Abstract
A castor oil-derived diglycidyl ester plasticizer (C26-DGE) was prepared and incorporated into poly(vinyl chloride) (PVC) for the first time. The chemical structure of the product was characterized by Fourier transform infrared spectroscopy (FTIR), proton nuclear magnetic resonance (1H-NMR), and carbon-13 nuclear magnetic resonance (13C-NMR). The plasticizing effects of C26-DGE as a primary or secondary plasticizer for the commercial plasticizer dioctyl phthalate (DOP) were studied. The mechanical properties, thermal stability, and migration stabilities of PVC films were investigated using dynamic mechanical analysis, thermogravimetric analysis (TGA), TGA-FTIR analysis, and PVC film surface analysis. Tensile, volatility, and extraction tests were also done. The castor oil-based plasticizer was found to endow the PVC matrix with enhanced compatibility and flexibility. With partially or completely substituted DOP, C26-DGE significantly increased the thermal stability of PVC blends. Furthermore, the volatility and extraction resistance of the novel plasticizers were generally superior to those of DOP. The interaction between the C26-DGE and PVC molecules and the thermal degradation process of PVC blends were also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its high versatility and relatively low cost, PVC is one of the most common polymers in a wide range of applications [1]. Nevertheless, pure PVC has a high glass transition temperature (Tg), low thermal stability, and is rigid at room temperature [1,2,3,4,5]. Plasticizers (e.g., phthalates, phosphates, citrates, etc.) are employed to modify the flexibility and workability of PVC. Plasticizer molecules in filtrate the polymer matrix and find attractive forces within the polar groups of the PVC molecule [6,7,8]. These attractive forces decrease chain–chain interactions of the matrix and increase the free volume of the polymer amorphous region, decreasing Tg [7, 8].
In recent decades, plasticizers—particularly phthalate esters [i.e., DOP and dibutyl phthalate (DBP)]—have become controversial due to their migration phenomenon, bioaccumulation in the environment, and potential toxicity to humans [9,10,11,12,13,14]. Furthermore, the use of phthalates in PVC products such as toys, medical devices, and food packing has been gradually restricted in many countries [1]. Thus, researchers are paying increased attention to biobased renewable plasticizers [15,16,17,18,19]. Plant oils represent a promising alternative for renewable plasticizers because of their improved biodegradability and low toxicity. Plant oil-based plasticizers include epoxidized oils and epoxidized fatty acid esters from soybean oil [20, 21], rubber seed oil [22], linseed oil [18], and sunflower oil [23].
Most of the major commercial plasticizers are esters, which allow specific polymer interactions (electrostatic, hydrogen bonds, or van der Waals) [1, 15]. The epoxy groups of the epoxidized plant oil-based plasticizers mentioned above can act as HCl scavengers and co-stabilizers to bring thermal stability to PVC [24]. The present work, therefore, means to develop a novel, plant oil-based plasticizer with esters acting as cohesive blocks and functional alicyclic and epoxy groups. Such a novel alternative plasticizer would offer outstanding thermal and migration stabilities as well as outstanding plasticizing effects.
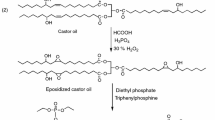
In this work, a novel castor oil-based C26-DGE plasticizer has been synthesized (Fig. 1). Plasticizing properties such as dynamic mechanical properties, mechanical properties, and the thermal and migration stabilities of PVC films were researched and compared to those of DOP. The interaction between the C26-DGE and PVC molecules and the thermal degradation process of PVC blends were also investigated.
Experimental section
Materials
Castor oil fatty acid (97%), cyclohexanedicarboxylic anhydride (99%), dioctyl phthalate (99.5%), sodium hydroxide (97+%), epichlorohydrin (99%), benzyltriethylammonium chloride (97%), and p-toluenesulfonic acid (98%) were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). Zinc stearate and calcium stearate were provided by Changzhou Huaren Chemical Co., Ltd., (Changzhou, China). PVC (S-1000) was supplied by the Sinopec Qilu Co., Ltd., (Zibo, China).
Preparation of C26-DA
To a 100-mL flask equipped with a reflux condenser, thermometer and magnetic stirrer, castor oil acid (30.00 g), cyclohexanedicarboxylic anhydride (15.40 g), and p-toluenesulfonic acid (0.09 g) were charged. Then, the reaction was continued at 130 °C for 2 h. After cooling, the reaction mixture was soluble in ethyl acetate and washed with distilled water to neutral. Finally, the water was removed by distillation under vacuum at 80 °C. Then, the C26-DA was obtained.
