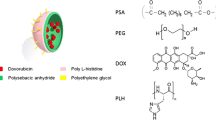
Abstract
The ideal drug delivery system (DDS) should be able to deliver a therapeutic dose of drug to the target site in the body and maintain appropriate concentration over a desired period of time. Thermo/pH-responsive targeted polymeric nanocapsules (p(NIPA-co-AAc-co-GAA)) for delivery of anticancer drug (doxorubicin, DOX) were fabricated by W/O/W double emulsion solvent evaporation technique. The morphology, hydrodynamic diameter, size distribution, thermo/pH responsiveness, targeted property, anti-tumor efficacy, encapsulation efficiency, drug loading and controlled release of drug were systematically investigated. The results demonstrated that p(NIPA-co-AAc-co-GAA) nanocapsules minimized the drug leakage and reduced the toxic and side effects on normal tissues, while triggered and accelerated the release of drug in tumor tissues. It is very interesting for the site-specific release of drug to improve curative effects and reduce side effects, implying that p(NIPA-co-AAc-co-GAA) nanocapsule is a attractive candidate for DDS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the advent of new drugs and deep understanding of the theory of chemotherapy, the efficacy of chemotherapy for cancer therapy has been greatly improved. However, drugs are often distributed all over the body after oral administration or intravenous injection due to their non-selectivity. Subsequently, drugs kill tumor cells as well as cause damages to normal cells and tissues [1]. Moreover, drug concentration eventually drops to a very low level when they reach the lesions due to the short half-life and often losing in the non-target tissue as a result of the uncontrolled release rate in the circulatory system of human body. In order to maintain or improve curative effect, increasing the dosage or repeated dosing must be done [2, 3]. Therefore, it is an urgent need to develop efficient DDS for cancer therapy.
Micro-nano particles have received a significant amount of attention as drug carriers owing to their distinct advantages. They can prevent the incorporated labile drugs from degradation, improve the bioavailability of drugs, control drug release and target drug to specific site [4,5,6,7]. Among these micro-nano particles, polymeric capsules with hollow core and polymeric shell have significant advantages over other microspheres when using as drugs delivery carriers. They can accommodate higher dose of drugs due to the large apartment. Meanwhile, the encapsulation efficiency and drug loading can also be dramatically enhanced [8, 9].
The ideal DDS should be able to deliver a therapeutic dose of drug to the target site in the body and then maintain the appropriate concentration over a desired period of time. In order to minimize the side effects and maximize the pharmic efficacy, novel drug delivery systems with multifunction should be developed to deliver drugs to the target site in a sustained or controlled release manner. One effective approach is to combine the drugs with targeted carriers; Therefore, the drugs could be delivered selectively to the target region with the guide of carriers. At present, many studies have focused on using folic acid as a target mediating substance in developing targeted vehicles. For example, Li et al. [10] developed a folic acid-conjugated multi-stimuli responsive microsphere for delivery of anticancer drug. Liu et al. [11] described a dual-responsive targeted drug carrier based on mesoporous silica for laryngeal carcinoma treatment. These targeted drug carriers were often fabricated by conjugating folic acid molecules onto the surface of microspheres through the reaction of NH2 groups of folic acid with COOH groups of microspheres. This method is difficult to be operated, and only a small amount of folic acid can be grafted onto the surface of the basement microspheres. In this study, we developed a novel method to avoid this disadvantage. On one hand, glycyrrhetic acid (GA), an effective component in Chinese medicine, has been found to be a high affinity ligand for hepatic cell. Covalent binding of GA to the cargos is very efficient, and the GA-binded cargo can be internalized by hepatocyte via receptor-mediated endocytosis. For example, Tian prepared GA-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery and the obtained particles were found to highly enriched in rat liver [12]. Therefore, we synthesized polymeric nanocapsules linked with GA as a tumor-targeted drug carrier in this paper. On the other hand, most solid malignancies show weakly acidic and hyperthermal characteristics inside cells when compared with normal tissues and bloodstream due to the hypoxia-induced coordinated upregulation of glycolysis [13, 14]. To achieve effective and controlled release of drugs, it is necessary to develop intelligent vehicles with responsiveness to these tumor-associated biological stimuli. Stimulus-responsive carriers can respond to the abnormal changes of temperature and chemical environment at the disease site and thereby spontaneously release the required dose of drugs [15, 16]. When the body is in a normal state, smart carriers restore the original state to suppress drug release. It is also known that temperature-sensitive polymer poly(N-isopropylacrylamide) (pNIPA) has been widely applied in the field of drug delivery systems, which exhibits a dramatic phase transition from expanded to shrunken conformation around its volume phase transition temperature (VPTT) near 32 °C in aqueous media. Many temperature-sensitive micro-nanoparticles based on pNIPA have been developed and applied in DDS [17,18,19].
