Abstract
Rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates are synthesized by a simple sol–gel method. The almost single rhombohedral phase nature of Li2.4Na0.6V2(PO4)3@C along with nanoplate structure leads to high specific capacity and rate capability, and a remarkable cycling performance. As cathode materials for lithium-ion batteries, rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates deliver a high initial discharge capacity of 121.6 mAh g−1 and an excellent capacity retention of 95.6% after 200 cycles at a rate of 1 C in a potential range of 3.0–4.3 V. Even at a high rate of 10 C, an initial discharge capacity of 115.3 mAh g−1 and a capacity retention of 76.7% after 500 cycles are observed. The outstanding electrochemical performance is attributed to the synergetic effect of stable rhombohedral phase, nanoplate structure and uniform carbon coating layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rechargeable lithium-ion batteries (LIBs) have been used in hybrid electric vehicles (HEVs), electric vehicles (EVs) and portable electronic equipment owing to their high voltage, high energy density and excellent cycling life [1,2,3]. Recently, lithium transition metal phosphates such as LiFePO4 [4, 5], LiMnPO4 [6, 7], LiCoPO4 [8] and Li3V2(PO4)3 [9,10,11,12] have attracted a great interest as potential cathode materials for lithium-ion batteries. The NASICON-structured Li3V2(PO4)3 has been synthesized in two different crystallographic structures: the monoclinic Li3V2(PO4)3 (M-LVP) and rhombohedral Li3V2(PO4)3 (R-LVP) [13, 14]. For the M-LVP, a three-dimensional structure is composed of slightly distorted PO4 tetrahedral and VO6 octahedral by sharing the oxygen vertexes [13]. The M-LVP has several advantages, including high operating voltage, ionic mobility and theoretical specific capacity (197 mAh g−1). As a cathode material, the M-LVP displays three voltage plateaus in charge–discharge curves corresponding to extraction/insertion of two lithium ions in a potential range of 3.0–4.3 V [15]. In contrast to the M-LVP, the R-LVP shows only a single voltage platform at 3.75 V, corresponding to the V3+/V4+ redox couple [14, 16]. The theoretical capacity of the R-LVP is 133 mAh g−1, corresponding to extraction/insertion of two lithium ions. The R-LVP with a single operation potential and considerable capacity is very attractive because it can supply a stable output power for electronic devices and electric vehicles to run well. Compared with the M-LVP, however, it is very difficult to directly synthesize the R-LVP due to its poor stability. [17,18,19]. Up to now, several feasible methods have been proposed to obtain rhombohedral compounds [16, 18, 20]. For example, Nazar’s and Goodenough’s groups have synthesized the R-LVP via chemical ion-exchange method [16, 18]. The partial substitution of metal Na+ ions for Li+ ions in pristine LVP can obtain R-Li3-xNa x V2(PO4)3 (R-LNVP) by stabilizing the rhombohedral structure [20]. Nevertheless, the electrochemical performance of the R-LVP or R-LNVP as cathode materials is usually poor, which restricts their practical application in lithium-ion batteries.
Recently, a few strategies have been applied to improve the electrochemical performance of R-LVP or R-LNVP, such as carbon coating [14] and nanosized particles [19, 21]. Yao’s group has synthesized carbon-coated R-LVP by ion-exchange reaction as a cathode material for lithium-ion batteries, which shows the reversible capacities of 97.7 and 76 mAh g−1 at 2 C and 5 C, respectively, and a capacity retention of 77% after 300 cycles at a rate of 1 C [14]. Zhang et al. [19] have prepared porous nanosheet-structured rhombohedral Li2NaV2(PO4)3/C using a facile sol–gel method. This Li2NaV2(PO4)3/C cathode displays a remarkable high rate capability (80 mAh g−1 at 5 C and 68 mAh g−1 at 10 C) and long-term cyclability. (About 93% of the initial capacity is retained over 500 cycles at 2 C.) However, the electrochemical performance, especially high-rate performance, cannot meet the requirement of their practical applications in lithium-ion batteries and needs to be further enhanced.