Preparation of C26-DGE
C26-DA (20.00 g), benzyltriethyl ammonium chloride (0.32 g), and epichlorohydrin (85.00 g) were added to a flask equipped with amagnetic stirrer, thermometer, and reflux condenser. The reaction was continued at 117 °C for 2 h. After the reaction, the mixture was cooled to 60 °C, the sodium hydroxide (3.70 g) was charged. Then, the reaction was continued for 3 h at 60 °C. The mixture was separated by a Buchner funnel. Then, the excess epichlorophydrin in the filter liquor was recycled by distillation under vacuum. Finally, a yellowish liquid product was obtained. The acid value and epoxy value of the C26-DGE were 1.31 mg/g and 3.6%, respectively.
Preparation of PVC films
For comparison, pure PVC and plasticized PVC films with different plasticizers were prepared. Thermal stabilizers (Ca salts/Zn salts = 3/1), plasticizers and PVC were mixed by a mechanical mixer for 5 min at room temperature. Then, the blends were continuing mixed by double-roller blending rolls (Zhenggong Co., China) at 165 °C for 3 min. The PVC films with a thickness of 2 mm were obtained. The formulations are shown in Table 1.
The FTIR measurements
The chemical structures of the plasticizer were confirmed by Fourier transform infrared (FTIR) spectra (Nicolet IS10 instrument, USA, KBr pellet) over a range of 4000–500 cm−1.
Nuclear magnetic resonance
The 1H NMR and 13C NMR spectra of the plasticizer were recorded in deuterated chloroform (CDCl3) using a Bruker ARX 300 spectrometer (BrukerCo., Germany) at room temperature.
DMA measurement
Dynamic mechanical analysis (DMA) of the PVC films was studied on a DMA Q800 (TA Instruments, New Castle, DE) in a dual-cantilever mode at a frequency of 1 Hz. The testing was swept with a heating rate of 3 °C/min from − 60 to 80 °C. In order to ensure the reproducibility of data, replicated tests were carried out for each sample.
Measurement of mechanical properties
Tensile properties of the PVC films were measured through a SANS CMT-4303 universal testing machine (Shenzhen XinsansiJiliang Instrument Co., China) with cross-head speed of 10 mm/min, according to ISO 527-2: 1993. Each specimen was conditioned at 50% humidity and 23 °C for 1 day prior to tensile testing. To obtain an average value, five samples were tested for each group.
TGA analysis
Thermogravimetric analysis (TGA) was carried out on a 409PC thermogravimetric analyzer (Netzsch Co., Germany). The PVC films were heated from 40 to 600 °C at a rate of 10 °C/min under N2 atmosphere.
TGA-FTIR analysis
The TGA-FTIR measurement was recorded on a NicoletiS10 FTIR (Nicolet Instrument Crop., USA) coupled with a 409PC thermal analyzer (Netzsch, Germany). Each PVC film was heated from 40 to 600 °C at a rate of 10 °C/min under N2 atmosphere. The spectrum was collected within the range of 4000–500 cm−1 at a resolution of 4 cm−1.
Volatility tests
Volatility tests were according to ISO 176:2005. The film with 120 cm3 of activated carbon spreading over it was placed on the bottom of a metal container with lid. Then, the container was placed in the convection oven (Shanghai Suo pu Instrument Co., China) at 70 °C ± 1 °C. The container was removed from the oven and cooled in a desiccator after 24 h. The PVC film was brushed and reweighed. The weight loss was measured before and after the heating. To obtain an average value, three samples were tested.
Extraction tests
Extraction tests were according to ASTMD 1239-98. The PVC film was immersed in soybean oil, petroleum ether, distilled water, 10% (w/w) sodium hydroxide and 30% (w/v) acetic acid at 23 ± 1 °C and 50 ± 5% relative humidity. The extracted PVC film was rinsed by distilled water and wiped up after 24 h. Then, each film was dried in a convection oven (Shanghai Suo pu Instrument Co., China) for 24 h at 30 °C and reweighed. The weight loss before and after the immersing was measured. Three samples were tested to get an average value.
