Abstract
In our research, a convenient exfoliation/restacking route was used to fabricate a sandwich-structured nanocomposite of Sr2Nb3O10/CoTMPyP [5,10,15,20-tetrakis(N-methylpyridinium-4-yl)porphyrinato cobalt] via electrostatic interaction between colloidal dispersion and cobalt porphyrin aqueous solution. The final self-assembled products were characterized by XRD, FTIR, UV–Vis, SEM, TEM, AFM, and ICP. During the exfoliation procedure, the well-dispersed Sr2Nb3O10− colloidal dispersion was obtained with the zeta potential value of − 44.2 mV. Additionally, the existence of unilamellar Sr2Nb3O10− nanosheet was evidenced by AFM, and zeta potential values of the reassembly process with the addition of CoTMPyP aqueous solution into the colloidal dispersion were measured by a Zetasizer Nano apparatus. Above all, the final Sr2Nb3O10/CoTMPyP hybrid film displayed excellent electro-catalytic activities towards hydrazine hydrate oxidation with peak potential at 0.158 V in pH 7.0 PBS indicated by CV measurements; moreover, a detection limit of 3.52 × 10−5 M was obtained in the concentration range of 5 × 10−5–9.9 × 10−4 M at a signal-to-noise ratio of 3.0.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrazine hydrate was not only widely applied as a strong reducing agent, pharmaceutical intermediates, antioxidants, etc., but also considered as a type of fuel cell with high energy density, except for methanol, formic acid fuel cell [1,2,3,4]. Comparing with the efficient oxidation of methanol and formic acid limited to noble metal electro-catalysts only, the possible electro-catalytic oxidation of hydrazine hydrate on non-noble metal electrodes became the advantage of direct hydrazine fuel cell. Therefore, much attention was focused on the exploration of efficient non-noble electro-catalysts towards hydrazine hydrate oxidation [5,6,7,8,9,10]. It is worth mentioning that a well-known class of naturally occurring compounds in biological systems, porphyrins and their derivatives played an important role in the development of electro-catalysts for different reactions because of the intriguing redox properties [11]; in particular, transition metal porphyrins complexes were reported to exhibit excellent catalytic activity towards hydrazine hydrate oxidation [8]. To overcome the own limitations, or even to improve their properties, metalloporphyrin complexes have been usually immobilized into the rigid inorganic matrix [12, 13]. However, the tedious and time-resuming work-up was required for introducing functional guest spices in large volume into the interlayer by virtue of the traditional ion-exchange process; consequently, the convenient and effective route for fabricating functional hybrids was urgent to be developed.
On the other hand, two-dimensional layered metal oxide semiconductors (LMOSs) nanosheets derived from the parent layered materials have gained popularity in fundamental research [14,15,16,17,18] since the production of graphene with amazing functionalities. Consequently, an interesting osmotic swelling/exfoliation route was advanced for producing unilamellar LMOSs nanosheet in high quality through the permeation of bulky tetraalkylammonium cations (TAA+) and large amounts of H2O molecules into the interlayer region to greatly weaken the interactions between neighbouring sheets [19, 20]. Owing to the structural diversity and extraordinary electronic properties, the obtained nanosheets with atomic or molecular thickness and micrometer-order lateral sizes were thus exploited for application in electronic, magnetic, optical, electrochemical, and catalytic nanodevices [17]; more interestingly, the negatively charged nanosheets can serve as promising building blocks for the construction of functional hybrid materials [21]. At present, the hybridization of a broad spectrum of guest species including transition metal complex [22, 23], transition metal nanoparticles [24, 25], and LDHs nanosheets [26, 27] with exfoliated LMOSs nanosheets have been achieved through electrostatic self-assembly reaction; nevertheless, the introduction of bulky organic functional pigments into the interlayer of LMOSs using the exfoliation/restacking method remained less studied [28].
