Abstract
We have synthesized a series of conjugated random copolymers—P1-COOR′, P2-COOR″, and P3-COOR″—containing electron-donating units based on (4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b′]dithiophene), alkyl 3-thiophene carboxylate, and thiophene by Stille polycondensation method. The polymers were characterized by 1H NMR spectroscopy, gel permeation chromatography (GPC), UV–Vis absorption spectroscopy, and cyclic voltammetry. The optical band gaps of the polymers varies from 1.88 to 1.93 eV and their absorption characteristics can be tuned by adjusting the ratio of the two electron-donating units: (4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b′]dithiophene) and thiophene. These polymers exhibit broad and strong absorption between 300 and 700 nm with good solubility and thermal stability. Electrochemical studies indicate sufficiently deep HOMO levels and it gives high open-circuit voltage in the bulk heterojunction device when fullerene derivatives are used as electron acceptors. By changing the ratio of the two donor monomers, the highest occupied molecular orbital energy levels of polymers varies between −5.04 and −5.19 eV and the lowest unoccupied molecular orbital energy levels range from −3.16 to −3.26 eV. Preliminary results on solar cell fabrication using the synthesized polymers show open-circuit voltage in the range of 0.62–0.63 V.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic semiconducting polymers have been of intense study in recent years due to their potential application in low-cost optoelectronic devices such as organic thin-film transistors and photovoltaic cells [1–7]. The power conversion efficiency (PCE) of organic photovolatics has reached 12 % recently [8]. To achieve high efficiency of PSCs, one of the most critical challenges at the molecular level is to develop ideal p-type conjugated polymers that simultaneously possess sufficient solubility, low band gap, and high hole mobility to ensure high PCE for organic photovoltaics. Among the polymers, polythiophene derivatives are the most commonly used due to their higher charge carrier mobility. Solubility can be improved by incorporation of suitable substituents attached at the 3-position of the thiophene. Most of the polythiophene analogs contain either alkyl side chains or electron-donating substituents. Polythiophenes with electron-withdrawing ester groups attached at the 3-position have already been reported [9, 10]. These polymers have lower HOMO energy levels which provide better oxidative doping stability than the conventional solution-processable polythiophenes. Introducing donor and acceptor monomers together in the polymer backbone is a promising strategy to obtain polymers with low band gap. In most cases, low band gap polymers achieved by the donor–acceptor (D−A) approach do not show broad absorption, but instead the absorption maxima are red-shifted, decreasing the number of absorbed photons in the visible region and ultimately limiting the achievable photocurrent. Combining two kinds of conjugated polymers with different broad absorption spectra and band structures have been conducted for the development of high performance photovoltaic solar cells due to the improved harvest of the solar emission and the control of band gap [11]. Incorporation of multiple chromophores in one polymer has received less attention even though it has been successfully shown that a randomized polymer structure in combination with the D/A strategy can lead to broadened absorption and efficiencies above 5 % [12–18].
In this study, we describe the synthesis and the optoelectronic characterization of a novel family of random copolymers bearing multiple monomer units. There have been reports on two-donor-one-acceptor random copolymers [19–21]. But the resulting absorption spectra were not so broad, and hence fabricated polymer solar cells resulted in low power conversion efficiencies. Later research has been focused on two-acceptor-one-donor random copolymers where the presence of two acceptors and donor with complementary absorption resulted in low band gap polymers with a broad absorption range in the visible region and near infrared (NIR) region, and higher power conversion efficiencies [22–27]. In the present study, we describe the application of a weak electron-withdrawing unit—alkyl 3-thiophene carboxylate—to enhance the open-circuit voltage of cyclopentadithiophene-based copolymers by random copolymerization method. The synthesis and the optoelectronic characterization of a novel family of random copolymers bearing varying molar ratio of two electron-donating units and a weak electron accepting unit have been studied. Carboxylate functionalized polymers have already been applied in organic solar cells to achieve high open-circuit voltage and better operational stability of the devices after thermo cleavage of the ester group [28–33]. The presence of 4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene and alkyl 3-thiophene carboxylate units are expected to lead copolymers with sufficiently deep HOMO/LUMO energy level and high charge carrier mobility for photovoltaic applications. The presence of alkyl 3-thiophene carboxylate unit can lower HOMO energy level to maximize the gap between the HOMO level of the electron-donating polymer and the LUMO level of the electron acceptor in order to increase the open-circuit voltage. Cyclopentadithiophene (CPDT) is used as a good electron-donating moiety due to the presence of rigid coplanar structure with easy π–π intermolecular interaction and side chain manipulation which can result in high charge carrier mobility essential for good fill factor. Also the two units show different electrochemical properties such as the frontier molecular orbital energy levels and band gaps. Hence, it is expected that random copolymerization of the two units can tune the HOMO and LUMO energy levels of the resulting polymer. The random copolymers composed of 4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene/thiophene/alkyl thiophene 3-carboxylate as the electron donors show intense light absorption from 300 to 700 nm and low-lying HOMO levels.