Results and discussion
Synthesis and characterization
The FTIR spectra of CA, C26-DA, and C26-DGE are shown in Fig. 2. In the CA spectrum, the absorption peaks at 3550–3300 cm−1 were due to the presence of O–H stretching vibrations. The peak at 1710 cm−1 corresponded to the carboxylic acid group. Compared to the FTIR spectrum of CA, it can be seen that the absorption spectrum of the –OH group completely disappeared in the C26-DA spectrum. Furthermore, the strong peaks at around 1706 cm−1 corresponded to the double carboxylic acid groups. In the C26-DGE spectrum, the absorption at around 1728 cm−1 was attributed to carboxylic eater groups. Peaks of the glycidyl ester groups were observed at 908, 849, 763, and 724 cm−1.The results indicate an epoxidation reaction of double carboxylic acid groups with epichlorohydrin.
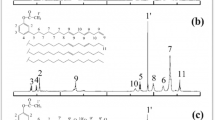
The 1HNMR and 13C NMR spectra of C26-DA and C26-DGE are shown in Figs. 3 and 4. In Fig. 3a, the methyl protons are shown at around 0.90 ppm (peak 18). The methylene protons can be seen at around 1.32–1.39 ppm (peaks 4–7 and 13–17). The chemical shifts at around 1.43–1.81 ppm correspond to the methylene protons attached to a six-membered alicyclic ring and-CH2COOH (peaks 20–23 and 3).The methylene group hydrogens attached to –COOH at 2.01 ppm. The chemical shifts around 2.24–2.38 ppm (peaks 8, 11, and 12) corresponded to the methylene and methyne associated with –CH=CH–. The methyne group protons attached to –O–O– and –C=O at 2.78 and 2.87 ppm. The peaks at around 4.89–5.45 ppm were attributed to the hydrogens of CH=CH (peaks 9 and 10). Proton signals at 2.63–4.41 ppm (peaks 26–31) and associated with the diglycidyl ester groups appeared in the spectrum (Fig. 2b), indicating an esterification reaction of C26-DA with epichlorohydrin. This result was in accordance with the FTIR results. Figure 4 shows the 13C NMR spectra of C26-DA and C26-DGE. New peaks at 42.44–51.30 and 64.74 ppm (Fig. 4b) suggest the existence of glycidyl ester groups. The results indicate the successful preparation of C26-DGE.
Dynamic mechanical analysis
Dynamic mechanical analysis technology was employed to study pure PVC and the PVC films. In Fig. 5, a single peak is observed in each curve, suggesting homogeneity and good plasticizer compatibility with PVC [15, 25]. An important metric of plasticizing efficiency, glass transition temperature (Tg) is the temperature at the maximum of the tan δ curve. As shown in Table 2, the Tg values of films S0–S4 were 41.46, 34.85, 40.42, 44.46, and 47.55 °C, respectively—lower than that of pure PVC (92.29 °C). It can be seen that the Tg of films S1 and S2 (with 10 and 20 phrC26-DGE, respectively) are lower than that of film S0. An explanation for this can be seen in Fig. 6. The polar glycidyl ester groups and the six-membered alicyclic ring of C26-DGE interacted with the polar portion of the PVC molecule, while the long alkyl chain of C26-DGE lubricated the PVC matrix, increasing the free volume of the polymer amorphous region, resulting in a decrease in Tg. However, with increased C26-DGE content, the corresponding Tg increased. Because C26-DGE has a greater molecular weight (477.8) than DOP (391), the number of polar groups in the plasticizing system was relatively reduced, thereby decreasing PVC plasticity.
Mechanical properties
Table 3 shows the tensile strength, elongation at break, and modulus of elasticity of the PVC films. It can be seen that PVC films plasticized with C26-DGE (films S0–S4) had similar tensile strength, better elongation at break, and a higher modulus of elasticity compared with film plasticized with pure DOP (film S0). The elongation at break of films S0–S4 generally increased from 210.7 to 310.6%, indicating a remarkable effect of C26-DGE on flexibility. This may be attributed primarily to lubrication of the PVC chains by the long alkyl chains ofC26-DGE. Lubrication reduces the polymer interaction force, increasing the free volume of the polymer amorphous region and potentially enhancing the flexibility of the PVC blends [26, 27]. It could be concluded that lower amounts of C26-DGE would be required to reach the same flexibility as is achieved with DOP.
Thermal stability
Figure 7 shows the TGA results for DOP and C26-DGE heated at 10 °C/min in nitrogen. The thermal degradation data, including the initial decomposition temperature (Ti) and 10 and 50% mass loss temperatures (T10 and T50), are summarized in Table 2. Compared with DOP, the Ti, T10, and T50 of C26-DGE increased 123.6, 94.1, and 141.4 °C, respectively. Hence, C26-DGE produced higher thermal stability, which might be attributed to the high thermal stability of the diglycidyl ester groups.