In this paper, rhombohedral Li2.4Na0.6V2(PO4)3@C (R-LNVP) nanoplates were successfully synthesized through a simple sol–gel method. The synthesized rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates as cathode materials have a remarkable electrochemical performance with excellent rate capability and long cycle life, delivering an initial discharge capacity of 121.6 mAh g−1 and a capacity retention of 95.6% after 200 cycles at 1 C rate in a potential range of 3.0–4.3 V. Even at a high rate of 10 C, the initial discharge capacity is 115.3 mAh g−1 and keeps 76.7% after 500 cycles.
Experimental
Preparation of the materials
Rhombohedral Li2.4Na0.6V2(PO4)3@C (R-LNVP) nanoplates were synthesized via a simple sol–gel method. In a typical procedure, stoichiometric amount of NH4VO3, C2H2O4·H2O, LiOH·H2O, NH4H2PO4, Na2CO3 and glucose was dissolved in 50 mL of deionized water under vigorous stirring at 70 °C, and the mixed solution was stirred continually till the green precursor gel was obtained. The obtained precursor gel was dried in a vacuum oven at 120 °C for 12 h to achieve a dry gel. Afterward, the dried precursor was pre-annealed at 350 °C for 4 h and then calcined at 750 °C for 10 h in an atmosphere of Ar gas containing 10% H2 to yield black R-LNVP nanoplates. Hybrid-phase Li2.4Na0.6V2(PO4)3@C (H-LNVP) nano/microspheres were prepared by a solvothermal method using NH4VO3, oxalic acid and polyvinylpyrrolidone (PVP) as starting materials. First, PVP was dissolved in 80 mL of ethylene glycol, and 0.01 mol NH4VO3 and 0.015 mol C2H2O4·H2O were added to the mixed solution under vigorous stirring at 70 °C. Stoichiometric LiOH·H2O, NH4H2PO4 and Na2CO3 dissolved completely into the mixed solution. The amount of PVP was based on 10 wt% residual carbon in Li2.4Na0.6V2(PO4)3@C. Then, the mixed solution was transformed into a 100-mL Teflon-lined stainless steel autoclave and heated at 180 °C for 2 h. After the solution cooled to room temperature, the solution was dried at 80 °C until the ethylene glycol was completely vaporized and dried at 120 °C for 12 h in a vacuum oven to achieve the precursor powders. Here, PVP not only served as a template, but also acted as a carbon source to enhance the electronic conductivity of Li2.4Na0.6V2(PO4)3. The sintering process was same as the synthesis of R-LNVP nanoplates.
Characterization of the materials
The crystal structure of the as-synthesized samples was identified using a PIXcel3D X-ray diffractometer with Cu Kα source. A field emission scanning electron microscope (FE-SEM, S-4800, 15 kV) was used to observe the morphologies of the samples. The microstructure of the samples was investigated by using a Tecnai G2 F20 H-800 transmission electron microscope with an accelerating voltage of 200 kV. The carbon content of the samples was determined by thermogravimetric (TG) analysis. X-ray photoelectron spectroscopy of the samples was collected using an ESCALAB-250Xi spectrometer. The Raman spectra were obtained using a HORIBA LabRAM HR800 equipped with a 514-nm Ar-ion laser.
Electrochemical measurements
The electrochemical properties of the samples were performed using CR2032 coin cells. The electrode was prepared by mixing active materials, acetylene black and polyvinylidene fluoride (PVDF) in a weight ratio of 80:10:10 in N-methyl-2-pyrrolidone (NMP). The slurry of mixture was coated onto aluminum foils using a film-coating machine and dried at 110 °C for 12 h in vacuum condition. The dried film was pressed in a fixed pressure, and then, the electrode sheet was punched into disks with a diameter of 14 mm. The electrolyte was 1 M LiPF6 in a mixture solution of ethylene carbonate (EC), dimethyl carbonate (DMC) and ethyl methyl carbonate (EMC) in a volume ratio of 1:1:1. The prepared electrode was used as the working electrode. The pure lithium plate served as the anode, and a polypropylene membrane (Celgard 2400) was used as the separator. The coin cells were assembled in an argon-filled glove box. Galvanostatic charge/discharge measurements were made on an Arbin battery testing system. Electrochemical impedance spectroscopy (EIS) testing was conducted using a Solartron electrochemical workstation with an AC signal of 5 mV and a frequency range from 10 mHz to 100 kHz. The cyclic voltammetry (CV) tests were also carried out using the Solartron electrochemical workstation at various scanning rates in the potential range of 3.0–4.3 V.