Experimental part
Materials
2,6-Dibromo-4H-cyclopenta[2,1-b:3,4-b′]dithiophene (1) was purchased from Luminescence Technology Corp. Anhydrous solvents and 2,5-bis(tri-n-butyltin)-thiophene were purchased from Aldrich. The precursors 2,6-dibromo-4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene (2) and alkyl 2,5-dibromo thiophene 3-carboxylates (5) were prepared as per reported in the literature [34, 35].
Characterization
1H NMR spectra were recorded on a Bruker 500 MHz spectrometer using deuterated solvents with tetramethylsilane (TMS) as a reference. UV–Vis absorption spectra were recorded in chloroform solution (10−5 mol L−1) on a Shimadzu 2401 PC spectrophotometer. Thermal analyses were carried out on a Mettler Toledo TGA/SDTA851e, DSC 822e analyzer under a dynamic atmosphere of nitrogen with a heating/cooling rate of 15°min−1. The molecular weights of the polymers were determined by gel permeation chromatography (GPC) against polystyrene standard with THF as eluent at 30 °C. GPC analysis was performed on a Perkin Elmer, Series 200, USA GPC system equipped with a refractive index detector. The cyclic voltammetric study was conducted on an Autolab PGSTAT302 N at a constant scan rate of 20 mV s−1. Platinum wires were used as both the counter and working electrodes, and Ag/AgCl electrode was used as a reference electrode. Polymer thin films were formed by drop-casting chloroform solution (5 mg mL−1) onto the working electrode, and then dried in the air at 80 °C. 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6) in acetonitrile was used as the electrolyte. The morphology of the active layers was investigated through atomic force microscopy (AFM) in tapping-mode under ambient conditions using Nanoscope-5 Bruker ALX.
Fabrication of polymer solar cells
The organic photovoltaic cells were fabricated with ITO glass as the anode, Ca and Al as the cathode, and the blend films of polymer: PC60BM as the BHJ active layer. ITO-coated glass substrate was pre-treated with acetone, trichloroethylene, and isopropanol in an ultrasonic bath. A thin layer of MoO3 (ca 10 nm) was thermally evaporated on the ITO anode. The active layer was prepared by spin-coating a blend solution of the polymers and PC60BM in chlorobenzene (CB) with the concentration of 30 mg mL−1. The active layer thickness was determined to be ca 80 nm. As a cathode, a thin layer of Ca and a film of Al (ca 100 nm) were evaporated in sequence under the vacuum of 10−6 Torr. This all resulted in the device configuration as ITO/MoO3/BHJ active layer/Ca/Al with pixel area of the device ~2 × 3 mm.The device was then annealed at 130 °C for 10 min. The current density–voltage (J–V) curves were obtained using a Keithley 2400 source-measure unit interfaced with a PC.
