Abstract
The sandwich-structured MnTMPyP/NbWO6 nanocomposites were synthesized by the electrostatic self-assembly of the manganese porphyrin (MnTMPyP) cations with the exfoliated niobium tungstate [NbWO6]− nanosheets. Various analytical techniques such as X-ray diffraction patterns, scanning electron micrograph, transmission electron microscope, energy-dispersive spectroscopy, UV–Vis absorption spectra and Fourier transform infrared spectra were used to determine the structure, composition and morphology of the as-prepared samples. It can be concluded that MnTMPyP cations were inserted into interlayer spacing of the [NbWO6]− nanosheets and arranged in an inclined single layer at 58°. The MnTMPyP/NbWO6 nanocomposites modified electrode exhibited excellent electro-catalytic oxidation activity toward nitrite in 0.2 mol L−1 and pH 7.0 phosphate buffer solution. Additionally, the oxidation peak current is proportional to the square root of scan rates, indicating that the redox reaction of nitrite is a typical diffusion-controlled process. Also, the sensitivity and detection limit for nitrite at the modified electrode was evaluated as 3.80 × 10−5 mol L−1 over a concentration range from 1.20 × 10−4 to 3.57 × 10−3 mol L−1 by using differential pulse voltammetry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrite is a kind of the widespread inorganic pollutant in environment, agriculture and chemical industry. It is also a common additive and preservative in the food industry [1,2,3]. Nitrite can react with hemoglobin in the blood to form methemoglobin, and the process is irreversible, leading to insufficient oxygen supply to the body [4, 5]. As a result, nitrite as a potentially toxic substance is harmful to the human health. This has also drawn the attention of the World Health Organization, which has set a maximum of 1 mg L−1 nitrite in drinking water [6]. Hence, the quantitative analysis of nitrite becomes particularly important.
So far, various analytical techniques have been used for the detection of nitrite, such as electrochemical sensor [7, 8], fluorescent spectroscopy [9], spectrophotometry [10], capillary electrophoresis [11] and chromatography [12]. Among the above analytical techniques, the electrochemical testing method attracts more attention and is widely adopted to detect nitrite owing to advantages of simple, convenient, inexpensive, high sensitivity and great selectivity [13,14,15].
In recent years, more and more layered metal oxide semiconductors (LMOSs) were focused due to their unique layered structure, ion-exchange capacity, superior thermal stability and great electrical conductivity, such as KTiNbO5 [16, 17], CsTi2NbO7 [18], LiTaWO6 [19], LiNbWO6 [20]. In consideration of the above advantages, a lot of modified electrodes based on these LMOSs have been prepared to study their applications in the field of electrochemistry [21,22,23]. However, the pure LMOSs are not suitable for the direct detection in electrochemical experiments because they exhibit very weak electrochemical activity and rather-low efficiency. Hence, some effective measures should be taken to enhance the performance of LMOSs. According to some previous reports [24,25,26], LMOSs were used as the host materials, and their negatively charged nanosheets can be obtained successively through acidification and exfoliation processes. A number of positively charged species have been discovered and introduced into the negative nanosheet layers by using electrostatic self-assembly technique, such as methylene blue cation [27] and metalloporphyrin cation [28]. The properties of the acquired nanocomposites can be improved, and the prepared electrochemical sensors are very stable. Moreover, this technology possesses the merits of quick, reliable, practical and simple operation.
As an important natural compound, porphyrins and porphyrin derivatives exist widely in biological systems and have regulated structure and outstanding properties [29, 30]. In addition, metalloporphyrin exhibits prospective application in electrochemical sensors as a consequence of its superior electro-catalytic capability for molecular oxygen reduction [31, 32]. As a result, various porphyrin-based modified electrodes have been performed in detection of nitrite [33], oxygen [34], ascorbic acid [35] and other biochemicals. Thereby the nanocomposites formed by the immobilization of porphyrin derivatives in various LMOSs have focused the attention of researchers.
In this study, LiNbWO6 with orthorhombic structure was selected to be host material, and the corresponding schematic structure of it is shown in Fig. S1 [20]. Also, MnTMPyP was chosen as guest material. Therefore, the MnTMPyP/NbWO6 nanocomposites were synthesized by combining the host [NbWO6]− nanosheets with the guest MnTMPyP cations via the exfoliation/restacking method. A novel and sensitive nanocomposites-based electrochemical sensor was developed to determine the nitrite. The as-prepared samples were characterized by X-ray diffraction (XRD), scanning electron micrograph (SEM), transmission electron microscope (TEM), energy-dispersive spectroscopy (EDS), infrared vibrational spectroscopy and UV–Vis spectroscopy. Furthermore, the MnTMPyP/NbWO6 modified electrode reveals excellent electro-catalytic oxidation activity, stability and anti-interference toward nitrite via cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The results indicate that the proposed modified electrode shows promising application in electrochemical sensor.