Results and discussion
Material characterization
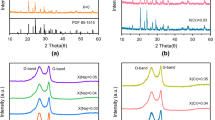
XRD patterns of H-LNVP and R-LNVP samples are shown in Fig. 1a, b. All the diffraction peaks of the H-LNVP sample (Fig. 1a) could be indexed to a two-phase mixture of R-LVP and M-LVP. According to Rietveld refinement, the mole ratio of M-LVP to R-LVP is 58:42. The lattice parameters of the M-LVP are a = 8.664 Å, b = 12.212 Å, c = 0.8603 Å, and those of the R-LVP are a = b=8.339 Å, c = 22.454 Å, which are in good agreement with those of the previous studies [16, 18]. The obtained parameters are Rexp = 3.03%, Rwp = 6.03% and Rp = 4.62%, which represent the satisfactory quality of the Rietveld refinement. The R-LNVP sample (Fig. 1b) is nearly pure R-LVP phase with a space group of \( R\overline{3} C \) analyzed by Rietveld refinement with satisfactory reliability factors (Rexp = 2.07%, Rwp = 4.98% and Rp = 3.5%). The unit cell parameters are a = b=8.341 Å and c = 22.458 Å, and the atomic positions of the R-LNVP sample are listed in Table 1. The absence of rhombohedral Na3V2(PO4)3 in these two samples suggests that small amount Na can replace completely Li of pristine LVP structure. No diffraction peaks of carbon phase are observed in the XRD patterns of these two samples, indicating that the residual carbon is amorphous. XPS measurements were made to study the content and oxidation states of the elements of the prepared samples, as displayed in Fig. 1(c). The peaks at the binding energy of 55.1, 133.6, 190.8, 285.4, 531.2 and 1071.2 eV corresponds to Li1s, P2p, P2s, C1s, O1s and Na1s, respectively. The high-resolution V2p3/2 XPS spectrum in Fig. 1d shows a peak at 516.9 eV, which represents the oxidation state of V3+ and is in good agreement with the previously reported papers [22, 23]. The content of Na in H-LNVP and R-LNVP determined by XPS is approximately identical, with a molar ratio value about 0.55 and 0.56, respectively. In order to identify the structural characteristics of the carbon coated on the surface of LNVP particles, Raman spectroscopy measurements were made, as shown in Fig. 1e. Two intense broad peaks located at about 1347 and 1586 cm−1 in the Raman spectrum of the R-LNVP sample can be assigned to the disorder-induced phonon mode (D-band) and graphite band (G-band), respectively. The ratio of ID/IG is 0.97, indicating that a high graphitization degree of carbon existed in the carbon matrices [24]. The carbon content is determined to be around 6.5 and 8.9 wt% for the H-LNVP and R-LNVP samples (Fig. 4f), respectively, using the TG analysis technique.
Figure 2 shows SEM and TEM images of the samples. As illustrated in Fig. 2a, the H-LNVP sample consists of nano/micro-sized spheres. From Fig. 2b, the R-LNVP sample exhibits nanoplate-like particles with a thickness of about 20 nm and a width of around 500 nm. The morphology of two samples is confirmed by TEM (Fig. 2c, d) to be nano/microspheres and nanoplates, respectively. High-resolution TEM (HRTEM) images (Fig. 2e, f) reveal an amorphous carbon layer with a thickness of about 4.8 nm and 5.8 nm covering on the surface of the H-LNVP and R-LNVP particles, which is expected to provide a high electronic conductivity to the materials. The characteristic of two-phase coexistence in the H-LNVP sample is verified in Fig. 2e. The lattice fringes with a spacing of 0.440 and 0.373 nm correspond to the (012) planes of M-LVP and the (113) planes of R-LVP, respectively. In addition, the two-phase boundary is clearly observed in the HRTEM image of the H-LNVP sample. In contrast, a typical well-crystalline R-LVP is clearly identified in the R-LNVP sample. As shown in Fig. 2f, a lattice fringe spacing of 0.433 nm in the single-phase region is well matched to that of the (110) planes of R-LVP.