Synthesis of polymers
Synthesis of poly{[(4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-thiophene-2,5-diyl]-co-[4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-3-hexylthiophenecarboxylate-2,5-diyl] (P1-COOR′)
The monomers 2 (0.200 g, 0.357 mmol), 5 (0.132 g, 0.357 mmol), and 6 (0.478 g, 0.721 mmol) were dissolved in a mixture of anhydrous toluene/DMF (8 mL) under N2 atmosphere. The reaction mixture was purged with nitrogen for 30 min and then Pd (PPh3)4 (17 mg, 2mol%) was added. The reaction mixture was heated with stirring for 48 h at 115 °C. 2-Bromothiophene (0.118 g, 0.723 mmol) and 2-(tributylstannyl)thiophene (0.269 mg, 0.721 mmol) were added as end capping agent to the mixture and stirred for 12 h under the same conditions. After cooling to room temperature, the viscous solution was poured into 300 mL of methanol. The precipitated polymer was isolated by filtration. It was further purified by Soxhlet extraction with acetone followed by reprecipitation from methanol. The polymer was dried under reduced pressure to yield P1-COOR′ as violet-colored solid (0.160 g, 68 %). 1H NMR (500 MHz, CDCl3): δ H = 7.08–7.13 (m, 5H, br), 7.48–7.51 (m, 2H, br), 4.32 (t, 2H, br), and 1.92–0.63 (m, 47, br). GPC analysis (PS standard): M n = 7.23 × 104 gmol−1, PDI = 1.59.
Synthesis of poly{[(4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-thiophene-2,5-diyl]-co-[4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-3-heptylthiophenecarboxylate-2,5-diyl] (P2-COOR″)
The monomers 2 (0.250 g, 0.446 mmol), 5 (0.086 g, 0.223 mmol), and 6 (0.443 g, 0.669 mmol) were dissolved in a mixture of anhydrous toluene/DMF (6.5 mL) under N2 atmosphere. The reaction mixture was purged with nitrogen for 30 min and then Pd (PPh3)4 (16 mg, 2 mol%) was added. The reaction mixture was heated with stirring for 48 h at 115 °C. 2-Bromothiophene (0.109 g, 0.699 mmol) and 2-(tributylstannyl)thiophene (0.250 mg, 0.699 mmol) were added as end capping agent to the mixture and stirred for 12 h under the same conditions. After cooling to room temperature, the viscous solution was poured into 300 mL of methanol. The precipitated polymer was isolated by filtration. It was further purified by Soxhlet extraction with acetone followed by reprecipitation from methanol. The polymer was dried under reduced pressure to yield P2-COOR″ as violet-colored solid (0.140 g, 52 %). 1H NMR (500 MHz, CDCl3): δ H = 6.93–7.14 (m, 9H, br), 7.44–7.51 (m, 2H, br), 4.29 (t, 4H, br), 1.89–0.65 (m, 89, br). GPC analysis (PS standard): M n = 8.159 × 104 gmol−1, PDI = 1.57.
Synthesis of poly{[(4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-thiophene-2,5-diyl]-co-[4,4-bis(2-ethylhexyl)-cyclopenta-[2,1-b:3,4-b′]dithiophene)-2,6-diyl-alt-3-heptylthiophenecarboxylate-2,5-diyl] (P3-COOR″)
The monomers 2 (0.146 g, 0.260 mmol), 5 (0.200 g, 0.521 mmol), and 6 (0.517 g, 0.781 mmol) were dissolved in a mixture of anhydrous toluene/DMF 7.5 mL) under N2 atmosphere. The reaction mixture was purged with nitrogen for 30 min and then Pd (PPh3)4 (18 mg, 2 mol%) was added. The reaction mixture was heated with stirring for 48 h at 115 °C. 2-Bromothiophene (0.127 g, 0.781 mmol) and 2-(tributylstannyl) thiophene (0.291 mg, 0.781 mmol) were added as end capping agent to the mixture and stirred for 12 h under the same conditions. After cooling to room temperature, the viscous solution was poured into 300 mL of methanol. The precipitated polymer was isolated by filtration. It was further purified by Soxhlet extraction with acetone followed by reprecipitation from methanol. The polymer was dried under reduced pressure to yield P3-COOR″ as violet-colored solid (0.200 g, 68 %). 1H NMR (500 MHz, CDCl3): δ H = 6.96–7.14 (m, 6H, br), 7.47–7.51 (m, 4H, br), 4.32 (t, 4H, br), and 1.92–0.68 (m, 62, br). GPC analysis (PS standard): M n = 9.145 × 104 gmol−1, PDI = 1.45.