As we all know, Dion–Jacobson (D–J)-type layered perovskites were highly favoured among two-dimensional LMOSs, one representative example of which was KSr2Nb3O10 characteristic of triple-octahedral arrangements along the host layer (schematic illustration of the structure is in Fig. 1) [29], and the protonated product can be also dispersed in the TAAOH aqueous solution to form unilamellar nanosheet maintaining excellent properties of semiconductor from the parent [30]; herein, the promising inorganic matrix of KSr2Nb3O10 was selected as the starting material, and Sr2Nb3O10− nanosheets were obtained by dispersing the protonated product into TBAOH [(C4H9)4NOH] aqueous solution; what is more, the final Sr2Nb3O10/CoTMPyP nanocomposites were fabricated via electrostatic interaction between Sr2Nb3O10− nanosheets colloidal dispersion and cobalt porphyrin aqueous solution (Fig. 2), and electro-catalytic measurements towards hydrazine hydrate oxidation adopting the Sr2Nb3O10/CoTMPyP assembled hybrid film were taken.
Experimental
Preparation of Sr2Nb3O10/CoTMPyP nanocomposite
The host material of KSr2Nb3O10 was synthesized through a conventional solid-state reaction referring to a previously reported literature [31]. The obtained product (1 g) was then converted into the protonic form of HSr2Nb3O10 in 6 M HNO3 (30 ml) for three times (24 h/per time). Concerning the preparation of exfoliated Sr2Nb3O10− nanosheets, it was realized by dispersing the acidified product into stoichiometric amounts of 10 (wt)% TBAOH aqueous solution with uniform agitation for 5 days (the molar ratio of TBA+/H+ = 1 was adopted), and the final translucent colloidal suspension was centrifuged at 3000 rpm for 10 min to remove the unexfoliated particles. In order to fabricate the Sr2Nb3O10/CoTMPyP nanocomposite, 1 mM CoTMPyP aqueous solution (2, 3, 4 ml) was separately added into the obtained Sr2Nb3O10− nanosheets colloidal dispersion above (4, 3, 2 ml), and then the flocculated precipitates were washed with distilled water and anhydrous ethanol repeatedly and dried at 50 °C in a vacuum oven for 24 h. The final nanocomposites were labelled as Sr2Nb3O10/CoTMPyP0.5, Sr2Nb3O10/CoTMPyP1, Sr2Nb3O10/CoTMPyP2, according to the corresponding volume ratio of CoTMPyP aqueous solution and the colloidal dispersion (CoTMPyP/Sr2Nb3O10− = 0.5, 1, 2).
Characterization
Shimadzu FTIR-8400S spectrometer was employed for collecting infrared spectra of the products with the use of KBr pellets. UV–Vis absorption spectra were recorded with a UV–Vis spectrometer (UV-2550). A RINT 2000 diffractometer (Cu Kα radiation, λ = 0.154 nm, Rigaku) was used to obtain X-ray diffraction patterns of the samples with 2θ going from 2° to 40°. The scanning electron microscopic images of the samples were acquired by a JSM-5600 apparatus (JEOL), atomic force microscope (AFM) images were taken with a Bruker dimension edge SPM apparatus adopting the peak force tapping mode, and high-resolution transmission electron microscope (HRTEM) images were taken with the apparatus (JEOL, JEM-2010); the specimens were prepared by sonicating the products in ethanol for 10 min in advance and evaporating a drop of the resulting suspension onto a copper grid. Zeta potential was measured by a Malvern Zetasizer Nano instrument, and water at 25 °C was selected as the dispersion solvent. The concentration of Co element in the final hybrid solution was analysed by an inductively coupled plasma (ICP) spectrometer (CAP6300).
Electrochemical measurements
Electrochemical measurements were performed in a conventional three-electrode cell using a CHI660c electrochemical workstation, platinum wire electrode and a saturated calomel electrode (SCE) were used as the auxiliary electrode and the reference electrode, respectively, and the Sr2Nb3O10/CoTMPyP1 hybrid thin-film modified glass carbon electrode (GCE) was used as the working electrode by coating 7.5 μl 1 mg/ml Sr2Nb3O10/CoTMPyP1 aqueous dispersion on GCE and dried naturally for 24 h. With the purpose of investigating electro-catalytic properties of the final hybrid film towards hydrazine hydrate oxidation, the measuring techniques of cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were adopted in this experiment. All buffer solutions including Britton–Robinson (B–R) buffer solution and phosphate buffer solution (PBS) in the electrochemical measurements were purged with nitrogen for 20 min prior to the experiments.
