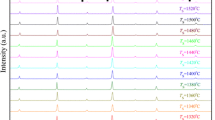
The room temperature X-ray diffraction patterns of BTH ceramics sintered at series \( T_{\text{s}} \) are shown in Fig. 3, and the results indicate that all samples have a pure perovskite phase without visible impurity phase (Fig. 3a). The detailed structural characterization of peaks around 39°, 45°, and 65° is also carried out in Fig. 3b–d.
The standard XRD spectra of BT show that: (1) Cubic (C) phase: Single diffraction peaks appear near 39°, 45°, and 65°, corresponding to (111), (200), and (220) planes, respectively; (2) T phase: Single peak appear near 39°, double peaks of left low and right high near 45°, and double peaks of left high and right low near 65°; (3) O phase: Single peak appear near 39°, double peaks of right low and left high near 45°, and triple peaks near 65°; and (4) R phase: Double peaks appear near 39°, single peak near 45°, and double peaks of right high and left low near 65°. By the standard spectrum of BT and the method in Refs. [13, 16], the fitting results of diffraction peaks near 39°, 45° and 65° of BTH ceramics (Fig. 3b–d) show that the T–O phases coexist in ceramics for \( T_{\text{s}} = 1400 \) °C and 1320 °C, while T–O–R phases for \( T_{\text{s}} = 1370 \) °C and 1320 °C. We would like to point out that, compared with \( T_{\text{s}} = 1320 \) °C, the overall high-angle peak shift of the sample for \( T_{\text{s}} = 1400 \) °C means the cell volume being smaller (Fig. 3a). One possible origination is the different surface effects of grains with different g, and another is the different proportions of T and O phases in the ceramics at different \( T_{\text{s}} \).
Combining with the corresponding values of \( d_{33} \) and g, it is found that the smaller g correspond to higher \( d_{33} \) when ceramics have the same phase compositions. Therefore, it is necessary to consider the effect of phase compositions when discussing the relationship between \( d_{33} \) and g.
Figure 4 illustrates \( \varepsilon^{\prime } \) and \( \varepsilon^{\prime \prime } \) of \( \varepsilon^{*} \) for BTH ceramics measures at various frequencies and temperatures (\( T \)). The results indicate that, similar to pure [46, 47] and low Hf-doped BaTiO3 ceramics [25, 28, 48, 49], there are three phase transitions, i.e., C–T, T–O, and O–R, in 100–430 K, and the corresponding transition temperatures (\( T_{\text{C}} \), \( T_{\text{TO}} \), and \( T_{\text{OR}} \)) vary with \( T_{\text{s}} \). According to the variation of \( \varepsilon^{\prime } \) and \( \varepsilon^{\prime \prime } \) versus T, it could be seen that the T–O phase transition is diffuse and broad, which may be caused by the uneven distribution of Hf, \( g \) distribution, and influence of grain boundaries. It leads to T–O phase coexistence near room temperature. Accordingly, the characteristic peaks of T and O phase were found in XRD spectra of BTH ceramics (Fig. 3c, d).
Based on the peak heights of \( \varepsilon^{'} \) and \( \varepsilon^{\prime \prime } \), the amount of transition between R and O phases and the room temperature content of O phase in the BTH ceramic for \( T_{\text{s}} = 1320 \) °C are larger than the other, and it is one reason for the larger \( d_{33} \) of the ceramic because O phase can bring about exceptionally low elastic modulus [50]. When \( T_{\text{s}} = 1350 \) °C and 1370 °C, the relative variation of \( \varepsilon^{\prime \prime } \) from \( T_{\text{TO}} \) to \( T_{\text{OR}} \) is small, and it can be inferred that O–R phase transition diffuses to higher temperatures due to possible random internal stress, which causes a few amount of R phase to exist when the temperature is much higher than \( T_{\text{OR}} \). Therefore, the characteristic peaks of R phase can be found in the XRD spectra (Fig. 3b). While \( T_{\text{s}} = 1400 \) °C, there is no characteristic peaks of R phase in the XRD spectra (Fig. 3b) due to the smaller dispersion of O–R phase transition.