Experimental
Exfoliation of niobium tungstate nanosheets
A traditional solid-state method was employed to synthesize layered LiNbWO6 according to the previous literature [36]. The LiNbWO6 was obtained by calcining stoichiometric mixture of Li2CO3 (Sinopharm), Nb2O5 (Sinopharm) and WO3 (Sinopharm) with molar ratio of 1:1:2 at 800 °C in air for 12 h twice. The as-prepared LiNbWO6 (2 g) was treated with 2 mol L−1 HNO3 aqueous solution (60 mL) at room temperature for 3 days and replaced with a fresh acid solution every day during the proton exchange reaction. The protonated product (HNbWO6) was centrifugal washed three times to remove the excess NO3−, and then dried at 80 °C in a vacuum oven overnight. In order to obtain [NbWO6]− nanosheets [37], 0.1 g of acidified product HNbWO6 was added to a solution containing 42 mL of distilled water and 700 μL of tetrabutylammonium hydroxide (TBAOH, 10% in water). After stirring for a week, a translucent colloidal dispersion was obtained. The resulting colloidal dispersion was centrifuged at 9000 rpm for 10 min to remove the unexfoliated particles. The supernatant solution is [NbWO6]− nanosheets.
Self-assembly of [NbWO6]− nanosheets and MnTMPyP
To obtain MnTMPyP/NbWO6 nanocomposites, 1 mmol L−1 MnTMPyP aqueous solution was evenly dispersed in the [NbWO6]− nanosheets colloidal solution with a volume ratio of 1:1 between MnTMPyP aqueous solution and [NbWO6]− nanosheets. After shaking, large amounts of brown precipitate were generated, owing to the electrostatic self-assembly of cationic MnTMPyP cations and the anionic [NbWO6]− nanosheets. After settling for 2 h, the precipitate of MnTMPyP/NbWO6 was centrifuged at 9000 r min−1 for 5 min, washed with distilled water for 3 times and then dried at 50 °C in a vacuum oven for 24 h.
Fabrication of MnTMPyP/NbWO6 nanocomposites modified electrode
Two milligrams of MnTMPyP/NbWO6 nanocomposites were dispersed in 1 mL distilled water and then treated with ultrasonic treatment for 30 min. Then, 7 μL of the obtained suspension was taken to coat on the surface of the glassy carbon electrode (GCE). The modified GCE is denoted as MnTMPyP/NbWO6/GCE.
Characterization
A RINT 2000 diffractometer (Rigaku Corporation, Tokyo, Japan) by using Cu Kα radiation (λ = 0.154 nm) with 2θ going from 1.5° to 70° was collected to obtain XRD data. Analysis of element types and contents of nanocomposite was measured by EDS equipped with a FEI Tecnai G2F30S-TWIN transmission electron microscope. SEM images of the Au-coated samples were acquired with a JSM-5600 apparatus (JEOL). TEM image of nanocomposite was photographed with a JEM-2010 instrument (JEOL). Atomic force microscope (AFM) image was taken with a Bruker dimension edge SPM equipment. Britain’s Malvern Zetasizer Nano instrument was used to monitor the Zeta potential value of nanosheets colloidal dispersion. FT-IR spectra were recorded by FTIR-8400S spectrometer (Shimadzu Corporation, Japan) with KBr pellets. UV–Vis absorption spectra were measured by a UV–Vis spectrometer (UV-2550). CV and DPV measurements were taken in a CHI660C electrochemical workstation with a conventional three-electrode system including saturated calomel electrode (SCE), platinum wire electrode and glassy carbon electrode (GCE).
Results and discussion
XRD analysis
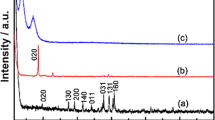
The XRD patterns of the obtained products are shown in Fig. 1. LiNbWO6 is orthorhombic structure, space group P-421 m, lattice parameters a = b = 0.4681 nm and c = 0.9275 nm [20]. It can be clearly seen that the original LiNbWO6 has narrow and strong diffraction peaks, indicating its well crystallinity. A characteristic (001) peak of LiNbWO6 is at 9.56°, which is in accordance with the standard XRD data (JCPDS 84-1764) [38]. An exchange of Li+ with H3O+ causes the shift of (00l) characteristic peak to a lower angle (6.87°), corresponding to the protonated product (HNbWO6). After the self-assembly of MnTMPyP cations and the [NbWO6]− nanosheets, the (00l) characteristic peak of the obtained MnTMPyP/NbWO6 nanocomposites was shifted to 3.88°. According to the 2θ angle of the (00l) peaks, the basal spacing values of the three products can be calculated as 0.92 nm, 1.29 nm and 2.28 nm (Table 1), respectively. The interlayer spacing values (Δd) of the protonated product and the self-assembled product are calculated to be 0.53 nm and 1.52 nm by subtracting the thickness of [NbWO6]− nanosheets (0.76 nm) [39]. Larger interlayer spacing indicates that the interlayer cations have been successfully inserted between the layers of the [NbWO6]− nanosheets. In addition, the arrangement of MnTMPyP cations in the galley of the self-assembled product was further studied. Considering the size of the MnTMPyP cations (1.8 nm × 1.8 nm × 0.75 nm), one conclusion can be drawn that MnTMPyP cations are arranged at an angle of about 58° between the [NbWO6]− nanosheets (Fig. S2).