Results and discussion
XRD analysis
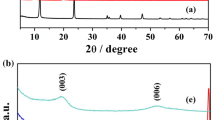
The sintered product of KSr2Nb3O10 was identified by X-ray diffraction analysis in Fig. 3, the basal spacing (d002) of the starting material was calculated as 1.46 nm on the basis of the angle (6.03°) of (002) diffraction peak, and the formation of H3O+ in the interlayer led the basal spacing of the acidified product an increase by 1.62 nm; upon the reassembly of Sr2Nb3O10− nanosheets and CoTMPyP cations, the (002) diffraction peaks of the three obtained nanocomposites shifted towards almost the same lower 2θ angle of ~ 4.3°, indicating that the basal spacings of Sr2Nb3O10/CoTMPyP hybrids increased to ~ 2.05 nm, correspondingly. As observed in Fig. 3c, the XRD pattern of Sr2Nb3O10− nanosheets colloidal suspension, the disappearance of sharp peaks suggested the collapse of layered structure [32, 33]. On the other hand, the arrangement model of the Sr2Nb3O10/CoTMPyP1 hybrid can be postulated by the inclined angle of CoTMPyP molecules in the galley. According to the interlayer distance of the Sr2Nb3O10/CoTMPyP1 hybrid (the value was calculated as 0.59 nm by subtracting the Sr2Nb3O10− layer thickness of 1.44 nm) [16] and the CoTMPyP molecular dimension (18 × 18 Å, estimated by MM2 calculation), it was concluded that CoTMPyP molecules were located in the galley of the Sr2Nb3O10/CoTMPyP1 hybrid by an inclined angle of 19° to form a monolayer in Fig. 2.
Zeta potential analysis
As can be seen from Fig. 4a, it presented the photograph of the reassembly process between Sr2Nb3O10− nanosheets colloidal suspension and CoTMPyP aqueous solution, the dark red flocculent precipitates appear at once as soon as CoTMPyP aqueous solution was added into Sr2Nb3O10− nanosheets colloidal dispersion, and the generation of the flocculent precipitates (i.e. the Sr2Nb3O10/CoTMPyP nanocomposite) can be illustrated as the electrostatic interaction between Sr2Nb3O10− nanosheets with negative charge and cobalt porphyrin cations. Furthermore, the reassembly process can be finished within 20 min, and this is the advantage of exfoliation/restacking route over the traditional ion-exchange method.
Tyndall light scattering phenomenon of the Sr2Nb3O10− colloidal dispersion is exhibited in Fig. 4b, suggesting the occurrence of exfoliation to a certain degree [34]; in order to further investigate the delamination/restacking process, the measurement of the surface potential was made by a Zetasizer Nano instrument. The zeta potential value of Sr2Nb3O10− nanosheets colloidal dispersion was − 44.2 mV in the light of the zeta potential report in Figure S3; regarding the formation of Sr2Nb3O10− nanosheets, it can be explained as the penetration of TBA+ ions in large volume into the interlayer of the acidified product, and expansion of the interlayer spacing to greatly weaken the interactions of neighbouring nanosheets [20]. Additionally, the influence of the volume ratio between CoTMPyP aqueous solution and Sr2Nb3O10− nanosheets colloidal suspension on the zeta potential values was investigated; it can be observed that as the increase in CoTMPyP aqueous solution volume added into Sr2Nb3O10− nanosheets colloidal dispersion, the zeta potential values increased gradually (Fig. 5) and the pH values decreased from 12.21 to 6.55. The zeta potential absolute value was approximately zero at the volume ratio of 0.22, and the zeta potential value change in the reassembly process also provides the support for the occurrence of flocculation phenomenon between Sr2Nb3O10− nanosheets colloidal dispersion and CoTMPyP aqueous solution.
IR spectra analysis
The successful introduction of CoTMPyP molecules into the galley of Sr2Nb3O10− laminate can also be identified by IR spectra analysis (Figure S1). The absorption peaks of KSr2Nb3O10 host material were mainly in the range of 1000–400 cm−1 (Nb–O stretching vibration); furthermore, the IR spectra of CoTMPyP molecules exhibited typical characteristic peaks such as 1640 cm−1, and 1560, 1459, 1401 cm−1, which were assigned to C=N stretching vibration in the pyridine substituent, and C=N, C=C stretching vibration in the porphyrin rings, respectively. Similarly, several absorption bands (1638, 1400, 1121, 924, and 582 cm−1) were observed in the IR spectra of the Sr2Nb3O10/CoTMPyP1 nanocomposite, illustrating that the final hybrid contains not only the Sr2Nb3O10− host laminate, but also CoTMPyP molecules. Furthermore, the interaction between guest molecules and host materials caused a slight shift in the characteristic peaks for Sr2Nb3O10/CoTMPyP compared with IR spectrum of pure CoTMPyP [35].