As shown in Fig. 5a, with increasing \( T_{\text{s}} \), (1) \( T_{\text{C}} \) goes up first and then down; (2) \( T_{\text{TO}} \) always increases; and (3) \( T_{\text{OR}} \) decreases first, then goes up and finally down, while, with increasing g, (4) both \( T_{\text{C}} \) and \( T_{\text{TO}} \) first go up and then down; and (5) \( T_{\text{OR}} \) always decreases (Fig. 5b). In which, the increase in \( T_{\text{C}} \) with g for \( g \le 21.3 \) μm could be explained by the grain size effect [51]; however, the decrease in \( T_{\text{C}} \) for \( g \ge 21.3 \) μm must contain other influencing factors, such as oxygen vacancies [52] and internal stress [51]. For the T–O and O–R phase transitions related to the coupling between the spontaneous polarizations and spontaneous strains, the influence of g, oxygen vacancies and internal stress to the coupling is not quite clear now.
The room temperature \( \varepsilon^{\prime } \) at 100 Hz with g and phase compositions for BTH ceramics of series \( T_{\text{s}} \) is shown in Fig. 6a, and it could be seen that, (1) for the ceramics of the same phase compositions, the smaller the g is, the larger the \( \varepsilon^{\prime } \) is; and (2) the \( \varepsilon^{\prime } \) of the ceramics of T–O phases is larger than that of the T–O–R ones. One possible corresponding mechanism is that, (1) the samples of T–O phases may contain more T phase than those of T–O–R phases, and T phase has higher density of 90° domain walls, which leads to the increase in \( \varepsilon^{\prime } \) [53, 54]; and (2) when the phase compositions are the same, the smaller the g is, the higher the domain wall density of 90° domains is [54].
Figure 1d shows that, with \( T_{\text{s}} \), the change trend of \( P_{\text{r}} \) is contrary to that of cell volume (Fig. 3), and the cell volume is related to phase compositions and g. As indicated by Fig. 6b, although the values of g (21.3 μm for \( T_{\text{s}} = 1370 \) °C) and (21.6 μm for \( T_{\text{s}} = 1400 \) °C) are almost same, \( P_{\text{r}} \) of the ceramics with T–O phases is larger than that of T–O–R ones, i.e., \( P_{\text{r}} \) is different because of the different phase compositions. Moreover, when the ceramics have same phase compositions, \( P_{\text{r}} \) are also different with different g. Figure 6c clearly indicates that \( E_{\text{c}} \) decreases with the increase in g, which may be caused by the decrease in internal stress with the decrease in g [54, 55].
According to the above analyses, the variation of \( d_{33} \) for BTH ceramics with g and phase compositions are shown in Fig. 6d. It is found that, with increasing g, \( d_{33} \) decreases first and then increases, and when the ceramics have the same phase compositions, the larger the g is, the smaller the \( d_{33} \) is. Specifically, for \( g = 0.9 \), 21.3, 21.6, and 37.2 μm, the phase compositions of the ceramics are T–O, T–O–R, T–O, and T–O–R phases, respectively, and \( d_{33} \) decreases with increasing g, which indicates \( d_{33} \) is mainly affected by g when \( g \le 21.6 \) μm [18, 39]. However, \( d_{33} \) goes up when \( g \ge 21.6 \) μm, i.e., \( d_{33} \) of T–O–R phase coexistence is higher than that of T–O, so it is mainly affected by phase compositions, which is consistent with the conclusion that the multiphase coexistence of T–O–R leads to a low energy barrier for the polarization rotations, resulting in the high \( d_{33} \) [16, 42, 56, 57].
The author considers that the reasons for the increase in \( d_{33} \) with the decrease in g in ceramics of same phase compositions are as follows: (1) With the decrease in g, the ratio of surface layer to volume of the grains increases; and (2) the interaction energy between a permanent dipole and its neighbors in the surface layer is higher than that of the permanent dipole in grain interior, i.e., its stability is lower. In other words, under the same external stress, the dipoles in the surface layer are more likely to be oriented and polarized, resulting in higher piezoelectric coefficients.
It is worthwhile pointing out that the Ranjan et al. [28] reported \( d_{33} \) of BTH ceramic of O–T phases (sintered 1300 °C for 4 h and then 1450–1500 °C for 6 h) is 370 pC/N, much smaller than 475 pC/N in this work. It can be expected that g of the ceramic of Ranjan et al. is much larger than ours (sintered at 1320 °C for 4 h), which is consistent with the results of Fig. 6d in this paper.