Results and discussion
Synthesis and thermal study
Copolymerization of 2,6-dibromo-4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene (2), alkyl 2,5-dibromothiophene 3-carboxylate (5) and 2,5-bis(tri-n-butyltin) thiophene (6) in toluene/DMF mixture at 115 °C with Pd (PPh3)4 catalyst yielded the desired polymers, as illustrated in Scheme 1. The comonomer feed ratios are 1:1, 2:1, and 1:2, and the corresponding polymers are named as P1-COOR′, P2-COOR″, and P3-COOR″, respectively, based on the alkyl substituent on thiophene 3-carboxylate ester. P1-COOR′ contains hexyl group on the ester part, while P2-COOR″ and P3-COOR″ carries heptyl group on it. The yields of polymers varied from 68 % for P1-COOR′ to 52 % for P2-COOR″, and finally 68 % for P3-COOR″. All the polymers showed good solubility at room temperature in organic solvents such as THF, chloroform, and CB. The molecular structures of the polymers were characterized by 1H NMR spectroscopy and the NMR spectra of the polymers are given in Fig. 1. The actual incorporation ratio of the monomers could be analyzed by comparison of the 1H NMR signal intensities of protons of –CH2 group in 4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene moiety and –CH2 group in carboxylate ester part. The signals with chemical shifts of 4.3 ppm and 1.90 ppm are assigned, respectively, to protons of –OCH2 in alkyl thiophene 3-carboxylate ester moiety and –CH2 group directly connected to CPDT unit. The integration ratio of these two peaks is slightly different from the feeding molar ratio of 2 to 5, as given in Table 1. GPC analysis of the polymers using polystyrene (PS) standard showed the number average molecular weight (M n ) of 7.231 × 104 gmol−1 (PDI = 1.59), 8.159 × 104 gmol−1 (PDI = 1.57), and 9.145 × 104 gmol−1 (PDI = 1.45) for P1-COOR′, P2-COOR″, and P3-COOR″, respectively.
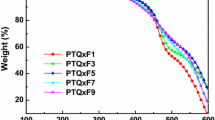
The thermal stability of the polymers was investigated by thermo-gravimetric analysis (TGA). TGA thermograms curves of polymers are shown in Fig. 2. TGA reveals good thermal stability of the copolymers with onset decomposition temperature higher than 300 °C. The decomposition temperature corresponding to 5 % weight loss temperatures (T d) for P1-COOR′, P2-COOR″, and P3-COOR″ are 338, 329, and 324 °C, respectively. The high-thermal stability is crucial for the application in PSCs. No distinct glass transition was observed from 25 to 300 °C in their DSC curves of the second heating and cooling runs (20 °C min−1). Table 1 summarizes the polymerization results including molecular weight, molecular weight distribution (PDI), thermal stability, and estimated compositions of the copolymers.
Optical properties
The UV–Vis absorption spectra of the copolymers in chloroform solution and thin-film formats are complied in Figs. 3 and 4, respectively. In solution, all the three polymers exhibit broad absorption in the region of 300–650 nm. Compared with their absorption spectra in solution, in films, the three polymers exhibit slightly broader and red-shifted feature due to enhanced interchain interactions in solid state.
The absorption maximum peak (λ max) of P1-COOR′ in visible region shows a red shift of 18 nm between solution and thin films, and in the case of P2-COOR″ and P3-COOR″ the absorption spectra in thin films exhibit a red shift of 24 nm each, which can be explained by the formation of stacking structure in the solid state that could π–π facilitate charge transportation for photovoltaic applications. Among the copolymers, the highest absorption maximum and higher red shift in absorption maximum is observed for copolymer incorporated with large amount of CPDT moiety, i.e., P2-COOR″.