UV–visible absorption spectra analysis
As shown in Figure S2, typically, the Soret band and Q band of CoTMPyP aqueous solution appeared at 437 and 549 nm, respectively; however, the self-assembly reaction of Sr2Nb3O10− nanosheets and CoTMPyP cations led to red shifts in the Soret bands and Q bands of the obtained dark-brown hybrid films; in detail, Sr2Nb3O10/CoTMPyP0.5, Sr2Nb3O10/CoTMPyP1, Sr2Nb3O10/CoTMPyP2 hybrids exhibited 8-, 9-, 10-nm red shifts in the Soret bands, and 1-, 2-, 3-nm red shifts in Q bands, respectively, demonstrating the intense interaction between Sr2Nb3O10− laminate and CoTMPyP cations [36]. Additionally, owing to the different intermolecular interactions of stacked metalloporphyrin molecules, the occurrence of a broadening phenomenon was in the absorption spectra of the final reassembly products when compared to free CoTMPyP absorption bands, which was coincident with several previous reports [13, 37, 38].
Morphology analysis
The surface morphology of KSr2Nb3O10, HSr2Nb3O10, and the reassembly product, Sr2Nb3O10/CoTMPyP1, was acquired by SEM and TEM as shown in Fig. 6, comparing with that of the origin material, KSr2Nb3O10, the smooth surface of the acidified product indicated higher crystallinity corresponding to XRD data of the acidified product, and the particle size of the protonated product ranges from 0.1 to ca. 10 μm. In order to confirm the existence of Sr2Nb3O10− nanosheets in the delamination process, the obtained colloidal dispersion was characterized by AFM adopting a peak force tapping mode. As can be seen clearly from Fig. 7a, there existed several nanosheets in irregular shape dispersing on the mica substrate, which provides strong evidence for the occurrence of exfoliation. On the side, the appearance of few stacked nanosheets reflected the randomness in the delamination process as well. On the basis of the height profile along the marked white line, the thickness of Sr2Nb3O10− nanosheet was measured as ca. 2.18 nm, and the additional thickness over the crystallographic thickness of the Sr2Nb3O10− laminate (1.44 nm) should be attributed to water molecules and counterions (TBA+) absorbed on the surface of the nanosheet [39,40,41], suggesting the production of unilamellar Sr2Nb3O10− nanosheet.
After the self-assembly of Sr2Nb3O10− nanosheets and CoTMPyP molecules, the overall crystallinity of the final hybrid has decreased; nevertheless, sandwich-structure Sr2Nb3O10/CoTMPyP1 nanocomposite was constructed via electrostatic interaction between Sr2Nb3O10− nanosheets and cobalt porphyrin cations (Fig. 6c, d). As labelled in the partial enlarged drawing (Fig. 6e), the thickness was approximately 4.08 nm with double basal spacings; namely the basal spacing of Sr2Nb3O10/CoTMPyP1 nanocomposite was ca. 2.04 nm, which corresponds well with XRD data (2.05 nm) [42,43,44,45]. Furthermore, the morphology of Sr2Nb3O10/CoTMPyP1 nanocomposite was also analysed by AFM shown in Fig. 7b, and exfoliated Sr2Nb3O10− nanosheets in the delamination process didn’t disturb the reconstruction of the lamellar structure of the final hybrid. The final hybrid consisted of several stacked nanoplates, and the basal spacing of Sr2Nb3O10/CoTMPyP1 was measured as ca. 2.0 and 2.26 nm according to the height profile along the marked white line, which was in good agreement with XRD data (2.05 nm). Besides, the quantitative analysis of the final hybrid was conducted using an ICP instrument, and 2.4 µmol Co element in per litre Sr2Nb3O10/CoTMPyP1 nanocomposite solution was detected.