Conclusions
BTH ceramics near MPB were prepared by traditional solid-state reaction at series \( T_{\text{s}} \). The room temperature \( d_{33} \), 2\( P_{\text{r}} \), \( 2E_{\text{c}} \), g, and phase compositions, as well as \( \varepsilon ' \) and \( \varepsilon^{\prime \prime } \) with temperature at series frequencies were tested and analyzed. The results indicate that, when \( T_{\text{s}} = 1320 \), 1350, 1370, and 1400 °C, \( d_{33} \) is 475, 327, 352, and 258 pC/N, \( g \) is 0.9, 37.2, 21.3, and 21.6 μm, and the corresponding phase compositions are T–O, T–O–R, T–O–R, and T–O phases, respectively. In other words, with increasing g, \( d_{33} \) decreases first and then increases, and when the ceramics have the same phase compositions, the larger the \( g \) is, the smaller the \( d_{33} \) is. Therefore, the phase compositions and g of the ceramics are interrelated and co-affect \( d_{33} \).
References
Jaffe H, Berlincourt DA (1965) Piezoelectric transducer materials. Proc IEEE 53(10):1372–1386. https://doi.org/10.1109/PROC.1965.4253
Cross E (2004) Materials science: lead-free at last. Nature 432(7013):24–25. https://doi.org/10.1038/nature03142
Zhang SJ, Li F, Yu FP, Jiang XN, Lee HY, Luo J, Shrout TR (2018) Recent developments in piezoelectric crystals. J Korean Ceram Soc 55(5):419–439. https://doi.org/10.4191/kcers.2018.55.5.12
Rödel J, Webber KG, Dittmer R, Jo W, Kimura M, Damjanovic D (2015) Transferring lead-free piezoelectric ceramics into application. J Eur Ceram Soc 35(6):1659–1681. https://doi.org/10.1016/j.jeurceramsoc.2014.12.013
Wu JG, Xiao DQ, Zhu JG (2015) Potassium–sodium niobate lead-free piezoelectric materials: past, present, and future of phase boundaries. Chem Rev 115(7):2559–2595. https://doi.org/10.1021/cr5006809
Shrout TR, Zhang SJ (2007) Lead-free piezoelectric ceramics: alternatives for PZT? J Electroceram 19(1):113–126. https://doi.org/10.1007/s10832-007-9047-0
Wang XP, Wu JG, Xiao DQ, Zhu JG, Cheng X, Zheng T, Zhang BY, Lou XJ, Wang XJ (2014) Giant piezoelectricity in potassium–sodium niobate lead-free ceramics. J Am Chem Soc 136(7):2905–2910. https://doi.org/10.1021/ja500076h
Trolier-McKinstry S, Zhang SJ, Bell AJ, Tan XL (2018) High-Performance piezoelectric crystals, ceramics, and films. Annu Rev Mater Res 48:191–217. https://doi.org/10.1146/annurev-matsci-070616-124023
Acosta M, Novak N, Rojas V, Patel S, Vaish R, Koruza J, Rossetti GA, Rödel J (2017) BaTiO3-based piezoelectrics: fundamentals, current status, and perspectives. Appl Phys Rev 4(4):41305. https://doi.org/10.1063/1.4990046
Zhao CL, Wu HJ, Li F, Cai YQ, Zhang Y, Song DS, Wu JG, Lyu X, Yin J, Xiao DQ, Zhu JG, Pennycook SJ (2018) Practical high piezoelectricity in barium titanate ceramics utilizing multiphase convergence with broad structural flexibility. J Am Chem Soc 140(45):15252–15260. https://doi.org/10.1021/jacs.8b07844
Liu WF, Ren XB (2009) Large piezoelectric effect in Pb-free ceramics. Phys Rev Lett 103(25):257602. https://doi.org/10.1103/PhysRevLett.03.257602
Chandrakala E, Paul Praveen J, Kumar A, James AR, Das D, Damjanovic D (2016) Strain-induced structural phase transition and its effect on piezoelectric properties of (BZT-BCT)-(CeO2) ceramics. J Am Ceram Soc 99(11):3659–3669. https://doi.org/10.1111/jace.14409