Morphology analysis
The SEM images of LiNbWO6, HNbWO6 and MnTMPyP/NbWO6 nanocomposites are shown in Fig. 2. As can be seen from Fig. 2a, the parent material LiNbWO6 has a layered structure, and the layers are tightly arranged but irregular. Compared to the origin LiNbWO6, the layered morphology of HNbWO6 (Fig. 2b) was obtained by acid treatment with an obvious smooth surface. After the self-assembly of [NbWO6]− nanosheets with manganese porphyrin cations, a two-dimensional layered structure was reconstructed. However, the surface of the resultant MnTMPyP/NbWO6 nanocomposites (Fig. 2c) becomes rough and the edges of it are no longer neat and regular, which is due to a large number of TBA+ cations and manganese porphyrin cations successively inserted between layers of HNbWO6 through exfoliation/restacking process. The TEM image was performed in Fig. 2d to further certify the structure of the MnTMPyP/NbWO6 nanocomposites, showing the clearly layered structure with many thin laminates. Furthermore, we also use AFM to study the morphology of the [NbWO6]− nanosheets. As shown in Fig. S3, the nanosheets exhibit many irregular blocky, single-layer and flat morphology, and each of them has a lateral dimension of approximately hundreds of nanometers and a vertical dimension is less than 2 nm, which suggests the occurrence of exfoliation of acidification products.
The element composition of the MnTMPyP/NbWO6 nanocomposites was examined by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and element mapping. In Fig. 3, there is a good spatial distribution corresponding to elements C, N, Mn which come from MnTMPyP cations and elements O, Nb, W which derived from the [NbWO6]− nanosheets. It demonstrates that MnTMPyP cations and [NbWO6]− nanosheets were recombined into a new MnTMPyP/NbWO6 nanocomposites [40, 41]. Moreover, the elements analysis results of the MnTMPyP/NbWO6 nanocomposites can be observed as follows: Mn (0.57 at.%), Nb (5.88 at.%) and W (7.55 at.%) (Table S1, Fig. S4). Therefore, we can infer that the chemical formula of MnTMPyP/NbWO6 nanocomposites is (MnTMPyP)0.08H0.68NbWO6·nH2O.
Zeta potential analysis
Zeta potential values of different volume ratios between MnTMPyP aqueous solution and [NbWO6]− colloidal suspension were surveyed to further investigate the exfoliation/restacking process. As shown in Fig. S5, the Zeta potential value of [NbWO6]− colloidal dispersion is − 45.7 mV, indicating the stable and well-dispersed niobium tungstate dispersion. As the volume ratio of MnTMPyP aqueous solution and [NbWO6]− nanosheets is gradually increased (Fig. 4), the Zeta potential value of mixed solution increases and large amounts of dark brown flocculent precipitates can be seen. It illuminates that the assembly process of MnTMPyP/NbWO6 nanocomposites is electrostatic force. When the volume ratio of MnTMPyP aqueous solution and [NbWO6]− nanosheets is 1:2.7, the Zeta potential value reaches nearly zero (0.028 mV). Furthermore, the inset exhibits the Tyndall light scattering phenomenon of [NbWO6]− nanosheets colloidal dispersion, suggesting the occurrence of exfoliation to a certain degree [42].
IR and UV–Vis spectra analysis
The FT-IR and UV–Vis tests were performed to further prove the preparation of MnTMPyP/NbWO6 nanocomposites. The FT-IR spectra of LiNbWO6, MnTMPyP and MnTMPyP/NbWO6 can be observed in Fig. 5a. The characteristic bands at 640 and 890 cm−1 in the LiNbWO6 are attributed to Nb=O and O–W–O stretching vibration [43]. For MnTMPyP, the characteristic bands at 1641, 1465, 1385 and 1108 cm−1 are assigned to stretching vibration of C=N bonds in the pyridine substituent and stretching vibration of C=N, C=C and C–N bonds in the porphyrin rings, respectively [44]. Similarly, the absorption bands mentioned above can be observed in MnTMPyP/NbWO6 nanocomposites, indicating that the hybrid contains both MnTMPyP cations and [NbWO6]− nanosheets. However, compared with MnTMPyP and [NbWO6]− nanosheets, these absorption bands are slightly offset because of the interaction between the two species.