According to above analysis, we designed a multifunctional intelligent DDS based on smart polymer combined with targeted GA. First, vinyl was introduced to GA to endow the polymerizable function, and then vinyl GA was copolymerized with N-isopropylacrylamide (NIPA) and acrylic acid (AAc) to form linear copolymer by free radical polymerization. At last, the targeted thermo/pH-triggered nanocapsules were fabricated with a W/O/W double emulsion solvent evaporation technique. The morphology, particle size, encapsulation efficiency, cytotoxicity and in vitro release of DOX were investigated. With this technique, large amount of GA could be conjugated onto nanocapsules and consequently improve the targeting ability of carriers to liver cancer cells. Furthermore, the double emulsion method is excellent candidate to fabricate the drug delivery vehicles since it is relatively simple and can be carried out at room temperature to avoid the damage of high temperature to medicine. To our knowledge, few studies have reported on introducing GA into smart polymer to develop thermo/pH-responsive targeted polymeric nanocapsules as a drug delivery system. This research may provide new ideas and practical basis for developing novel drug carriers with multiple functions.
Experimental
Materials
GA was purchased from Fujie Medical Co. (Xi’an, China). NIPA was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and purified by recrystallization from n-hexane. AAc was provided by Tianjin Chemical Reagent II Co. (Tianjin, China) and purified by vacuum distillation. N-hydroxysuccinimide (SuOH) and N,N-dicyclohexyl carbodiimide (DCC) were received by Sinopharm Chemical Reagent Co. (Shenyang, China). N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC·HCl) and N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) were the products of Shanghai Medpep Co., Ltd. (Shanghai, China). DOX was purchased from Wuhan Huamei Bioengineering Co., Ltd. (Wuhan, China). N,N,N,N-tetramethylenediamine (TEMED), ethylenediamine (EDA), azobisisobutyronitrile (AIBN), etc., were of analytical grade made in China.
Preparation of thermo/pH-responsive targeted linear polymer
Thermo/pH-responsive targeted polymer p(NIPA-co-AAc-co-GAA) was prepared according to the method of our previous work with some modifications [20]. Briefly, GA was modified by EDA in the presence of DCC and SuOH to form glycyrrhetic acid amine derivatives (GA-NH2). Then, GA-NH2 was reacted with AAc in the presence of sulfo-NHS and EDC to synthesize vinyl monomer (GAA). Finally, p(NIPA-co-AAc-co-GAA) linear polymer was synthesized by free radical copolymerization of NIPA, AAc and GAA using AIBN/TEMED as an initiating system. The molar ratio of NIPA, AAc and GAA was 13.8:4.6:1.6, and the molecular weight of the p(NIPA-co-AAc-co-GAA) polymer was measured with gel permeation chromatography, which was 3.4 × 104.
Preparation of thermo/pH-responsive targeted polymeric nanocapsules
Thermo/pH-responsive targeted polymeric nanocapsules containing DOX were prepared by a modified W1/O/W2 double emulsion technique. First, 200 μL of PEG with or without DOX aqueous solution (W1) was emulsified in 4 mL of dichloromethane and acetone solvent (3:1 v/v) containing p(NIPA-co-AAc-co-GAA) polymer by mixing with vortex to form a W1/O emulsion. Subsequently, this primary emulsion was poured into 80 mL of PVA aqueous solution (W2) under stirring at a fast rate for 3 h to produce a W1/O/W2 emulsion. The reaction temperature was kept constant at room temperature for 4 h to allow the solvent to evaporate and the particles to stabilize. Finally, thermo/pH-responsive targeted polymeric nanocapsules were obtained after filtration, washing and freeze-drying. The formation process of p(NIPA-co-AAc-co-GAA) nanocapsules and controlled release of drugs are summarized schematically in Fig. 1.