Zhou PF, Zhang BP, Zhao L, Zhao XK, Zhu LF, Cheng LQ, Li JF (2013) High piezoelectricity due to multiphase coexistence in low-temperature sintered (Ba, Ca)(Ti, Sn)O3-CuOx ceramics. Appl Phys Lett 103(17):172904. https://doi.org/10.1063/1.4826933
Long PQ, Liu XT, Long X, Yi ZG (2017) Dielectric relaxation, impedance spectra, piezoelectric properties of (Ba, Ca)(Ti, Sn)O3 ceramics and their multilayer piezoelectric actuators. J Alloys Compd 706:234–243. https://doi.org/10.1016/j.jallcom.2017.02.237
Jiang XP, Li L, Chen C, Tang J, Zheng KP, Li XH (2014) Structure and properties of (Ba0.85Ca0.15)(Ti0.9Zr0.1−xSnx)O3 lead-free ceramics with high piezoelectric constant. J Inorg Mater 29(1):33–37. https://doi.org/10.3724/sp.J.1077.2014.13213
Zhu LF, Zhang BP, Zhao XK, Zhao L, Yao FZ, Han X, Zhou PF, Li JF (2013) Phase transition and high piezoelectricity in (Ba, Ca)(Ti1−xSnx)O3 lead-free ceramics. Appl Phys Lett 103(7):72905. https://doi.org/10.1063/1.4818732
Wu JG, Xiao DQ, Wu WJ, Chen Q, Zhu JG, Yang ZC, Wang J (2012) Composition and poling condition-induced electrical behavior of (Ba0.85Ca0.15)(Ti1−xZrx)O3 lead-free piezoelectric ceramics. J Eur Ceram Soc 32(4):891–898. https://doi.org/10.1016/j.jeurceramsoc.2011.11.003
Hoshina T, Hatta S, Takeda H, Tsurumi T (2018) Grain size effect on piezoelectric properties of BaTiO3 ceramics. Jpn J Appl Phys 57(9):0902BB. https://doi.org/10.7567/jjap.57.0902bb
Chen ZH, Li ZW, Qiu JH, Zhao TX, Ding JN, Jia XG, Zhu WQ, Xu JJ (2018) Y2O3 doped Ba09Ca0.1Ti0.9Sn0.1O3 ceramics with improved piezoelectric properties. J Eur Ceram Soc 38(4):1349–1355. https://doi.org/10.1016/j.jeurceramsoc.2017
Chen ZH, Li ZW, Ma MG, Qiu JH, Zhao TX, Ding JN, Jia XG, Zhu KQ (2018) Enhanced piezoelectric properties in (Ba1−xCax)(Ti0.90Sn0.10)O3-0.08Dy2O3 lead-free ceramics. Mater Res Bull 105:330–333. https://doi.org/10.1016/j.materresbull.2018.05.004
Wu JG, Xiao DQ, Wu WJ, Chen Q, Zhu JG, Yang ZC, Wang J (2011) Role of room-temperature phase transition in the electrical properties of (Ba, Ca)(Ti, Zr)O3 ceramics. Scr Mater 65(9):771–774. https://doi.org/10.1016/j.scriptamat.2011.07.028
Wang P, Li YX, Lu YQ (2011) Enhanced piezoelectric properties of (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 lead-free ceramics by optimizing calcination and sintering temperature. J Eur Ceram Soc 31(11):2005–2012. https://doi.org/10.1016/j.jeurceramsoc.2011.04.023
Chandrakala E, Paul Praveen J, Hazra BK, Das D (2016) Effect of sintering temperature on structural, dielectric, piezoelectric and ferroelectric properties of sol-gel derived BZT-BCT ceramics. Ceram Int 42(4):4964–4977. https://doi.org/10.1016/j.ceramint.2015.12.009
Zhao ZH, Li XL, Ji HM, Dai YJ, Li T (2015) Microstructure and electrical properties in Zn-doped Ba0.85Ca0.15Ti0.90Zr0.10O3 piezoelectric ceramics. J Alloys Compd 637:291–296. https://doi.org/10.1016/j.jallcom.2015.02.093
Payne WH, Tennery VJ (1965) Dielectric and structural investigations of system BaTiO3–BaHfO3. J Am Ceram Soc 48(8):413–417. https://doi.org/10.1111/j.1151-2916.1965.tb14779.x
Dobal PS, Dixit A, Katiyar RS, Yu Z, Guo R, Bhalla AS (2001) Micro-Raman scattering and dielectric investigations of phase transition behavior in the BaTiO3–BaZrO3 system. J Appl Phys 89(12):8085–8091. https://doi.org/10.1063/1.1369399
Verbitskaia TN, Zhdanov GS, Venevtsev IN, Soloviev SP (1958) Electrical and X-ray diffraction studies of the BaTiO3–BaZrO3 system. Sov Phys Crystallogr 3:182–192