Electrochemical characterization
Figure 8 gives CV curves of CoTMPyP aqueous solution, Sr2Nb3O10/CoTMPyP1 hybrid film, and KSr2Nb3O10 modified GCE in pH 12.0 B–R buffer solution at the scan rate of 100 mV s−1. Owing to the sensitivity of metalloporphyrin molecules to pH conditions, undefined oxidation/reduction peaks were present in CV curves of CoTMPyP aqueous solution; on the contrary, the assembled hybrid film displayed two pairs of similar well-defined oxidation/reduction peaks, with the redox potentials of one couple of the oxidation/reduction peaks (Ia1, Ic1) at − 0.709 and − 0.754 V, and the redox potentials of the other couple of oxidation/reduction peaks (Ia2, Ic2) at − 0.853 and − 0.959 V, corresponding to CoIII/IITMPyP and CoII/ITMPyP redox couple, respectively [46]. The reversible redox process can be illustrated by the following expressions: CoIITMPyP4+ + e− → CoITMPyP3+ (peak Ic2), CoITMPyP3+ − e− → CoIITMPyP4+ (peak Ia2), CoIIITMPyP5+ + e− → CoIITMPyP4+ (peak Ic1), CoIITMPyP4+ − e− → CoIIITMPyP5+ (peak Ia1). The presence of well-defined oxidation/reduction peaks and the increase in the peak current intensity in the final hybrid film revealed that the immobilization of CoTMPyP molecules on the support matrix of Sr2Nb3O10− was an effective method for utilizing excellent electrochemical performance of metalloporphyrin in harsh conditions.
Electro-catalytic properties of Sr2Nb3O10/CoTMPyP hybrid film towards hydrazine hydrate oxidation
The electrochemical oxidation of N2H4·H2O using Sr2Nb3O10/CoTMPyP1 modified GCE was attempted in 0.2 M pH 7.0 PBS, and a comparison of KSr2Nb3O10/GCE, Sr2Nb3O10/CoTMPyP1 modified GCE and bare GC at 100 mV s−1 was made (Fig. 9A). Concerning the electro-catalytic oxidation effect of bare GC and the host material modified GCE, they barely work in the N2H4·H2O oxidation process, while the oxidation potential of Sr2Nb3O10/CoTMPyP1 modified GCE appeared at 0.158 V, suggesting that the obtained assembled hybrid film through the exfoliation/restacking route can promote electrochemical oxidation process towards N2H4·H2O effectively.
A CV curves of (a) bare GCE (dash line), (b) KSr2Nb3O10 modified GCE (dash dot line), and (c) Sr2Nb3O10/CoTMPyP1 modified GCE (solid line) in N2-saturated pH 7.0 PBS containing 3.92 mM N2H4·H2O at 100 mV s−1. B CV curves of Sr2Nb3O10/CoTMPyP1 modified GCE in N2-saturated pH 7.0 PBS containing 3.92 mM N2H4·H2O at 10, 20, 30, 40, 50, 60, 70, 100, 150, 200, 300, and 400 mV s−1; the inset is the relationship curve between Ipa and v1/2
Additionally, the influences of scan rate on CV behaviour of the final hybrid film were investigated in 0.2 M pH 7.0 PBS shown in (Fig. 9B). With the increase in the scan rate from 10 to 400 mV s−1, the oxidation peak current increases gradually, and the oxidation peak potential moved towards negative, indicating the irreversibility of hydrazine hydrate oxidation process on Sr2Nb3O10/CoTMPyP1 modified GCE. According to the linear equation of Ipa (μA) = 6.11 + 61.15 v1/2 (V1/2 s−1/2) (R = 0.9990) of the relationship curve plotted in the inset (Fig. 9B), a conclusion can be drawn that peak current (Ipa) was proportional to square root of the scan rate (v1/2), demonstrating that electrochemical oxidation process on the surface of Sr2Nb3O10/CoTMPyP1 modified GCE was controlled by hydrazine hydrate diffusion. In addition, the inverted shape peaks were found in the CV curves of Sr2Nb3O10/CoTMPyP towards hydrazine hydrate oxidation analogous to previous literatures [47, 48], which was probably ascribable to the formation of CoIIITMPyP–N2H4 complex in the electrochemical oxidation process, and thus, the possible mechanism of N2H4·H2O oxidation that occurred on the modified electrode was proposed as follows referring to related literatures [49, 50]:
P in the formula represents porphyrin ring, and the rate of the whole process is determined by the formation of CoIIITMPyP–N2H4 complex, namely the second step.