Characterization of thermo/pH-responsive targeted polymeric nanocapsules
The morphologies of the resultant polymer nanocapsules were visualized by scanning electron microscopy (SEM, XL30, Philips, Holland) and transmission electron microscopy (TEM, H-7650, Hitachi, Japan). The average hydrodynamic diameter and size distribution of the polymer nanocapsules (1.0 mg/mL) were measured by dynamic light scattering (DLS) (Autosize LocFc-963, Malvern Instrument) at different temperature and pH, respectively.
Targeted properties and anti-tumor efficacy assay
The targeted properties and anti-tumor efficacy were investigated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Human normal hepatocytes (HL-7702), hepatoma cells (HepG2) and mouse fibroblast cells (L929) were seeded in 96-well plates at a density of 104 cells/well and incubated for 24 h at 37 °C under a humidified atmosphere of 5% CO2, respectively. Then, the cells were treated with pNIPA nanocapsules, p(NIPA-co-AAc-co-GAA) nanocapsules, free DOX, DOX-loaded pNIPA nanocapsules and DOX-loaded p(NIPA-co-AAc-co-GAA) nanocapsules at the desired concentration. After incubation for 24 h, cell viabilities were determined using a standard MTT assay. Data are presented as the average (SD, n = 3).
Drug loading and encapsulation efficiency
The encapsulation efficiency (EE) and drug loading (DL) of nanocapsules were determined by extracting and quantifying the encapsulated DOX. Briefly, DOX-loaded microspheres were dissolved in dichloromethane–ethanol mixture (1:9, v/v) and then phosphate buffer saline (PBS, pH 7.4) was added into this solution to extract DOX for several times. The above suspension was centrifuged at 12000 r.p.m for 10 min to remove the polymeric debris. The concentration of free DOX in the supernatant was analyzed by ultraviolet absorption at their maximum wavelength (490 nm) on a UV-Vis spectrophotometer. Three samples were prepared for each testing. The EE% and DL% of nanocapsules were calculated according to the following equations:
where W initial drug is the weight of DOX for loading, W encapsulated drug is the weight of DOX encapsulated in the nanocapsules, and W nanoparticles is the weight of the nanocapsules for loading.
In vitro drug release studies
The in vitro release studies of DOX-loaded nanocapsules were performed by diffusion technique. DOX-loaded nanocapsules (20 mg) were placed into a dialysis bag and immersed in 100 mL PBS (pH = 3.0 and 7.4) at 37 and 45 °C, respectively, with gentle shaking. At predetermined time intervals, 3 mL of PBS was withdrawn and replaced with an equal volume of fresh PBS to maintain the constant volume. The amount of released DOX in PBS solutions was determined using a UV-Vis spectrophotometer at a maximal wavelength of 490 nm. The cumulative release was expressed as the total percentage of drug molecule released through the dialysis membrane over time, which was calculated according to the following equation:
where W t is the weight of DOX released at time t, and W 0 is the weight of DOX encapsulated in the nanocapsules.
Statistical analysis
Nanocapsules diameters, cytotoxicity and in vitro release were performed in triplicate. All obtained data were expressed as the mean value with standard deviation and analyzed using the T-test and one-way analysis of variance (ANOVA). In all tests, a p value of < 0.05 was regarded as statistically significant.
Results and discussion
Morphology and size of polymeric nanocapsules
The morphological analysis of polymeric nanoparticles was carried out by SEM and TEM. The images in Fig. 2 demonstrated that most of the nanoparticles had a uniform spherical shape and smooth surface. The SEM photographs of broken nanoparticles shown in Fig. 2b, c exhibited the presence of hollow structure in the nanoparticles. The large apartment of the obtained polymeric nanocapsules is beneficial to the encapsulation of drugs. The morphologies of p(NIPA-co-AAc-co-GAA) nanocapsules and DOX-loaded p(NIPA-co-AAc-co-GAA) nanocapsules were further observed by TEM. As shown in Fig. 2d, e, DOX-loaded p(NIPA-co-AAc-co-GAA) nanocapsules were darker than p(NIPA-co-AAc-co-GAA) nanocapsules due to the encapsulation of DOX in nanocapsules.