Electrochemical properties of the samples
CV curves of two samples were obtained at a scanning rate of 0.1 mV s−1 in the potential range of 3.0–4.3 V, as shown in Fig. 3a. Three anodic peaks at around 3.61, 3.69 and 4.1 V for the H-LNVP sample correspond to the extraction of lithium ion in the monoclinic phase, while the anodic peak at 3.82 V is attributed to the extraction reaction of lithium ions in the rhombohedral phase [14, 25]. Four corresponding cathodic peaks appear around 3.55, 3.63, 3.639 and 4.03 V, respectively. CV curve demonstrates the coexistence of monoclinic and rhombohedral phases in the H-LNVP sample. In contrast to the H-LNVP sample, the R-LNVP sample shows a pair of sharp redox peaks at around 3.81/3.69 V relating to the insertion/extraction of lithium ions in the rhombohedral phase and three very small auxiliary redox peaks caused by the insertion/extraction of lithium ions in the monoclinic phase, indicating that the R-LNVP sample consists of rhombohedral LVP and a negligible amount of monoclinic LVP. According to the CV curve, the mole ratio of R-LVP to M-LVP is estimated to be 95:5.
a Cyclic voltammograms of the H-LNVP and R-LNVP samples at a scanning rate of 0.1 mV s−1 in the potential range of 3.0–4.3 V. b, c Cyclic voltammograms of two samples at various scanning rates. d A linear relationship between the oxidation peak current and the square root of scanning rate for two samples
Figure 3b, c displays the CV curves of the H-LNVP and R-LNVP samples at various scanning rates of 0.1, 0.2, 0.5, 1 and 2 mV s−1, respectively. Even at a relatively high scanning rate (2 mV s−1), the well-defined and symmetrical redox peaks can be observed, implying the good lithium intercalation reversibility and rate performance of the samples. As shown in Fig. 3d, the highest oxidation peak current has a linear relationship with the square root of scanning rate, which is indicative of electrode reaction controlled by diffusion [26]. The lithium-ion diffusion coefficient D (cm2 s−1) can be calculated by the following Randles–Sevcik Eq. (1) [27]:
where ip is the peak current (A), n is the number of electrons involved during the redox process, A is the surface area of electrode (cm2), \( C_{\text{Li}}^{*} \) is the concentration of lithium ions in the electrode (mol cm−3), υ is the scan rate (V s−1), and D is the diffusion coefficient of lithium ions (cm2 s−1). Based on Eq. (1) and the slope of ip versus υ1/2 plots in Fig. 3d, the chemical diffusion coefficient of lithium ion in the H-LNVP and R-LNVP samples is calculated to be 5.06 × 10−10 and 1.01 × 10−9 cm2 s−1, respectively. The R-LNVP sample has a higher Li-ion chemical diffusion coefficient than the H-LNVP sample and LVP nanocrystals (10−10 cm2 s−1) [10], indicating better rate capability. It is resulted from a larger free volume of the interstitial space in rhombohedral LVP, supplying a faster lithium-ion movement [16].
The electrochemical performance of two samples was firstly evaluated by galvanostatic charge/discharge tests at 1 C (1 C = 133 mA g−1) in the potential range of 3.0–4.3 V. Figure 4a, b presents the charge/discharge curves of the H-LNVP and R-LNVP samples. The H-LNVP sample exhibits four pairs of charge/discharge plateaus, which agrees well with the redox peaks in the CV curves. The plateaus around 3.6, 3.68 and 4.10 V correspond to the reversible reaction of the lithium ions in M-LVP, and the plateau near 3.76 V is assigned to lithium-ion insertion/extraction in R-LVP. In comparison, the R-LNVP sample shows clearly a pair of flat charge/discharge platforms at 3.77/3.72 V, which is attributed to the V3+/V4+ redox couple in R-LVP [14, 16, 19]. The corresponding cycle performance of the two samples is presented in Fig. 4c. Both samples deliver high capacities and excellent cycling stability. An initial discharge capacity of the H-LNVP and R-LNVP samples is 115.9 and 121.6 mAh g−1, and a capacity retention is 100 and 95.6% after 200 cycles, respectively. Except the first several cycles, the coulombic efficiencies of two samples are close to 100% in the whole cycling processes. In order to evaluate objectively the superiority of the R-LNVP sample, a comparison of cycle performance of the H-LNVP and R-LNVP samples with same carbon content (8.9 wt%) is carried out, as illustrated in Fig. 4c. The H-LNVP sample (8.9 wt%C) was prepared by adding 2.4 wt% conductive carbon (acetylene black) to the H-LNVP sample (6.5 wt%C) and mixing uniformly. The capacities and cycle performance were investigated by charge–discharge testing at 1 C. The H-LNVP sample (8.9 wt%C) shows an initial discharge capacity of 117.5 mAh g−1, which is inferior to that of the R-LNVP sample (121.6 mAh g−1). In addition, the capacity retention of the H-LNVP sample (8.9 wt%C) is a little lower than that of the H-LNVP sample (6.5 wt%C). The results indicate that the effect of the increasing small carbon content (2.4 wt%) on the electrochemical performance of the samples is ignorable.