At last, the electrochemical determination of N2H4·H2O using Sr2Nb3O10/CoTMPyP1 modified GCE was attempted with the peak current (Ipa) and hydrazine hydrate concentration (c) in hydrazine hydrate concentration of 0.05–0.99 mM in 0.2 M pH 7.0 PBS. As can be inferred from the relationship curve of the inset (Fig. 10), the peak current was proportional to hydrazine hydrate concentration with the calibration equation of I (μA) = 0.06 + 4.05c (mmol L−1) (r = 0.9957); hence, a detection limit was calculated as 3.52 × 10−5 M at a signal-to-noise ratio of 3.0.
Conclusions
The successful introduction of functional metalloporphyrin molecules (CoTMPyP) in large volume into the interlayer of KSr2Nb3O10 through the convenient exfoliation/restacking route for the first time, which was confirmed by XRD, FTIR, UV–Vis, SEM, TEM, AFM, and ICP. Comparing with the crystallographic thickness of the Sr2Nb3O10− laminate (1.44 nm), the observed thickness of Sr2Nb3O10− nanosheet (2.18 nm) was likely to be homogeneously unilamellar, and the basal spacing of Sr2Nb3O10/CoTMPyP1 nanocomposite was measured as 2.0, 2.26 nm (AFM) and 2.04 nm (TEM) corresponding well to XRD data (2.05 nm). Besides, the well-dispersed and stable Sr2Nb3O10− nanosheets colloidal dispersion with the zeta potential value of − 44.2 mV was obtained, and the zeta potential value was close to 0 mV when the volume ratio of CoTMPyP aqueous solution to Sr2Nb3O10− nanosheets colloidal dispersion was 0.22. The fabrication of Sr2Nb3O10− and CoTMPyP cations through electrostatic self-assembly will facilitate the development of a variety of novel functional nanocomposites based on D–J-type perovskite nanosheets. Eventually, the final self-assembled hybrid film prepared by drop-coating method exhibited excellent electro-catalytic activities towards N2H4·H2O oxidation in pH 7.0 PBS, which makes it a promising electro-catalyst towards N2H4·H2O oxidation; meanwhile, this paper also provides theoretical basis for oxidation of N2H4·H2O by virtue of functional nanocomposites associated with metalloporphyrin complex. Finally, a detection limit was estimated as 3.52 × 10−5 M according to a signal-to-noise ratio of 3.0.
References
Rosca V et al (2009) Nitrogen cycle electrocatalysis. Chem Rev 109:2209–2244
Aldous L, Compton RG (2011) The mechanism of hydrazine electro-oxidation revealed by platinum microelectrodes: role of residual oxides. Phys Chem Chem Phys 13:5279–5287
Serov A, Kwak C (2010) Direct hydrazine fuel cells: a review. Appl Catal B Environ 98:1–9
Li Z, Han C, Shen J (2006) Reduction of Ni2+ by hydrazine in solution for the preparation of nickel nano-particles. J Mater Sci 41:3473–3480. https://doi.org/10.1007/s10853-005-5874-z
Sanabria-Chinchilla J et al (2011) Noble metal-free hydrazine fuel cell catalysts: EPOC effect in competing chemical and electrochemical reaction pathways. J Am Chem Soc 133:5425–5431
Asazawa K et al (2007) A platinum-free zero-carbon-emission easy fuelling direct hydrazine fuel cell for vehicles. Angew Chem Int Edit 46:8024–8027
Kumar AVN, Joseph J (2014) New Zn–NiHCF hybrid electrochemically formed on glassy carbon: observation of thin layer diffusion during electro-oxidation of hydrazine. J Phys Chem C 119:296–304
Yamazaki SI et al (2012) Electrochemical oxidation of hydrazine derivatives by carbon-supported metalloporphyrins. J Power Sources 204:79–84
Geraldo D et al (2002) Volcano correlations between formal potential and Hammett parameters of substituted cobalt phthalocyanines and their activity for hydrazine electro-oxidation. Electrochem Commun 4:182–187
Hosseini M, Momeni MM (2010) Silver nanoparticles dispersed in polyaniline matrixes coated on titanium substrate as a novel electrode for electro-oxidation of hydrazine. J Mater Sci 45:3304–3310. https://doi.org/10.1007/s10853-010-4347-1