Dynamic light scattering analysis
The hydrodynamic diameters and size distribution of the polymeric nanocapsules were measured by DLS at different temperature and pH as shown in Fig. 3. Generally speaking, the diameters of p(NIPA-co-AAc-co-GAA) nanocapsules decreased and their size distribution became narrow when the environmental temperature was increased from 25 to 50 °C (Fig. 3a). It is worth noting that the diameters and size distribution of nanocapsules dramatically changed from 37 to 40 °C, while they exhibited slight change above 40 °C and under 37 °C. For example, the particle size was about 450 nm and the size distribution was 200–800 nm at 25 and 37 °C. However, they were approximately 350 nm and 200–500 nm at 40, 45 and 50 °C, respectively. The diameters of nanocapsules decreased significantly from 450 to 350 nm with the increase in temperature from 37 to 40 °C. It is well known that the VPTT of pNIPA in aqueous media is 32 °C [21]. pNIPA chains form extended molecular conformation by hydration below 32 °C, while they will undergo a sharp conformational transition from extended to contractive configuration by dehydration when the temperature is above 32 °C. The VPTT of pNIPA can be adjusted by introducing components with different hydrophilic/hydrophobic properties. In our study, the VPTT of p(NIPA-co-AAc-co-GAA) nanocapsules was enhanced to 37–40 °C due to the copolymerization of hydrophilic AAc monomer. The presence of massive carboxyl groups in the acrylic acid molecular resulted in the formation of abundant hydrogen bonds between macromolecular chains of nanocapsules and water molecules. The conformational transition of macromolecular chains required more energy to destroy these hydrogen bonds. Therefore, the VPTT of p(NIPA-co-AAc-co-GAA) was higher than that of pNIPA in aqueous media. Similar result was also observed in other work [22].
With regard to the effect of pH on particle diameter and size distribution, as shown in Fig. 3b, the significant decrease in particle diameter was related to the decrease in pH value. The presence of carboxylic acid units attached to the polymer chains is the main reason for the response of particle diameter to pH changes. The carboxylic acid groups of p(NIPA-co-AAc-co-GAA) will be dissociated into carboxylic acid and hydrogen ions in water to form a ionization equilibrium. This equilibrium state is influenced by the pH value of the solution. Under alkaline conditions, the carboxylic acid groups of p(NIPA-co-AAc-co-GAA) are in the form of a negative COO− and produce an electrostatic repulsion in the interior of nanocapsules, which results in a stretching of the coiled polymer chains. With the decrease in pH value, the increase in H+ concentration inhibits the ionization of carboxylic acid groups. The decrease in COO− concentration reduces the electrostatic repulsion of polymer chains and facilitates the aggregation of polymer chains [23, 24]. Moreover, the COOH groups in polymer chains are easy to form hydrogen bonds with the CONH groups of pNIPA. This intramolecular hydrogen bond makes the three-dimensional network structure of nanocapsules become more tight [25].
Targeted properties and anti-tumor efficacy assay
As a drug carrier material, the toxicity of p(NIPA-co-AAc-co-GAA) nanocapsules should be evaluated due to its potential further application in biomedical fields. The cell viability of human hepatocytes (HL-7702), hepatoma cells (HepG2) and mouse fibroblast cells (L929) treated with PNIPA and p(NIPA-co-AAc-co-GAA) nanocapsules was determined by MTT assay, respectively. As shown in Fig. 4a, pNIPA nanocapsules had no obvious toxicity to three kinds of cells; however, p(NIPA-co-AAc-co-GAA) nanocapsules showed different toxicity to them. Specifically, p(NIPA-co-AAc-co-GAA) nanocapsules not only had no toxic effects on HL7702 and L929, but promoted cell activity. Meanwhile, we could clearly see the reduction of cell viability of HepG2 treated with p(NIPA-co-AAc-co-GAA) nanocapsules. Glycyrrhetic acid (GA), as one of the effective components in Chinese medicine, can inhibit the proliferation of hepatoma cells and induce its differentiation into benign cells and promote the regeneration of normal liver cells. GA has been widely used in the treatment of liver diseases and liver protection [26, 27]. Therefore, the cell viability of normal liver cell HL7702 was much higher than that of hepatoma cells (HepG2) in our experimental results. It could also be seen that the cell viability of L929 treated with p(NIPA-co-AAc-co-GAA) nanocapsules was higher than that of control but lower than that of HL7702 treated with p(NIPA-co-AAc-co-GAA) nanocapsules. This result indicated that p(NIPA-co-AAc-co-GAA) nanocapsules exhibited good biocompatibility to normal cells. Furthermore, the presence of strong affinity between hepatocytes and GA resulted in the enrichment of HL7702 to p(NIPA-co-AAc-co-GAA) nanocapsules, which enhanced the cell viability. These characteristics of GA made it a suitable candidate for hepatic targeted drug delivery system (HTDDS) as carrier materials.