As shown in Fig. 5b, UV–Vis absorption spectrum of MnTMPyP/NbWO6 hybrid film shows a strong Soret band at 469 nm and a weak Q band at 570 nm. The Soret band and Q band of the hybrid film undergo redshift of 7 nm and 10 nm, respectively, owing to the strong interaction of MnTMPyP cations in the tight accumulation between the host material layers [37], comparing with the spectrum of MnTMPyP aqueous solution. Furthermore, the broadening phenomenon of the two absorption bands in the MnTMPyP/NbWO6 nanocomposites may be due to the superposition and aggregation of metalloporphyrin cations between the [NbWO6]− layers [35]. According to the above results, MnTMPyP cations were successfully inserted into the interlayer of [NbWO6]− nanosheets.
Electrochemical characterization
The electrochemical behaviors of MnTMPyP/GCE and MnTMPyP/NbWO6/GCE were performed in N2-saturated 0.2 mol L−1 and pH 7.0 phosphate buffer solution (PBS) at a scan rate of 50 mV s−1 (Fig. 6a). For MnTMPyP/GCE, there is a couple of well-defined redox peaks at − 0.175 and − 0.259 V corresponding to a single electron reversible redox process of MnIIITMPyP5+/MnIITMPyP4+ [26], and the potential difference between the two peaks (ΔEp) is 84 mV. Similarity, a pair of redox peaks of MnTMPyP/NbWO6/GCE appear at − 0.175 and − 0.238 V, respectively; the current of the redox peaks is larger than MnTMPyP and the ΔEp reduces to 63 mV. This demonstrates that the semiconductor layers ([NbWO6]− nanosheets) improve the electron transfer ability of MnTMPyP in the process of electrode testing [45].
a CV curves of MnTMPyP/GCE (dash line) and CoTMPyP/NbWO6/GCE (solid line) in N2-saturated 0.2 mol L−1 and pH 7.0 PBS at a scan rate of 50 mV s−1; b CV curves of CoTMPyP/NbWO6/GCE in N2-saturated pH 7.0 PBS at 50, 60, 80, 100, 150, 200, 250 mV s−1 from inner to outer; c the linear relationship between I and v1/2; d CV curves of bare electrode (dash line), LiNbWO6/GCE (short dash line), MnTMPyP/GCE (dash dot line) and MnTMPyP/NbWO6/GCE (solid line) in N2-saturated 0.2 mol L−1 and pH 7.0 PBS containing 6 mmol L−1 NaNO2 at a scan rate of 100 mV s−1
The CV curves of MnTMPyP/NbWO6/GCE measured at different scan rates in N2-saturated pH 7.0 PBS are given in Fig. 6b. It can be clearly seen that the oxidation peaks and reduction peaks were moved toward the positive and negative directions, respectively, as the scan rate increasing. Meanwhile, the value of ΔEp was increased from 63 to 112 mV as the scan rate increases from 50 to 250 mV s−1 due to the slow electron transfer process at high scan rates. Two linear relationships between anodic peak currents (Ipa), cathodic peak currents (Ipc) and the square root of the scan rate (v1/2) are plotted in Fig. 6c. The liner equations are Ipa (μA) = − 14.97 + 2.92 v1/2 (v1/2 s−1/2) (n = 7, |R| = 0.9985) and Ipc (μA) = − 10.09 − 2.31 v1/2 (v1/2 s−1/2) (n = 7, |R| = 0.9986), indicating that the redox reaction of the hybrid undergoes a diffusion-controlled process. Here n and R represent the number of scans and linear correlation coefficients, respectively.
In order to investigate the electrochemical oxidation behavior of the modified electrode, a comprehensive comparison of the CV curves of bare electrode, LiNbWO6/GCE, MnTMPyP/GCE and MnTMPyP/NbWO6/GCE in N2-saturated 0.2 mol L−1 and pH 7.0 PBS containing 6 mmol L−1 NaNO2 at a scan rate of 100 mV s−1 is shown in Fig. 6d [46, 47]. There is a strong oxidation process on the surface of the four electrodes, which corresponds to a two-electron oxidation process from NO2− to NO3− [48]. However, the reduction process is absent in the reverse scan. This may be ascribed to the irreversibility of the electron transfer process and the coupling of fast irreversible post-electron-transfer chemical reactions [49]. Obviously, the oxidation peak potential (Epa) of the MnTMPyP/NbWO6/GCE moves toward negative than bare electrode, indicating the electro-catalytic oxidation of nitrite. On the one hand, the Epa values of the MnTMPyP/NbWO6/GCE (0.89 V) were negatively shifted to 270, 170 and 120 mV, respectively, compared with that of the bare electrode (1.16 V), LiNbWO6/GCE (1.06 V) and MnTMPyP/GCE (1.01 V). On the other hand, the oxidation peak current of the MnTMPyP/NbWO6/GCE is relatively high. All the characteristics mentioned above are electrochemical catalytic oxidation process, suggesting that the MnTMPyP/NbWO6 nanocomposites can enhance electro-catalytic oxidation of nitrite efficiently.