Kalyani AK, Brajesh K, Senyshyn A, Ranjan R (2014) Orthorhombic-tetragonal phase coexistence and enhanced piezo-response at room temperature in Zr, Sn, and Hf modified BaTiO3. Appl Phys Lett 104(25):252906. https://doi.org/10.1063/1.4885516
Avrahami Y, Tuller HL (2004) Improved electromechanical response in rhombohedral BaTiO3. J Electroceram 13(1–3):463–469. https://doi.org/10.1007/s10832-004-5143-6
Zhou C, Liu WF, Xue DZ, Ren XB, Bao HX, Gao JH, Zhang LX (2012) Triple-point-type morphotropic phase boundary based large piezoelectric Pb-free material-Ba(Ti0.8Hf0.2)O3–(Ba0.7Ca0.3)TiO3. Appl Phys Lett 100(22):222910. https://doi.org/10.1063/1.4724216
Wang DL, Jiang ZH, Yang B, Zhang ST, Zhang MF, Guo FF, Cao WW (2014) Phase transition behavior and high piezoelectric properties in lead-free BaTiO3–CaTiO3–BaHfO3 ceramics. J Mater Sci 49(1):62–69. https://doi.org/10.1007/s10853-013-7650-9
Wang DL, Jiang Z, Yang B, Zhang ST, Zhang MF, Guo FF, Cao WW (2014) Phase diagram and enhanced piezoelectric response of lead-free BaTiO3–CaTiO3–BaHfO3 system. J Am Ceram Soc 97(10):3244–3251. https://doi.org/10.1111/jace.13137
Zhao CL, Wu WJ, Wang H, Wu JA (2016) Site engineering and polarization characteristics in (Ba1−yCay)(Ti1−xHfx)O3 lead-free ceramics. J Appl Phys 119(2):24108. https://doi.org/10.1063/1.4939762
Zhao CL, Feng YM, Wu HP, Wu JG (2016) Phase boundary design and high piezoelectric activity in (1 − x)(Ba0.93Ca0.07)TiO3–xBa(Sn1−yHfy)O3 lead-free ceramics. J Alloys Compd 666:372–379. https://doi.org/10.1016/j.jallcom.2016.01.105
Zhao CL, Wang H, Xiong J, Wu JG (2016) Composition-driven phase boundary and electrical properties in (Ba0.94Ca0.06)(Ti1−xMx)O3 (M = Sn, Hf, Zr) lead-free ceramics. Dalton Trans 45(15):6466–6480. https://doi.org/10.1039/c5dt04891e
Di Loreto A, Machado R, Frattini A, Stachiotti MG (2017) Improvement in the sintering process of Ba0.85Ca0.15Zr0.1T i0.9O3 ceramics by the replacement of Zr by Hf. J Mater Sci: Mater Electron 28(1):588–594. https://doi.org/10.1007/s10854-016-5562-6
Yang Y, Zhou Y, Ren J, Zheng QJ, Lam KH, Lin DM (2018) Coexistence of three ferroelectric phases and enhanced piezoelectric properties in BaTiO3–CaHfO3 lead-free ceramics. J Eur Ceram Soc 38(2):557–566. https://doi.org/10.1016/j.jeurceramsoc.2017.09.023
Tian HY, Wang Y, Miao J, Chan HLW, Choy CL (2007) Preparation and characterization of hafnium doped barium titanate ceramics. J Alloys Compd 431(1–2):197–202. https://doi.org/10.1016/j.jallcom.2006.05.037
Wang JC, Zheng P, Yin RQ, Zheng LM, Du J, Zheng L, Deng JX, Song KX, Qin HB (2015) Different piezoelectric grain size effects in BaTiO3 ceramics. Ceram Int 41(10):14165–14171. https://doi.org/10.1016/j.ceramint.2015.07.039
Zhi Y, Chen A, Guo RY, Bhalla AS (2002) Piezoelectric and strain properties of Ba(Ti1−xZrx)O3 ceramics. J Appl Phys 92(3):1489–1493. https://doi.org/10.1063/1.1487435
Li W, Xu ZJ, Chu RQ, Fu P, Zang GZ (2010) Dielectric and piezoelectric properties of Ba(ZrxTi1−x)O3 lead-free ceramics. Braz J Phys 40(3):353–356. https://doi.org/10.1590/S0103-97332010000300018
Yao YG, Zhou C, Lv DC, Wang D, Wu HJ, Yang YD (2012) Large piezoelectricity and dielectric permittivity in BaTiO3–xBaSnO3 system: the role of phase coexisting. EPL (Europhys Lett) 98(2):27008. https://doi.org/10.1209/0295-5075/98/27008