To evaluate the rate capability, the H-LNVP and R-LNVP samples are also tested at various current rates of 0.1 C, 0.5 C, 1 C, 5 C, 10 C and 20 C in the potential range of 3.0-4.3 V. The rate capability of two samples is displayed in Fig. 4d. The discharge capacities of the H-LNVP sample are 114.1, 115.6, 113.8, 105.4, 97.4 and 80.1 mAh g−1 at 0.1 C, 0.5 C, 1 C, 5 C, 10 C and 20 C, respectively. Compared with the H-LNVP sample, the R-LNVP sample exhibits higher discharge capacities of 121.6, 119.3, 115.6, 108.4, 102.9 and 94.7 mAh g−1 under the same testing procedures, indicating an outstanding rate capability. After 55 cycles, the current density recovers to 0.5 C; the discharge capacity of the R-LNVP sample can remain 116.7 mAh g−1, as high as 96.0% of the first capacity (121.6 mAh g−1). The gravimetric energy density of the R-LNVP sample (451.5 Wh kg−1) is higher than that of the H-LNVP sample (435.1 Wh kg−1) and LiMn2O4 (~ 430 Wh kg−1) [28]. The remarkable rate capability of the R-LNVP sample may be attributed to the synergetic effect of nanoplate-like structure and stable rhombohedral LVP. Compared with spherical H-LNVP, the R-LNVP nanoplates can provide a higher surface area and a shorter lithium-ion diffusion path, in favor of improving the rate capability. The stabilized NASICON framework of rhombohedral LVP could provide a better lithium-ion transport in LVP, leading to enhance the rate capability and cycle stability [16]. Furthermore, the uniform and thick carbon coating layer on the surface of the R-LNVP particles effectively increases its electronic conductivity and improves the electrochemical performance.
The high-rate long-term cycle stability of the H-LNVP and R-LNVP samples at 10 C is shown in Fig. 4e. The R-LNVP sample exhibits higher cycle stability than the H-LNVP sample, with an initial discharge capacity of 115.3 mAh g−1 and a capacity retention of 76.7% after 500 cycles. The excellent long-term cycle performance of the R-LNVP sample is due to the synergetic effect of nanoplate-like structure, stable rhombohedral LVP and uniform carbon coating layer. The electrochemical performance of the R-LNVP sample is comparable to previous reported plate-like V-based phosphate cathodes (Table 2), confirming the superiority of the R-LNVP sample developed in this work.
To further understand lithium-ion insertion/extraction kinetics of the samples, EIS spectra of two samples were tested in fully discharge state of the cells after different cycles at 1 C. The AC voltage amplitude of 5 mV was employed in a frequency range of 10 mHz–100 kHz. From Fig. 5, all EIS spectra consist of a depressed semicircle at the high-frequency region and a sloping line at the low-frequency region. All the EIS spectra were simulated by an equivalent circuit model using the Zview program [32]. The semicircle at the high-frequency region is related to the charge transfer resistance (Rct). The sloping line in the low-frequency region represents the diffusion impedance of lithium ions in active materials. The fitting results are listed in Table 3. The Rct value of the R-LNVP fresh cell is 24 Ω and then increases to 54, 52 and 50 Ω after 1, 5 and 200 cycles, respectively. The large increase in Rct value in the first cycle can be attributed to the formation of solid electrolyte interface (SEI) film [11], then slightly decreases after 5 cycles owing to the perfect carbon coating layer and keeps almost unchanged over 200 cycles due to the stable rhombohedral structure. It can be found that the Rct value of the R-LNVP sample (50 Ω) is lower than that of the H-LNVP sample (70 Ω), suggesting the nanoplate-structured R-LNVP has fast charged species transport and enhanced rate capability.