As illustrated in Fig. 7a, the influence of the scan rate on MnTMPyP/NbWO6/GCE oxidation peak current was studied by CV curves in PBS containing 6 mmol L−1 NaNO2 at the scan rate range of 10–150 mV s−1. With the increase in the scan rate, the value of Epa was positively moved and the Ipa was gradually increased. Additionally, the Ipa is proportional to the v1/2, indicating that the electro-catalytic oxidation of nitrite on the surface of MnTMPyP/NbWO6/GCE is a typical diffusion-controlled process. The linear relationship between Ipa and Epa is displayed in Fig. 7b, and the liner equations can be expressed as Ipa (μA) = 21.2 − 18.9 v1/2 (v1/2 s−1/2) (n = 10, |R| = 0.9993). According to previous studies [50], the electro-catalytic mechanism of nitrite oxidation on MnTMPyP/NbWO6/GCE is proposed as follows:
a CV curves of MnTMPyP/NbWO6/GCE in N2-saturated 0.2 mol L−1 and pH 7.0 PBS containing 6 mmol L−1 NaNO2 at scan rates of 20,30,40, 50, 60, 70, 80, 90, 100 and 150 mV s−1; b the relationship curve between the oxidation peak current (Ipa) and the square root of scan rate (v1/2); c DPV curves of MnTMPyP/NbWO6/GCE in N2-saturated 0.2 mol L−1 and pH 7.0 PBS with different concentrations of NaNO2; d the relationship between NaNO2 concentration (C) and oxidation peak current (Ipa)
According to the characteristic that DPV technology is sensitive to nitrite detection. Hence, this technology was conducted for the quantitative determination of nitrite on the MnTMPyP/NbWO6/GCE. In N2-saturated 0.2 mol L−1 and pH 7.0 PBS with different concentrations of NaNO2, DPVs of NaNO2 at MnTMPyP/NbWO6/GCE are presented in Fig. 7c. With gradient concentrations of NaNO2 from 1.20 × 10−4 to 3.57 × 10−3 mol L−1, the oxidation peak current increases gradually and the oxidation peak potential moves toward high potential. In Fig. 7d, a linear regression equation between the peak current (Ipa) and the concentration (C) can be obtained as Ipa (μA) = 2.94 C + 0.40 (C) (mmol L−1) (n = 12, |R| = 0.9998), with a detection limit of 3.80 × 10−5 mol L−1 at a signal-to-noise ratio of 3.0 [34]. Moreover, the comparison with some previously reported tests for nitrite is listed in Table 2. It can be observed that the MnTMPyP/NbWO6/GCE has a great detection performance for the electro-catalytic oxidation of nitrite.
Stability and reproducibility of the MnTMPyP/NbWO6/GCE
For evaluating the stability and reproducibility of the MnTMPyP/NbWO6/GCE, a string of DPV measurements were taken in N2-saturated 0.2 mol L−1 and pH 7.0 PBS containing 2.47 mmol L−1 NaNO2. In Fig. 8a, this bar chart reflects the results of ten consecutive measurements with little change in current, and the relative standard deviation (RSD) is calculated to be 0.74%. Moreover, the stability test of NaNO2 at the MnTMPyP/NbWO6/GCE within 1 month is displayed in Fig. 8b. After 9 days of storage, the peak current was decreased by only 1.53%. And the current value was still retained 88.18% for a month later. Meanwhile, the hybrid thin film on the modified electrode can be continuously tested for several hours without shedding in the process of electrochemical measurement. These research results suggest that the excellent stability of the MnTMPyP/NbWO6/GCE enables it to be subjected to extensive testing. In addition, from Fig. 8c, the reproducibility of the MnTMPyP/NbWO6/GCE was also investigated at five different electrodes with the RSD of 0.3%. The great reproducibility indicates that the fabrication of the MnTMPyP/NbWO6 nanocomposites is credible.
a The oxidation currents of repetitive determinations on MnTMPyP/NbWO6/GCE in N2-saturated 0.2 mol L−1 and pH 7.0 PBS containing 2.47 mmol L−1 NaNO2; b the oxidation peak currents of 2.47 mmol L−1 NaNO2 detected at different storage times; c the oxidation currents of 2.47 mmol L−1 NaNO2 at different electrodes; d the oxidation peak currents of 0.22 mmol L−1 NaNO2 detected with 100-fold interference species of AA, Glucose, KCl, NaNO3, and NaCl
Interference
The selectivity and suitability of the senor built on MnTMPyP/NbWO6 nanocomposites were assessed by comparing it with some common substances related to food and environment, including AA, Glucose, KCl, NaNO3, and NaCl. As shown in Fig. 8d, the concentrations of these possible interferents are 100-fold than NaNO2, and the current response changes are calculated to be 4.6, 4.2, 3.0, 5.1 and 3.1%, respectively. These interferents show the little effect on the determination of nitrite, demonstrating that the sensor has a strong anti-interference ability.