Biesaga M et al (2000) Porphyrins in analytical chemistry. A review. Talanta 51:209–224
Goubert-Renaudin SN et al (2010) Synthesis and characterization of carbon-supported transition metal oxide nanoparticles—cobalt porphyrin as catalysts for electroreduction of oxygen in acids. Electrochem Commun 12:1457–1461
Halma M et al (2008) Synthesis, characterization, and catalytic activity of anionic iron(III) porphyrins intercalated into layered double hydroxides. J Catal 257:233–243
Allen MR et al (2009) Evolution of physical and photocatalytic properties in the layered titanates A2Ti4O9 (A = K, H) and in nanosheets derived by chemical exfoliation. Chem Mater 22:1220–1228
Omomo Y et al (2003) Redoxable nanosheet crystallites of MnO2 derived via delamination of a layered manganese oxide. J Am Chem Soc 125:3568–3575
Osada M, Sasaki T (2012) Two-dimensional dielectric nanosheets: novel nanoelectronics from nanocrystal building blocks. Adv Mater 24:210–228
Ma R, Sasaki T (2010) Nanosheets of oxides and hydroxides: ultimate 2D charge-bearing functional crystallites. Adv Mater 22:5082–5104
Yin S et al (2013) Effects of reaction parameters on solution combustion synthesis of lepidocrocite-like K0.80Ti1.733Li0.267O4: phase formation and morphology evolution. J Mater Sci 48:1533–1542. https://doi.org/10.1007/s10853-012-6908-y
Sasaki T, Watanabe M (1998) Osmotic swelling to exfoliation. Exceptionally high degrees of hydration of a layered titanate. J Am Chem Soc 120:4682–4689
Maluangnont T et al (2013) Osmotic swelling of layered compounds as a route to producing high-quality two-dimensional materials. A comparative study of tetramethylammonium versus tetrabutylammonium cation in a lepidocrocite-type titanate. Chem Mater 25:3137–3146
Sasaki T et al (2001) Layer-by-layer assembly of titania nanosheet/polycation composite films. Chem Mater 13:4661–4667
Unal U et al (2003) Electrostatic self-assembly deposition of titanate(IV) layered oxides intercalated with transition metal complexes and their electrochemical properties. J Phys Chem B 107:12680–12689
Ebina Y et al (2005) Photocatalyst of lamellar aggregates of RuOx-loaded perovskite nanosheets for overall water splitting. J Phys Chem B 109:17212–17216
Lin B et al (2013) Mesoporous cobalt-intercalated layered tantalotungstate with high visible-light photocatalytic activity. Microporous Mesoporous Mater 172:105–111
Oshima T et al (2015) Intercalation of highly dispersed metal nanoclusters into a layered metal oxide for photocatalytic overall water splitting. Angew Chem Int Edit 54:2698–2702
Gunjakar JL et al (2011) Mesoporous layer-by-layer ordered nanohybrids of layered double hydroxide and layered metal oxide: highly active visible light photocatalysts with improved chemical stability. J Am Chem Soc 133:14998–15007
Li L et al (2007) Layer-by-layer assembly and spontaneous flocculation of oppositely charged oxide and hydroxide nanosheets into inorganic sandwich layered materials. J Am Chem Soc 129:8000–8007
Pan B et al (2016) Research on the self-assembly of exfoliated perovskite nanosheets (LaNb2O7 −) and cobalt porphyrin utilized for the electrocatalytic oxidation of ascorbic acid. RSC Adv 6:46388–46393
Ebina Y et al (2012) Synthesis and in situ X-ray diffraction characterization of two-dimensional perovskite-type oxide colloids with a controlled molecular thickness. Chem Mater 24:4201–4208
Takagaki A et al (2003) Exfoliated nanosheets as a new strong solid acid catalyst. J Am Chem Soc 125:5479–5485
Dion M et al (1981) Nouvelles familles de phases MIM II2 Nb3O10 a feuillets “perovskitesh”. Mater Res Bull 16:1429–1435
Sun L et al (2007) Preparation of exfoliated epoxy/α-zirconium phosphate nanocomposites containing high aspect ratio nanoplatelets. Chem Mater 19(7):1749–1754