Relative cell viability treated with a pNIPA and p(NIPA-co-AAc-co-GAA) nanocapsules, b free DOX, DOX-loaded pNIPA and DOX-loaded p(NIPA-co-AAc-co-GAA). The cell viability without any treatment was used as a control and set to 100%. The concentration of nanocapsules was 500 μg/mL. Statistical significances between HL-7702 and HepG2 treated with p(NIPA-co-AAc-co-GAA) nanocapsules were evaluated: p < 0.01. Statistical significances between free DOX and DOX-pNIPA, free DOX and DOX-p(NIPA-co-AAc-co-GAA) were evaluated: p > 0.05 and p < 0.01, respectively
The MTT assay was further used to investigate the cell viability of Hep2 treated with free DOX, DOX-loaded pNIPA and DOX-loaded p(NIPA-co-AAc-co-GAA). As shown in Fig. 4b, all of the three groups exhibited increasing inhibition against hepatoma cells with the increasing concentrations of DOX. Moreover, at the same concentrations of DOX, the cell viability of HepG2 treated with DOX-loaded p(NIPA-co-AAc-co-GAA) was lower than that of HepG2 treated with free DOX and DOX-loaded pNIPA. As discussed above, p(NIPA-co-AAc-co-GAA) nanocapsules have thermo/pH-responsive, liver-targeted and toxic properties to hepatoma cells. On one hand, the synergistic effects of DOX and GA in p(NIPA-co-AAc-co-GAA) nanocapsules greatly enhanced the lethality to HepG2. On the other hand, DOX-loaded p(NIPA-co-AAc-co-GAA) could be easily taken up by Hep2 through receptor-mediated endocytosis and efficiently released DOX in weakly acidic and hyperthermal characteristics inside cancer cells. DOX-loaded p(NIPA-co-AAc-co-GAA) nanocapsules could avoid the damage of free DOX to normal cells and significantly improve the therapeutic efficiency of DOX to tumor cells.
Drug loading and in vitro drug release
The encapsulation efficiency (EE) and drug loading (DL) of nanocapsules are important parameters to evaluate the properties of drug carriers. In our experiment, DOX was encapsulated as a model drug to evaluate the potential application of p(NIPA-co-AAc-co-GAA) nanocapsules as a drug delivery vehicle. As shown in Table 1, the EE and DL of DOX in p(NIPA-co-AAc-co-GAA) nanocapsules were affected by the initial concentrations of p(NIPA-co-AAc-co-GAA), DOX and PVA. The EE increased with the increase in initial concentration of p(NIPA-co-AAc-co-GAA), DOX and PVA. The higher concentration of p(NIPA-co-AAc-co-GAA) yielded higher viscosity of primary W/O emulsion, which accelerated the solidification rate, prompted the formation of denser structure and reduced the drug loss during evaporation [28]. Meanwhile, the increase in concentration of PVA increased the viscosity of outer water phase, hindered the diffusion of DOX and further improved the EE [29]. On the other hand, the DL increased with the increase in initial concentration of DOX and PVA, but decreased with the increase in initial concentration of p(NIPA-co-AAc-co-GAA). For example, if all other parameters were kept constant, the DL was 24.31 and 15.26% when the initial concentrations of p(NIPA-co-AAc-co-GAA) were 5 and 10 mg/mL, respectively. Based on the calculating equation of DL, the increase in polymer content in the feed ratio led to the decrease of DL. The result also suggested that the encapsulation of DOX did not reach its saturation. These results are in agreement with previous work [29, 30].