Conclusions
In this work, the MnTMPyP/NbWO6 nanocomposites were fabricated through a rapid and convenient self-assembly method. The element composition and structure of the nanocomposites were confirmed by XRD, SEM, HAADF-STEM, EDS, UV–Vis and IR. Herein, a novel electrochemical sensor based on MnTMPyP/NbWO6 nanocomposites held the capacity for the detection of nitrite. From the results of CVs and DPVs of the proposed modified GCE, the sensor exhibited excellent electro-catalytic oxidation activity toward nitrite and a great detection limit of 3.80 × 10−5 mol L−1 in neutral solution. Furthermore, the sensitivity, stability, reproducibility and anti-interference of the prepared sensor were also displayed remarkable. Therefore, the electrochemical detection method could serve as a simple, sensitive, stable and repeatable assay to determine nitrite. It is significant for the sensor to be applied to the environmental sample analysis.
References
Rosca V, Duca M, de Groot MT, Koper MT (2009) Nitrogen cycle electrocatalysis. Chem Rev 109:2209–2244
Zhang ML, Huang DK, Cao Z, Liu YQ, He JL, Xiong JF, Yin YL (2015) Determination of trace nitrite in pickled food with a nano-composite electrode by electrodepositing ZnO and Pt nanoparticles on MWCNTs substrate. LWT Food Sci Technol 64:663–670
Daniel WL, Han MS, Lee JS, Mirkin CA (2009) Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J Am Chem Soc 131:6362–6363
Wu L, Zhang X, Wang M, He L, Zhang Z (2018) Preparation of Cu2O/CNTs composite and its application as sensing platform for detecting nitrite in water environment. Measurement 128:189–196
Wang H, Wan N, Ma L, Wang Z, Cui B, Han W, Chen Y (2018) A novel and simple spectrophotometric method for detection of nitrite in water. Analyst 143:4555–4558
Hussain I, Ahamad KU, Nath P (2016) Low-cost, robust, and field portable smartphone platform photometric sensor for fluoride level detection in drinking water. Anal Chem 89:767–775
Zhu N, Xu Q, Li S, Gao H (2009) Electrochemical determination of nitrite based on poly (amidoamine) dendrimer-modified carbon nanotubes for nitrite oxidation. Electrochem Commun 11:2308–2311
Liu QH, Yan XL, Guo JC, Wang DH, Lei L, Yan FY, Chen LG (2009) Spectrofluorimetric determination of trace nitrite with a novel fluorescent probe. Spectrochim Acta 73:789–793
Zhang Y, Su Z, Li B, Zhang L, Fan D, Ma H (2016) Recyclable magnetic mesoporous nanocomposite with improved sensing performance toward nitrite. ACS Appl Mater Int 8:12344–12351
Yue XF, Zhang ZQ, Yan HT (2004) Flow injection catalytic spectrophotometric simultaneous determination of nitrite and nitrate. Talanta 62:97–101
Kikura-Hanajiri R, Martin RS, Lunte SM (2002) Indirect measurement of nitric oxide production by monitoring nitrate and nitrite using microchip electrophoresis with electrochemical detection. Anal Chem 74:6370–6377
Tsikas D (2000) Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 72:4064–4072
Rajalakshmi K, John SA (2015) Highly sensitive determination of nitrite using FMWCNTs-conducting polymer composite modified electrode. Sens Actuators B Chem 215:119–124
Zhang S, Li B, Sheng Q, Zheng J (2016) Electrochemical sensor for sensitive determination of nitrite based on the CuS–MWCNT nanocomposites. J Electroanal Chem 769:118–123
Mani V, Periasamy AP, Chen SM (2012) Highly selective amperometric nitrite sensor based on chemically reduced graphene oxide modified electrode. Electrochem Commun 17:75–78
Liu C, Zhu H, Zhu Y, Dong P, Hou H, Xu Q, Hou W (2018) Ordered layered N-doped KTiNbO5/gC3N4 heterojunction with enhanced visible light photocatalytic activity. Appl Catal B Environ 228:54–63
Li J, Zhang X, Pan B, Xu J, Liu L, Ma J, Tong Z (2016) Application of a nanostructured composite material constructed by self-assembly of titanoniobate nanosheets and cobalt porphyrin to electrocatalytic reduction of oxygen. Chin J Chem 34:1021–1026
Liu C, Zhang C, Wang J, Xu Q, Chen X, Wang C, Hou W (2018) N-doped CsTi2NbO7@gC3N4 core–shell nanobelts with enhanced visible light photocatalytic activity. Mater Lett 217:235–238
Li J, Pan B, Xu J, Wang M, Zhang X, Liu L, Tong Z (2017) Nanotubes formed by exfoliation of HTaWO6. Chem Lett 46:597–598