The thermogravimetric results for PVC films with varied C26-DGE content are shown in Fig. 8. Thermal data for Ti, T10, and T50 weight loss at different times and residuals are summarized in Table 2. According to previous reports, two main degradation steps are observed in each TGA curve [28,29,30]. In the first stage, dehydrochlorination of PVC accelerated and the greatest weight loss (70.08%) occurred between 200 and 350 °C. These cond degradation step were connected with the decomposition of the aromatic compounds (400–550 °C) and had the greatest weight loss 22.53% [31]. Ti, T10, and T50 gradually increased when DOP was replaced with C26-DGE. When 30 phr DOP was substituted with C26-DGE, the Ti, T10, and T50 were 4.9, 16.7, and 14.2 °C higher than S0, respectively. The film (S4) modified by 40 phr C26-DGE had the highest Ti, T10, and T50 of 275.5, 279.5, and 322.0 °C, respectively. This finding is primarily due to the multi-glycidylester groups of C26-DGE which can react with HCl, delaying thermal decomposition [32]. Hence, C26-DGE offers greater thermal stability in PVC blends than DOP.
The TGA-FTIR spectra of the gas products of the thermal degradation in the PVC films plasticized with DOP and C26-DGE are shown in Fig. 9a, b. The gaseous phases of the pyrolysis of films S0 and S4 were hydrogen chloride (HCl), water (H2O), carbon dioxide (CO2), carbon monoxide (CO), and benzene (C6H6); these were similar to one another. In the first degradation stage (at ~ 200–350 °C), the HCl (~ 2800 cm−1), H2O (3500–4000 cm−1), and C6H6 (~ 3000 and 1500 cm−1) were released [33, 34]. Figure 8b also showed that glycidylesters (1716, 1096, 802, and 668 cm−1) were released due to the addition of C26-DGE. It is seen that more H2O was obtained in the decomposition of film S4 than in film S0. Furthermore, with the incorporation of C26-DGE to PVC, a decline was observed in HCl concentration compared to that of pure DOP. However, the intensity of the absorption band of C6H6 in film S0 was higher than that of film S4, indicating that the addition of C26-DGE reduced C6H6 concentrations in PVC films during decomposition. These results were in agreement with the TGA results. Figure 10 shows the thermal degradation process of diglycidyl ester as a plasticizer for PVC. The ester groups, epoxy groups, and alicyclic structure of C26-DGE could, therefore, endow PVC with excellent thermal stability.
Surface characterization
The interaction between the plasticizers and PVC could be explored by FTIR according to the characteristic bands of the PVC matrix [35]. Figure 11 shows the FTIR spectra of the PVC films. In Fig. 11a, the peaks at 688 and 1426 cm−1 corresponded to the C–H and C–Cl of PVC, respectively [36]. As shown in Fig. 11b, c, obvious variations can be seen in the spectra of films S0–S4. The C–Cl and C–H absorption peaks visibly shifted toward the higher frequency at 1–5 cm−1. This result could be explained by the relationship of the force constant to the vibration frequency through Hooke’s Law [3] (Eq. (1)). The force constant increases with increased wave number, suggesting that the introduced electrons of the diglycidyl ester and carboxylic ester groups in the plasticizers formed hydrogen bonds with the –C–H– of the PVC molecules. Further, the finding indicated that the C26-DGE had a stronger interaction force with PVC than with DOP. This result is in agreement with the proposed plasticization mechanism shown in Fig. 6.
where k is the force constant, v is the vibration frequency, and m is the mass.
Volatility and extraction resistance
Figure 12a shows weight loss in the PVC films following volatility testing. It can be seen that weight loss during volatility testing decreased in the order of F0, F1, F2, F3, and F4, suggesting volatilization loss generally declined with increased C26-DGE content. Figure 12b–f shows PVC film performance during extraction in petroleum ether, soybean oil, distilled water, 30% (w/w) acetic acid, and 10% (w/w) NaOH. The poorest resistance was observed in films leached in petroleum ether; this was attributed to the fact that the solvents were organic. Furthermore, it was clear that the migration resistance in soybean oil, 30% (w/w) acetic acid, and 10% (w/w) NaOH was improved when C26-DGE replaced DOP. The resistance of extraction by distilled water was similar to that of pure DOP. These results might be attributed to the chemical structure of the plasticizers [37] as well as to the intermolecular interactions between plasticizers and PVC.