The controlled release properties of DOX-loaded p(NIPA-co-AAc-co-GAA) nanocapsules were investigated under different temperature and pH values. As shown in Fig. 5, less than 15% of DOX was released from p(NIPA-co-AAc-co-GAA) nanocapsules at pH 7.4 and 37 °C after 50 h, which indicated that DOX was sufficiently stable in this nanocapsules under normal physiological conditions (i.e., in the blood circulation). In contrast, the release rate of DOX was much higher under an acidic pH than that under a physiological pH. For example, almost 60% of DOX was released from p(NIPA-co-AAc-co-GAA) nanocapsules at pH 3.0 and 37 °C after 30 h, which was 4 times higher than that at pH 7.4 and 37 °C after 50 h. When the DOX was encapsulated into p(NIPA-co-AAc-co-GAA) nanocapsules, the ionic pairs of polymer–COO− +DOX were formed between polymer and drug. However, the decrease in pH promoted the ionic exchange, weakened the affinity between DOX and nanocapsules and consequently accelerated the release of drug [31, 32]. Meanwhile, the system showed a more rapidly release behavior at high temperature. By keeping pH at 7.4, more than 70% of DOX was released within 24 h at 45 °C, which was much higher than that at 37 °C. Furthermore, the cumulative release amount of DOX reached up to 90% only within 12 h at an acidic pH (3.0) and high temperature (45 °C). As discussed above, the introduction of AAc monomer into nanocapsules enhanced the VPTT of p(NIPA-co-AAc-co-GAA) to 37–40 °C. When the environmental temperature was above the VPTT of p(NIPA-co-AAc-co-GAA), the shrinkage of the nanocapsules facilitated the escape of DOX from the matrix due to the pumping effect [33]. In addition, higher temperature accelerated the molecular thermodynamic movement of DOX and consequently led to a faster diffusion rate of drug [34]. In order to further understand the drug release behavior, the cumulative release data were evaluated on the basis of classical Ritger–Peppas equation to elucidate the DOX transport mechanism. The model is valid only for the description of the first 60% of release curve, i.e., M t /M ∞ ≤ 60%.
where W t and W ∞ are the total amount of drug released at time t and equilibrium time, respectively. k is the kinetic constant reflecting the drug release rate. n is an exponent characterizing the drug release mechanism. Generally, n ≤ 0.45 corresponds to a Fickian diffusion mechanism, 0.45 < n < 0.89 to non-Fickian diffusion, n = 0.89 to Case II (relaxation) transport and n > 0.89 to Super Case II transport [35, 36]. The k and n values are calculated by plotting the ln (W t /W ∞) versus ln t. Coefficients of correlation (R 2) are used to evaluate the accuracy of the fit. The linear regression results are summarized in Table 2. It could be seen that the coefficients of correlation (R 2) were best fit with the Ritger–Peppas models (0.921–0.962). The obvious increase of k with the increase in temperature and decrease in pH demonstrated that the drug release was triggered and accelerated under the weakly acidic and high-temperature environment. More importantly, the release exponent n < 0.45 at 37 °C indicated that the release mechanism of DOX followed Fick’s law. It means that the diffusion of drug depends on the concentration gradient of drug at 37 °C. However, the values of n were higher than 0.45 at 45 °C, which proved that the release of drug showed a non-Fickian release mechanism at 45 °C. As discussed above, the pumping effect of nanocapsules and the intense thermodynamic movement of DOX facilitated the release of DOX. In other words, the release of drug was not only dependent on the concentration gradient of drug; therefore, the diffusion of DOX deviated from Fick’s law at high temperature. The kinetic analysis of drug release further validated the results of our experiments.
Conclusions
Thermo/pH-responsive targeted polymeric nanocapsules (p(NIPA-co-AAc-co-GAA)) for delivery of anticancer drug were successfully synthesized by a W/O/W double emulsion solvent evaporation technique. Most of p(NIPA-co-AAc-co-GAA) nanocapsules had a uniform spherical shape and hollow structure, which contributed to the high encapsulation efficiency of drug. The hydrodynamic diameters of polymeric nanocapsules were about 450 nm at room temperature. Meanwhile, the particle size could be responsive to temperature and pH, which would trigger and accelerate the release of drug encapsulated in nanocapsules at appropriate environment. In particular, p(NIPA-co-AAc-co-GAA) nanocapsule could minimize the drug leakage and reduce the toxic and side effects on normal tissues in the process of blood circulation. However, the drug could be rapidly released from nanocapsules in the weakly acidic and high-temperature environment of tumor tissues to induce cancer cells death. Moreover, the introduction of GA with hepatic targeting property was hopeful to carry drug molecules across biological barrier and go straight to liver cancer cells, which avoided damage to the other normal cells and tissue. These results are very interesting for the site-specific release of drug to improve curative effects and reduce side effects, which indicates that p(NIPA-co-AAc-co-GAA) nanocapsule is a attractive candidate for DDS.