Ali Z, Khan I, Rahman M, Ahmad R, Ahmad I (2016) Electronic structure of the LiAA’O6 (A = Nb, Ta, and A’ = W, Mo) ceramics by modified Becke–Johnson potential. Opt Mater 58:466–475
Pan B, Xu J, Zhang X, Li J, Wang M, Ma J, Tong Z (2018) Electrostatic self-assembly behaviour of exfoliated Sr2Nb3O10 − nanosheets and cobalt porphyrins: exploration of non-noble electro-catalysts towards hydrazine hydrate oxidation. J Mater Sci 53:6494–6504. https://doi.org/10.1007/s10853-018-2033-x
Sun Y, Deng JP, Tu ZY, Ma JJ (2016) Preparation and electrochemical performance of manganese porphyrin/titanate intercalated nanocomposite. Mater Sci Eng 137:012031
Xu J, Pan B, Li J, Zhang X, Wang M, Tong Z (2017) Electrocatalytic activity towards oxygen reduction reaction of laminar nanocomposite LaNb2O7/CoIIITMPyP prepared via the exfoliation/restacking method. Micro Nano Lett 12:731–734
Miyamoto N, Yamamoto H, Kaito R, Kuroda K (2002) Formation of extraordinarily large nanosheets from K4Nb6O17 crystals. Chem Commun 0:2378–2379
Bizeto MA, Shiguihara AL, Constantino VR (2009) Layered niobate nanosheets: building blocks for advanced materials assembly. J Mater Chem 19:2512–2525
Ma J, Yang M, Chen Y, Liu L, Zhang X, Wang M, Tong Z (2015) Sandwich-structured composite from the direct coassembly of layered titanate nanosheets and Mn porphyrin and its electrocatalytic performance for nitrite oxidation. Mater Lett 150:122–125
Ma J, Zhang Z, Yang M, Wu Y, Feng X, Liu L, Tong Z (2016) Intercalated methylene blue between calcium niobate nanosheets by ESD technique for electrocatalytic oxidation of ascorbic acid. Microporous Mesoporous Mater 221:123–127
Xu J, Wang M, Pan B, Li J, Xia B, Zhang X, Tong Z (2017) Electrostatic self-assembly of exfoliated niobate nanosheets (Nb3O8 −) and cobalt porphyrins (CoIIITMPyP) utilized for rapid construction of intercalated nanocomposite and exploration of electrocatalysis towards oxygen reduction. Funct Mater Lett 10:1750070
Kung CW, Chang TH, Chou LY, Hupp JT, Farha OK, Ho KC (2015) Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem Commun 58:51–56
Wu H, Fan S, Jin X, Zhang H, Chen H, Dai Z, Zou X (2014) Construction of a zinc porphyrin-fullerene-derivative based nonenzymatic electrochemical sensor for sensitive sensing of hydrogen peroxide and nitrite. Anal Chem 86:6285–6290
Kemmegne-Mbouguen JC, Angnes L (2015) Simultaneous quantification of ascorbic acid, uric acid and nitrite using a clay/porphyrin modified electrode. Sens Actuators B Chem 212:464–471
Winnischofer H, de Souza Lima S, Araki K, Toma HE (2003) Electrocatalytic activity of a new nanostructured polymeric tetraruthenated porphyrin film for nitrite detection. Anal Chim Acta 480:97–107
Xu J, Xia B, Wang M, Fan Z, Zhang X, Ma J, Tong Z (2018) A biosensor consisting of Ca2Nb3O10 − substrates and functional molecule manganese porphyrins (MnTMPyP) utilized for the determinations of nitrite. Funct Mater Lett 11:1850053
Wang M, Liu Y, Zhang X, Fan Z, Tong Z (2018) Development of sandwich-structured cobalt porphyrin/niobium molybdate nanosheets catalyst for oxygen reduction. J Mater Res 33:4199–4206
Wang M, Xu J, Zhang X, Fan Z, Tong Z (2018) Fabrication of a new self-assembly compound of CsTi2NbO7 with cationic cobalt porphyrin utilized as an ascorbic acid sensor. Appl Biochem Biotechnol 185:834–846
Hu LF, Li R, He J, Da LG, Lv W, Hu JS (2015) Structure and photocatalytic performance of layered HNbWO6 nanosheet aggregation. J Nanophotonics 9:093041
Pan B, Zhao W, Zhang X, Li J, Xu J, Ma J, Tong Z (2016) Research on the self-assembly of exfoliated perovskite nanosheets (LaNb2O7 −) and cobalt porphyrin utilized for the electrocatalytic oxidation of ascorbic acid. RSC Adv 6:46388–46393
He J, Li QJ, Tang Y, Yang P, Li A, Li R, Li HZ (2012) Characterization of HNbMoO6, HNbWO6 and HTiNbO5 as solid acids and their catalytic properties for esterification reaction. Appl Catal A Gen 443:145–152
Prasad GK, Takei T, Arimoto K, Yonesaki Y, Kumada N, Kinomura N (2006) Nanocomposites based on exfoliated NbWO6 nanosheets and ionic polyacetylenes. Solid State Ionics 177:197–201