Yu J et al (2015) One-step direct synthesis of layered double hydroxide single-layer nanosheets. Nanoscale 7(21):9448–9451
Liu Z et al (2006) Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128:4872–4880
Park IY, Kuroda K, Kato C (1989) Preparation of a layered double hydroxide-porphyrin intercalation compound. Chem Lett 11:2057–2058
Yao K et al (2003) Spectroscopic and photoelectrochemical study of sensitized layered niobate K4Nb6O17. Langmuir 19:321–325
Yao K et al (2001) Spectroscopic and photoelectrochemical differences between racemic and enantiomeric [Ru(phen)3]2+ ions intercalated into layered niobate K4Nb6O17. J Electroanal Chem 510:144–148
Unal U et al (2006) Visible light photoelectrochemical activity of K4Nb6O17 intercalated with photoactive complexes by electrostatic self-assembly deposition. J Solid State Chem 179:33–40
Ozawa TC et al (2007) Preparation and characterization of the Eu3+ doped perovskite nanosheet phosphor: La0.90Eu0.05Nb2O7. Chem Mater 19:6575–6580
Ozawa TC et al (2008) A new nanosheet phosphor with the high intrananosheet site photoactivator concentration. J Phys Chem C 112:1312–1315
Ida S et al (2006) Photoluminescence properties of multilayer oxide films intercalated with rare earth ions by the layer-by-layer technique. Chem Commun 34:3619–3621
Zhai Z et al (2011) Thermostable nitrogen-doped HTiNbO5 nanosheets with a high visible-light photocatalytic activity. Nano Research 4(7):635–647
Zhai Z et al (2012) Novel mesoporous NiO/HTiNbO5 nanohybrids with high visible-light photocatalytic activity and good biocompatibility. Nanoscale 4(2):547–556
Zhai Z et al (2012) Nitrogen-doped mesoporous nanohybrids of TiO2 nanoparticles and HTiNbO5 nanosheets with a high visible-light photocatalytic activity and a good biocompatibility. J Mater Chem 22(36):19122–19131
Liu C et al (2014) The nanocomposite of polyaniline and nitrogen-doped layered HTiNbO5 with excellent visible-light photocatalytic performance. Phys Chem Chem Phys 16(26):13409–13417
Chen SM et al (2001) The catalytic and photocatalytic autoxidation of S 2−x to SO4 2− by water-soluble cobalt porphyrin. J Mol Catal A: Chem 166(2):243–253
Ardiles P et al (2001) Electrocataltyic oxidation of hydrazine at polymeric iron-tetraaminophthalocyanine modified electrodes. J Mol Catal A: Chem 165(1):169–175
Green MP et al (1989) In situ scanning tunneling microscopy studies of the underpotential deposition of lead on gold (111). J Phys Chem 93(6):2181–2184
Ozoemena KI, Nyokong T (2005) Electrocatalytic oxidation and detection of hydrazine at gold electrode modified with iron phthalocyanine complex linked to mercaptopyridine self-assembled monolayer. Talanta 67:162–168
Muthukumar P, John SA (2014) Efficient oxidation of hydrazine using amine-functionalized cobalt and nickel porphyrin-modified electrodes. J Solid State Electrochem 18(9):2393–2400
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 21401062, 21201070, 51202079), Natural Science Fund of Jiangsu Province (BK20161294, BK20140447, BK20141247, SBK201220654), University Science Research Project of Jiangsu Province (13KJB430005, 12KJD150001, 15KJB430004), Key R&D Project of Jiangsu Province (CG1602, CG1622), HHIT Research Project (Z2015011, Z2014004), and 521 High-level Personnel Training Research Project of Lianyungang City (KK15041).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, B., Xu, J., Zhang, X. et al. Electrostatic self-assembly behaviour of exfoliated Sr2Nb3O10− nanosheets and cobalt porphyrins: exploration of non-noble electro-catalysts towards hydrazine hydrate oxidation. J Mater Sci 53, 6494–6504 (2018). https://doi.org/10.1007/s10853-018-2033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2033-x