Conclusions
A novel, renewable castor oil-based plasticizer (C26-DGE) was successfully prepared and applied to a PVC matrix. The plasticizing effects of C26-DGE partially or completely substituting for DOP were investigated. The results of DMA analysis and tensile tests indicated are markable increase in flexibility in PVC films plasticized with C26-DGE. The values of elongation at break of the PVC films (S0-S4) increased from 210.7 to 310.6%. When 10 and 20 phr DOP were replaced with C26-DGE, the Tg values decreased by 6.61 and 1.04 °C, respectively, compared to pure DOP. TGA and TGA-FTIR analyses suggested that C26-DGE could significantly increase the thermal stability of PVC blends. With 30 phr C26-DGE, the Ti, T10, and T50 were 4.9, 16.7, and 14.2 °C higher, respectively, than those of DOP. The volatility and extraction tests showed that the migration stabilities of this plasticizer were mainly superior to those of DOP. The present study indicates that this castor oil-derived plasticizer is a promising alternative plasticizer for PVC and may be an excellent phthalate substitute in terms of human health and environmental protection.
References
Daniels PH (2009) A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J Vinyl Addit Technol 15:219–223
Roy KJ, Anjali TV, Sujith A (2017) Asymmetric membranes based on poly(vinyl chloride): effect of molecular weight of additive and solvent power on the morphology and performance. J Mater Sci 52:5708–5725. https://doi.org/10.1007/s10853-017-0807-1
Marcilla A, Garcia S, Garcia-Quesada JC (2008) Migrability of PVC plasticizers. Polym Test 27:221–233
Erythropel HC, Shipley S, Börmann A, Nicell JA, Maric M, Leask RL (2016) Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 89:18–27
Yang Y, Huang J, Zhang R, Zhu J (2017) Designing bio-based plasticizers: effect of alkyl chain length on plasticization properties of isosorbide diesters in PVC blends. Mater Des 126:29–36
Alexander M, Thachil ET (2006) A comparative study of cardanol and aromatic oil as plasticizers for carbon-black-filled natural rubber. J Appl Polym Sci 102:4835–4841
Scientists R, Hygienists I (2012) Handbook of plasticizers. William Andrew, New York
Sears JK, Darby JR (1982) The technology of plasticizers. Wiley, New York
Sampson J, De KD (2011) DEHP-plasticised PVC: relevance to blood services. Transfus Med 21:73–83
Lithner D, Larsson A, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409:3309–3324
Drake PL, Rojas M, Reh CM, Mueller CA, Jenkins FM (2001) Occupational exposure to airborne mercury during gold mining operations near El Callao, Venezuela. Int Arch Occ Env Hea 74:206–212
Wams TJ (1987) Diethylhexylphthalate as an environmental contaminant–a review. Sci Total Environ 66:1–61
Wang WL, Wu QY, Wang C, He T, Hu HY (2015) Health risk assessment of phthalate esters (PAEs) in drinking water sources of China. Environ Sci Pollut Res 22:3620–3630
Gardner ST, Wood AT, Lester R, Onkst PE, Burnham N, Perygin DH, Rayburn J (2016) Assessing differences in toxicity and teratogenicity of three phthalates, diethyl phthalate, Di-n-propyl phthalate, and Di-n-butyl phthalate, using Xenopus laevis embryos. J Toxicol Environ Health A 79:71–82
Koziara BT, Nijmeijer K, Benes NE (2015) Optical anisotropy, molecular orientations, and internal stresses in thin sulfonated poly(ether ether ketone) films. J Mater Sci 50:3031–3040. https://doi.org/10.1007/s10853-015-8844-0
Ferri JM, Samper MD, García-Sanoguera D, Reig MJ, Fenollar O, Balart R (2016) Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J Mater Sci 51:5356–5366. https://doi.org/10.1007/s10853-016-9838-2
Wu D, Chang PR, Ma X (2011) Preparation and properties of layered double hydroxide–carboxymethylcellulose sodium/glycerol plasticized starch nanocomposites. Carbohydr Polym 86:877–882
Ollett AL, Parker R, Smith AC (1991) Deformation and fracture behaviour of wheat starch plasticized with glucose and water. J Mater Sci 26:1351–1356. https://doi.org/10.1007/BF00544476