Luo B, Chen M, Zhang Z, Xu J, Li D, Xu D, Shi W (2017) Highly efficient visible-light-driven photocatalytic degradation of tetracycline by a Z-scheme gC3N4/Bi3TaO7 nanocomposite photocatalyst. Dalton Trans 46:8431–8438
Liu C, Wu Q, Ji M, Zhu H, Hou H, Yang Q, Hou W (2017) Constructing Z-scheme charge separation in 2D layered porous BiOBr/graphitic C3N4 nanosheets nanojunction with enhanced photocatalytic activity. J Alloy Compd 723:1121–1131
Liu Z, Ma R, Osada M, Iyi N, Ebina Y, Takada K, Sasaki T (2006) Synthesis, anion exchange, and delamination of Co–Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128:4872–4880
Wang Y, Dong R, Li A, Hu LF, He J (2016) Characterization of modified α-LiNbWO6 layered materials and their catalytic performance for toluene nitration. Optoelectron Adv Mater 10:102–107
Tao T, Zhang X, Liu L, Ma J, Zhang D, Pan B, Tong Z (2014) Preparation and electrochemical behaviour study of layered Bi2SrTa2O9 with a cationic manganese porphyrin. Micro Nano Lett 9:909–912
Ma J, Wu J, Zheng J, Liu L, Zhang D, Xu X, Tong Z (2012) Synthesis, characterization and electrochemical behavior of cationic iron porphyrin intercalated into layered niobite. Microporous Mesoporous Mater 151:325–329
Wu M, Wang Y, Wei Z, Wang L, Zhuo M, Zhang J, Ma J (2018) Ternary doped porous carbon nanofibers with excellent ORR and OER performance for zinc–air batteries. J Mater Chem A 6:10918–10925
Wang L, Wang Y, Wu M, Wei Z, Cui C, Mao M, Ma J (2018) Nitrogen, fluorine, and boron ternary doped carbon fibers as cathode electrocatalysts for zinc–air batteries. Small 14:1800737
Sousa AL, Santos WJ, Luz RC, Damos FS, Kubota LT, Tanaka AA, Tanaka SM (2008) Amperometric sensor for nitrite based on copper tetrasulphonated phthalocyanine immobilized with poly-l-lysine film. Talanta 75:333–338
Zhang X, Wang M, Li D, Liu L, Ma J, Gong J, Tong Z (2013) Electrochemical investigation of a novel metalloporphyrin intercalated layered niobate modified electrode and its electrocatalysis on ascorbic acid. J Solid State Electron 17:3177–3184
Armijo F, Goya MC, Reina M, Canales MJ, Arévalo MC, Aguirre MJ (2007) Electrocatalytic oxidation of nitrite to nitrate mediated by Fe(III) poly-3-aminophenyl porphyrin grown on five different electrode surfaces. J Mol Catal A Chem 268:148–154
do Carmo DR, Paim LL, Metzker G, Dias Filho NL, Stradiotto NR (2010) A novel nanostructured composite formed by interaction of copper octa (3-aminopropyl) octasilsesquioxane with azide ligands: preparation, characterization and a voltammetric application. Mater Res Bull 45:1263–1270
Ojani R, Raoof JB, Zarei E (2008) Poly (ortho-toluidine) modified carbon paste electrode: a sensor for electrocatalytic reduction of nitrite. Electroanalysis 20:379–385
Pan B, Ma J, Zhang X, Li J, Liu L, Zhang D, Tong Z (2015) A laminar nanocomposite constructed by self-assembly of exfoliated α-ZrP nanosheets and manganese porphyrin for use in the electrocatalytic oxidation of nitrite. J Mater Sci 50:6469–6476. https://doi.org/10.1007/s10853-015-9205-8
Hu F, Chen S, Wang C, Yuan R, Yuan D, Wang C (2012) Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal Chim Acta 724:40–46
Liu SY, Chen YP, Fang F et al (2008) Innovative solid-state microelectrode for nitrite determination in a nitrifying granule. Environ Sci Technol 42:4467–4471
Acknowledgements
This work was supported by Natural Science Foundation of Jiangsu Province (BK20161294, BK20160434), Lianyungang Science Project (CG1602), the University Science Research Project of Jiangsu Province (15KJB430004), Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX18_2607), the Jiangsu Marine Resources Development and Research Institute (LYG52105-2018045), China Postdoctoral Science Foundation (2018M632283), Industry-University-Research Collaboration Project of Jiangsu Province (BY2018281), and Huaihai Institute of Technology Graduate Practice Innovation Project (XKYCXX2017-5).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, Z., Sun, L., Wu, S. et al. Preparation of manganese porphyrin/niobium tungstate nanocomposites for enhanced electrochemical detection of nitrite. J Mater Sci 54, 10204–10216 (2019). https://doi.org/10.1007/s10853-019-03526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03526-4