References
Gandhi NS, Tekade RK, Chougule MB (2014) Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release 194:238–256
Kowalczuk A, Trzcinska R, Trzebicka B, Műller AHE, Dworak A, Tsvetanov CB (2014) Loading of polymer nanocarriers: factors, mechanisms and applications. Prog Polym Sci 39:43–86
Creixell M, Peppas NA (2012) Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today 7:367–379
Wang Y, Wan GY, Li ZY, Shi SR, Chen BW, Li CY, Zhang LY, Wang YS (2017) PEGylated doxorubicin nanoparticles mediated by HN-1 peptide for targeted treatment of oral squamous cell carcinoma. Int J Pharm 525:21–31
Esfanjani AF, Jafari SM, Assadpour E (2017) Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem 221:1962–1969
Assadpour E, Jafari SM, Maghsoudlou Y (2017) Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int J Biol Macromol 95:238–247
Assadpour E, Maghsoudlou Y, Jafari SM, Ghorbani M, Aalami M (2016) Evaluation of Folic acid nano-encapsulation by double emulsions. Food Bioprocess Technol 9:2024–2032
Haladjova E, Toncheva-Moncheva N, Apostolova MD, Trzebicka B, Dworak A, Petar P, Dimitrov I, Rangelov S, Tsvetanov CB (2014) Polymeric nanoparticle engineering: from temperature-responsive polymer mesoglobules to gene delivery systems. Biomacromol 15:4377–4395
Chang YW, Silas JA, Ugaz VM (2010) A direct probe of the interplay between bilayer morphology and surface reactivity in polymersomes. Langmuir 26(4):12132–12139
Li RR, Feng FL, Wang YS, Yang XY, Yang XL, Yang VC (2014) Folic acid- conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J Colloid Interface Sci 429:34–44
Liu X, Yu D, Jin CS, Song XW, Cheng JZ, Zhao X, Qi XM, Zhang GX (2014) A dual responsive targeted drug delivery system based on smart polymer coated mesoporous silica for laryngeal carcinoma treatment. New J Chem 38:4830–4836
Tian Q, Zhang CN, Wang XH, Wang W, Huang W, Cha RT, Wang CH, Yuan Z, Liu M, Wan HY, Tang H (2010) Glycyrrhetinic acid-modified chitosan/ poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials 31(17):4748–4756
Shirakura T, Kelson TJ, Ray A, Malyarenko AE, Kopelman R (2014) Hydrogel nanoparticles with thermally controlled drug release. ACS Macro Lett 3:602–606
Zhu J, Liao L, Bian XJ, Kong JL, Yang PY, Liu BH (2012) pH-controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small 8:2715–2720
Lu Y, Sun WJ, Gu Z (2014) Stimuli-responsive nanomaterials for therapeutic protein delivery. J Control Release 194:1–19
Stuart M, Huck WT, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S (2010) Emerging applications of stimuli-responsive polymer materials. Nat Mater 9:101–113
Constantina M, Bucatariu S, Ascenzi P, Simionescu BC, Fundueanu G (2014) Poly(NIPAAm-co-b-cyclodextrin) microgels with drug hosting and temperature- dependent delivery properties. React Funct Polym 84:1–9
Hu Y, Zhao NN, Li JS, Yang WT, Xu FJ (2012) Temperature-responsive porous polycaprolactone-based films via surface-initiated ATRP for protein delivery. J Mater Chem 22:21257–21264
Ma ML, Ma Y, Zhang BL, Zhang HP, Geng WC, Zhang QY (2015) Fabrication and characterization of 1 D Fe3O4/P(NIPAM-MAA-MBA) nanochains with thermo- and pH-responsive shell for controlled release for phenolphthalein. J Mater Sci 50:3083–3090. doi:10.1007/s10853-015-8868-5
He XL, Yu S, Dong YY, Yan FY, Chen L (2009) Preparation and properties of a novel thermo-responsive poly(N-isopropylacrylamide) hydrogel containing glycyrrhetinic acid. J Mater Sci 44:4078–4086. doi:10.1007/s10853-009-3588-3