Conclusions
In summary, rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates have been successfully synthesized by a simple sol–gel method. For comparison, hybrid-phase Li2.4Na0.6V2(PO4)3@C nano/microspheres have also been prepared by a solvothermal method. As cathode materials for lithium-ion batteries, the rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates show superior electrochemical properties than the hybrid-phase Li2.4Na0.6V2(PO4)3@C nano/microspheres. Rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates deliver a high initial discharge capacity of 121.6 mAh g−1 and an excellent capacity retention of 95.6% after 200 cycles at 1 C in the potential range of 3.0–4.3 V. Even at a high rate of 10 C, they also exhibit an initial discharge capacity of 115.3 mAh g−1 and a capacity retention of 76.7% after 500 cycles. The outstanding electrochemical performance is ascribed to the synergetic effect of nanoplate structure, stable rhombohedral LVP phase and uniform carbon coating layer.
References
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264
Yuan L-X, Wang Z-H, Zhang W-X, Hu X-L, Chen J-T, Huang Y-H, Goodenough JB (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4(2):269–284
Malik R, Abdellahi A, Ceder G (2013) A critical review of the Li insertion mechanisms in LiFePO4 electrodes. J Electrochem Soc 160(5):A3179–A3197
Pivko M, Bele M, Tchernychova E, Logar NZ, Dominko R, Gaberscek M (2012) Synthesis of nanometric LiMnPO4 via a two-step technique. Chem Mater 24(6):1041–1047
Aravindan V, Gnanaraj J, Lee Y-S, Madhavi S (2013) LiMnPO4—A next generation cathode material for lithium-ion batteries. J Mater Chem A 1(11):3518–3539
Oh S-M, Myung S-T, Sun Y-K (2012) Olivine LiCoPO4-carbon composite showing high rechargeable capacity. J Mater Chem 22(30):14932–14937
Cheng Y, Ni X, Feng K, Zhang H, Li X, Zhang H (2016) Phase-change enabled 2D Li3V2(PO4)3/C submicron sheets for advanced lithium-ion batteries. J Power Sources 326:203–210
Wang L, Bai J, Gao P, Wang X, Looney JP, Wang F (2015) Structure tracking aided design and synthesis of Li3V2(PO4)3 nanocrystals as high-power cathodes for lithium ion batteries. Chem Mater 27(16):5712–5718
Zhang L-L, Li Z, Yang X-L, Ding X-K, Zhou Y-X, Sun H-B, Tao H-C, Xiong L-Y, Huang Y-H (2017) Binder-free Li3V2(PO4)3/C membrane electrode supported on 3D nitrogen-doped carbon fibers for high-performance lithium-ion batteries. Nano Energy 34:111–119
Rajagopalan R, Zhang L, Dou SX, Liu H (2016) Lyophilized 3D lithium vanadium phosphate/reduced graphene oxide electrodes for super stable lithium ion batteries. Adv Energy Mater 6(1):1501760-1–1501760-8
Huang H, Yin SC, Kerr T, Taylor N, Nazar LF (2002) Nanostructured composites: a high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries. Adv Mater 14(21):1525–1528
Jian Z, Han W, Liang Y, Lan Y, Fang Z, Hu Y-S, Yao Y (2014) Carbon-coated rhombohedral Li3V2(PO4)3 as both cathode and anode materials for lithium-ion batteries: electrochemical performance and lithium storage mechanism. J Mater Chem A 2(47):20231–20236
Rui X, Yan Q, Skyllas-Kazacos M, Lim TM (2014) Li3V2(PO4)3 cathode materials for lithium-ion batteries: a review. J Power Sources 258:19–38
Lu Y, Wang L, Song J, Zhang D, Xu M, Goodenough JB (2013) Aluminum-stabilized NASICON-structured Li3V2(PO4)3. J Mater Chem A 1(1):68–72
Yin SC, Grondey H, Strobel P, Anne M, Nazar LF (2003) Electrochemical property: structure relationships in monoclinic Li3-yV2(PO4)3. J Am Chem Soc 125(34):10402–10411