Fenollar O, Sanchez-Nacher L, Garcia-Sanoguera D, López J, Balart R (2009) The effect of the curing time and temperature on final properties of flexible PVC with an epoxidized fatty acid ester as natural-based plasticizer. J Mater Sci 44:3702–3711. https://doi.org/10.1007/s10853-009-3495-7
Bouchareb B, Benaniba MT (2010) Effects of epoxidized sunflower oil on the mechanical and dynamical analysis of the plasticized poly(vinyl chloride). J Appl Polym Sci 107:3442–3450
Chen J, Li X, Wang Y, Huang J, Li K, Nie X, Jiang J (2016) Synthesis and application of environmental soybean oil based epoxidized glycidyl ester plasticizer for poly(vinyl chloride). Eur J Lipid Sci Technol 119:1600216–1600225
Joseph R, Alex R, Vinod VS, Premalatha CK, Kuriakose B (2003) Studies on epoxidized rubber seed oil as plasticizer for acrylonitrile butadiene rubber. J Appl Polym Sci 89:668–673
Chavan AP, Gogate PR (2015) Ultrasound assisted synthesis of epoxidized sunflower oil and application as plasticizer. J Ind Eng Chem 21:842–850
Krewson CF, Riser GR, Scott WE (1966) Euphorbia and Vernonia seed oil products as plasticizer-stabilizers for polyvinyl chloride. J Am Oil Chem Soc 43:377–379
Greco A, Brunetti D, Renna G, Mele G, Maffezzoli A (2010) Plasticizer for poly(vinyl chloride) from cardanol as a renewable resource material. Polym Degrad Stab 95:2169–2174
Sharma AK, Mahanwar PA (2010) Effect of particle size of fly ash on recycled poly (ethylene terephthalate)/fly ash composites. Int J Plast Tech 14:53–64
Lim H, Hoag SW (2013) Plasticizer effects on physical-mechanical properties of solvent cast soluplus films. AAPS PharmSciTech 14:903–910
Iván B, Kelen T, Tüdös F (1989) The main elementary events of degradation and stabilization of PVC. Macromol Symp 29:59–72
Jiménez A, Berenguer V, López J, Sánchez A (2010) Thermal degradation study of poly(vinyl chloride): kinetic analysis of thermogravimetric data. J Appl Polym Sci 50:1565–1573
Szarka G, Iván B (2013) Thermal properties, degradation and stability of poly(vinyl chloride) predegraded thermooxidatively in the presence of dioctyl phthalate plasticizer. J Macromol Sci A 50:208–214
Jellinek HGH (1989) Degradation and stabilization of polymers. Elsevier, Dutch
Ayrey G, Man FP, Poller RC (1979) Mechanism of thermal stabilization of PVC by organotin compounds: scavenging of chlorine atoms. J Organomet Chem 173:171–174
Botelho G, Queirós A, Liberal S, Gijsman P (2001) Studies on thermal and thermo-oxidative degradation of poly(ethylene terephthalate) and poly(butylene terephthalate). Polym Degrad Stab 74:39–48
Qu H, Xin L, Xu J, Ma H, Jiao Y, Xie J (2014) Investigation on thermal degradation of poly(1,4-butylene terephthalate) filled with aluminum hypophosphite and trimer by thermogravimetric analysis-Fourier transform infrared spectroscopy and thermogravimetric analysis-mass spectrometry. Ind Eng Chem Res 53:8476–8483
Coltro L, Pitta JB, Madaleno E (2013) Performance evaluation of new plasticizers for stretch PVC films. Polym Test 32:272–278
Marcilla A, Garcia S, Garcia-Quesada JC (2008) Migrability of PVC plasticizers. Polym Test 27:221–233
Kovačić T, Mrklić Ž (2002) The kinetic parameters for the evaporation of plasticizers from plasticized poly(vinyl chloride). Thermochim Acta 381:49–60
Acknowledgements
The authors are grateful for the financial support from the National “Twelfth Five-Year” Plan for Science & Technology Support (Grant Number: 2015BAD15B08) and the Key laboratory of biomass energy and materials of Jiangsu Province (Grant Number: JSBEM-S-201604).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Zengshe Liu: Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Chen, J., Liu, Z., Nie, X. et al. Synthesis and application of a novel environmental C26 diglycidyl ester plasticizer based on castor oil for poly(vinyl chloride). J Mater Sci 53, 8909–8920 (2018). https://doi.org/10.1007/s10853-018-2206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2206-7