Karaki T, Yan K, Adachi M (2007) Barium titanate piezoelectric ceramics manufactured by two-step sintering. Jpn J Appl Phys 46(10B):7035–7038. https://doi.org/10.1143/jjap.46.7035
Tsurumi T, Li J, Hoshina T, Kakemoto H, Nakada M, Akedo J (2007) Ultrawide range dielectric spectroscopy of BaTiO3-based perovskite dielectrics. Appl Phys Lett 91(18):182905. https://doi.org/10.1063/1.2804570
Hoshina T, Takizawa K, Li J, Kasama T, Kakemoto H, Tsurumi T (2008) Domain size effect on dielectric properties of barium titanate ceramics. Jpn J Appl Phys 47(9):7607–7611. https://doi.org/10.1143/jjap.47.7607
Jaffe B, Cook WR, Jafie H (1971) Piezoelectric ceramics. Academic Press, London
Devonshire AF (1949) XCVI. Theory of barium titanate-part 1. Philos Mag 40(309):1040–1063. https://doi.org/10.1080/14786444908561372
Tian HY, Wang Y, Miao J, Chan HLW, Choy CL (2007) Preparation and characterization of hafnium doped barium titanate ceramics. J Alloys Compd 431(1–2):197–202. https://doi.org/10.1016/j.jallcom.2006.05.037
Anwar S, Sagdeo PR, Lalla NP (2006) Crossover from classical to relaxor ferroelectrics in BaTi1−xHfxO3 ceramics. J Phys: Condens Matter 18(13):3455–3468. https://doi.org/10.1088/0953-8984/18/13/013
Zhou C, Ren XB, Tan XL, Guo HZ (2014) Unique single-domain state in a polycrystalline ferroelectric ceramic. Phys Rev B 89(10):100104. https://doi.org/10.1103/PhysRevB.89.100104
Mitoseriu L, Tura V, Papusoi C, Osaka T, Okuyama M (1999) A comparative study of the grain size effects on ferro-para phase transition in barium titanate ceramics. Ferroelectrics 223(1):99–106. https://doi.org/10.1080/00150199908260558
Kuwabara M, Matsuda H, Kurata N, Matsuyama E (1997) Shift of the curie point of barium titanate ceramics with sintering temperature. J Am Ceram Soc 80(10):2590–2596. https://doi.org/10.1111/j.1151-2916.1997.tb03161.x
Li BR, Wang XH, Li LT, Zhou H, Liu XT, Han XQ, Zhang YC, Qi XW, Deng XY (2004) Dielectric properties of fine-grained BaTiO3 prepared by spark-plasma-sintering. Mater Chem Phys 83(1):23–28. https://doi.org/10.1016/j.matchemphys.2003.08.009
Arlt G, Hennings D, de With G (1985) Dielectric properties of fine-grained barium titanate ceramics. J Appl Phys 58(4):1619–1625. https://doi.org/10.1063/1.336051
Mitoseriu L, Tura V, Papusoi C, Osaka T, Okuyama M (1999) A comparative study of the grain size effects on ferro-para phase transition in barium titanate ceramics. Ferroelectrics 223(1):99–106. https://doi.org/10.1080/00150199908260558
Zhu LF, Zhang BP, Zhao L, Li S, Zhou Y, Shi XC, Wang N (2016) Large piezoelectric effect of (Ba,Ca)TiO3–xBa(Sn,Ti)O3 lead-free ceramics. J Eur Ceram Soc 36(4):1017–1024. https://doi.org/10.1016/j.jeurceramsoc.2015.11.039
Zhao L, Zhang BP, Wang N, Chen JY (2017) High piezoelectricity in CuO-modified Ba(Ti0.90Sn0.10)O3 lead-free ceramics with modulated phase structure. J Eur Ceram Soc 37(4):1411–1419. https://doi.org/10.1016/j.jeurceramsoc.2016.11.028
Acknowledgements
This work is supported by the Natural Science Foundation of China (Grant No. 11664042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, HM., Xu, WJ., Zhou, HW. et al. Effects of phase composition and grain size on the piezoelectric properties of HfO2-doped barium titanate ceramics. J Mater Sci 54, 12392–12400 (2019). https://doi.org/10.1007/s10853-019-03726-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03726-y