Gaubicher J, Wurm C, Goward G, Masquelier C, Nazar L (2000) Rhombohedral form of Li3V2(PO4)3 as a cathode in Li-ion batteries. Chem Mater 12(11):3240–3242
Zhang Y, Nie P, Shen L, Xu G, Deng H, Luo H, Zhang X (2014) Rhombohedral NASICON-structured Li2NaV2(PO4)3 with single voltage plateau for superior lithium storage. RSC Adv 4(17):8627–8631
Wang W, Chen Z, Zhang J, Dai C, Li J, Ji D (2013) A comparative structural and electrochemical study of monoclinic Li3V2(PO4)3/C and rhombohedral Li2.5Na0.5V(2−2x/3)Nix(PO4)3/C. Electrochim Acta 103:259–265
Alfaruqi MH, Islam S, Song J, Kim S, Pham DT, Jo J, Kim S, Baboo JP, Putro DY, Mathew V, Kim J (2017) Carbon-coated rhombohedral Li2NaV2(PO4)3 nanoflake cathode for Li-ion battery with excellent cycleability and rate capability. Chem Phys Lett 681:44–49
Cui K, Hu S, Li Y (2016) Nitrogen-doped graphene nanosheets decorated Li3V2(PO4)3/C nanocrystals as high-rate and ultralong cycle-life cathode for lithium-ion batteries. Electrochim Acta 210:45–52
Sun P, Zhao X, Chen R, Chen T, Ma L, Fan Q, Lu H, Hu Y, Tie Z, Jin Z, Xu Q, Liu J (2016) Li3V2(PO4)3 encapsulated flexible free-standing nanofabric cathodes for fast charging and long life-cycle lithium-ion batteries. Nanoscale 8(14):7408–7415
Chen L, Yan B, Xu J, Wang C, Chao Y, Jiang X, Yang G (2015) Bicontinuous structure of Li3V2(PO4)3 clustered via carbon nanofiber as high-performance cathode material of Li-Ion batteries. ACS Appl Mater Interfaces 7(25):13934–13943
Qiao YQ, Wang XL, Mai YJ, Xiang JY, Zhang D, Gu CD, Tu JP (2011) Synthesis of plate-like Li3V2(PO4)3/C as a cathode material for Li-ion batteries. J Power Sources 196(20):8706–8709
Liu H, Yang G, Zhang X, Gao P, Wang L, Fang J, Pinto J, Jiang X (2012) Kinetics of conventional carbon coated-Li3V2(PO4)3 and nanocomposite Li3V2(PO4)3/graphene as cathode materials for lithium ion batteries. J Mater Chem A 22(22):11039–11047
Zuo Z-L, Deng J-Q, Pan J, Luo W-B, Yao Q-R, Wang Z-M, Zhou H-Y, Liu H-K (2017) High energy density of Li3−xNaxV2(PO4)3/C cathode material with high rate cycling performance for lithium-ion batteries. J Power Sources 357:117–125
Fang C, Huang Y, Zhang W, Han J, Deng Z, Cao Y, Yang H (2016) Routes to high energy cathodes of sodium-ion batteries. Adv Energy Mater 6(5):1501727-1–1501727-8
Teng F, Hu Z-H, Ma X-H, Zhang L-C, Ding C-X, Yu Y, Chen C-H (2013) Hydrothermal synthesis of plate-like carbon-coated Li3V2(PO4)3 and its low temperature performance for high power lithium ion batteries. Electrochim Acta 91:43–49
Wang Y, Tang Y, Zhong B, Liu H, Zhong Y, Guo X (2014) Facile synthesis of Li3V2(PO4)3/C nano-flakes with high-rate performance as cathode material for Li-ion battery. J Solid State Electrochem 18(1):215–221
Wei Q, Xu Y, Li Q, Tan S, Ren W, An Q, Mai L (2016) Novel layered Li3V2(PO4)3/rGO&C sheets as high-rate and long-life lithium ion battery cathodes. Chem Commun 52(56):8730–8732
Wang S, Zhang Z, Jiang Z, Deb A, Yang L, S-i Hirano (2014) Mesoporous Li3V2(PO4)3@CMK-3 nanocomposite cathode material for lithium ion batteries. J Power Sources 253:294–299
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51661009, 21363005 and 51371061) and the Natural Science Foundation of Guangxi Province (2016GXNSFGA380001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Zuo, Z., Deng, J. et al. Rhombohedral Li2.4Na0.6V2(PO4)3@C nanoplates as high-rate and long-life cathode materials for lithium-ion batteries. J Mater Sci 53, 10327–10337 (2018). https://doi.org/10.1007/s10853-018-2302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2302-8