Zha LS, Zhang Y, Yang WL, Fu SK (2002) Monodisperse temperature-sensitive microcontainers. Adv Mater 14:1090–1093
Zhou L, Lu ZY, Zhang X, Dai H (2006) The studies on the temperature sensitive of N-isopropylacrylamide copolymer. Polym Mater Sci Eng 22(2):165–168
Kraus K, Tieke B (2014) pH-and temperature-responsive hydrogels of acrylic acid, N-isopropylacrylamide and a non-ionic surfmer: phase behaviour, swelling properties and drug release. Colloid Polym Sci 292:3127–3135
Friedrich T, Tieke B, Stadler FJ, Bailly C (2011) Copolymer hydrogels of acrylic acid and a nonionic surfmer: pH-induced switching of transparency and volume and improved mechanical stability. Langmuir 27:2997–3005
Hu W, Zhang Y (2010) Synthesis and characterization of fluorescent composite microgels with the dual thermo- and pH-sensitive. Acta Chim Sinica 18:1855–1863
Wasserman MA, Sundell CL, Kunsch C, Edwards D, Meng CQ, Medford RM (2003) Chemistry and pharmacology of vascular protectants: a novel approach to the treatment of atherosclerosis and coronary artery disease. Am J Cardiol 91(3):34–40
Zhang J, Zhang QS, Chen XM, Tian GY (2003) Synthesis of targeting drug for antifibrosis of liver: a conjugate for delivering glycyrrhetin to hepatic stellate cells. Glycoconj J 19:423–429
Khoee S, Sattari A, Atyabi F (2014) Physico-chemical properties investigation of cisplatin loaded polybutyladipate (PBA) nanoparticles prepared by w/o/w. Mater Sci Eng, C 32:1078–1086
Liu MX, Chen LH, Zhao YH, Gan LH, Zhu DZ, Xiong W, Lv YK, Xu ZJ, Hao ZX, Chen LW (2012) Preparation, characterization and properties of liposome-loaded polycaprolactone microspheres as a drug delivery system. Colloid Surf A 395:131–136
Yang YY, Chia HH, Chung TS (2000) Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Control Release 69:81–96
Cuggino JC, Contreras CB, Jimenez-Kairuz A, Maetto BA, Igarzabal CIA (2014) Novel poly(NIPA-co-AAc) functional hydrogels with potential application in drug controlled release. Mol Pharm 11:2239–2249
Zhang XJ, Achazi K, Steinhilber D, Kratz F, Dernedde J, Haag R (2014) A facile approach for dual-responsive prodrug nanogels based on dendritic polyglycerols with minimal leaching. J Control Release 174:209–216
Fundueanu G, Constantin M, Oanea I, Harabagiu V, Ascenzi P, Simionescu BC (2010) Entrapment and release of drugs by a strict “on-off ” mechanism in pullulan microspheres with pendant thermosensitive groups. Biomaterials 31(36):9544–9553
Salehi R, Rasouli S, Hamishehkar H (2015) Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int J Pharm 487:274–284
Korsmeyer RW, Peppas NA (1984) Solute and penetrant diffusion in swellable polymers. III. Drug release from glassy poly(HEMA-co-NVP) copolymers. J Control Release 1(2):89–98
Yang XL, Luo YL, Xu F, Chen YS (2014) Thermosensitive mPEG-b-PA-g-PNIPAM comb block copolymer micelles: effect of hydrophilic chain length and camptothecin release behavior. Pharm Res 31:291–304
Acknowledgements
This work was supported by Natural Science Foundation of Tianjin (Nos. 16JCYBJC23800, 16JCZDJC37500) and Training Program of Innovation and Entrepreneurship for Undergraduates (201610058064).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, X., Liang, F., Wang, F. et al. Targeted delivery and thermo/pH-controlled release of doxorubicin by novel nanocapsules. J Mater Sci 53, 2326–2336 (2018). https://doi.org/10.1007/s10853-017-1679-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1679-0