The optical band gap was calculated from the onset wavelength of absorption by \( E_{\text{g}}^{\text{opt}} \) = 1240 nm/λ onset, where \( E_{\text{g}}^{\text{opt}} \) is the optical band gap and λ onset is the onset wavelength of absorption. The optical and electrochemical properties of the polymers are given in Table 2.
Electrochemical properties
The electrochemical properties of the polymers were investigated by using cyclic voltammetry (CV) to determine the energy levels of the HOMO and LUMO energy levels of the polymers. Figure 5 shows the CV diagrams of the copolymers using 0.1 M TBAPF6 as supporting electrolyte in acetonitrile solution.
The HOMO level values of the copolymers were estimated from the roughly evaluated onset oxidation potential according to the following empirical formula E HOMO = −e(E ox + 4.40) (eV). The E ox is the onset oxidation potential versus Ag/Ag+. The lowest unoccupied molecular orbital (LUMO) level values of the copolymers were estimated as E LUMO = E HOMO−E g, where E g is the optical band gap. The onset oxidation potentials (E ox), which are related with the constitution of polymers, varied from 0.79 V for P1-COOR′ to 0.64 V for P2-COOR″ and 0.73 V for P3-COOR″.
The CV results illustrated that these new polymers are good candidates as donor materials. The LUMO energy levels of are in the range of −3.16 to −3.26 eV which are higher than that of PC61BM (−4.2 eV), guaranteeing the photoinduced electrons transfer from the donor to acceptor, that is, from polymers to PC61BM. For a clear comparison, the energy levels of the polymers determined by the electrochemical method are shown in the energy level diagram in Fig. 5b. P1-COOR′ has a band gap of 1.93 eV, whereas P2-COOR″ and P3-COOR″ show band gap of 1.88 and 1.91 eV, respectively.
Photovoltaic properties
Preliminary results of a conventional photovoltaic device made of the bulk heterojunction composite of P1-COOR′ with PC60PM show an open-circuit voltage (V oc) of 0.63 V, a current density of 0.45 mA cm−2 and a fill factor 35 %. The open-circuit voltage of the all the polymers is comparable to that of well-known alternating copolymer based on cyclopentadithiophene and benzothiadiazole (PCPDTBT) [36]. The J–V characteristics of solar cells prepared with polymer and PC60BM in 1:1 ratio is shown in Fig. 6 and solar cell parameters are summarized in Table 3.
The poor PCE of 0.1 % was resulted for P1-COOR′ from the very low J sc values. Although the relatively high band gap have decreased the light harvesting ability of the polymer, the significant factor attributed to the poor J sc is possibly due to the lack of interpenetrating network of donor and acceptor in the blend, which adversely affect exciton dissociation and transport of electrons and holes to the respective electrodes.
The nanoscale morphologies of the polymer/PC60BM films were studied using tapping-mode AFM. AFM tapping-mode height images were taken for each film and are shown in Fig. 7. Surface roughness values measured from the height images were 2.038 nm for P1COOR′, 2.497 nm for P2COOR″, and 0.497 nm for P3COOR″. Further investigations are needed to understand the effect of random structure of the polymers on crystallization and carrier mobility to shed light on low value of short circuit current. The low fill factor indicates the further device performance improvements by optimization of the film morphology by thermal annealing, solvent/vapor annealing, or the addition of solvent additives.
Conclusions
In summary, new random copolymers based on three different donor moieties have been synthesized by Stille polycondensation method. The structures have been confirmed by 1H NMR spectroscopy and molecular weights have been determined by GPC. The polymers have intense and broad absorption between 300 and 700 nm with optical band gap of 1.88–1.93 eV. The presence of alkyl 3-thiophene carboxylate units decreases the value of HOMO energy levels of the random copolymers. Thus, the HOMO and LUMO energy levels can be tuned by the feeding molar ratios of the two donor monomers—4,4-diethylhexyl-cyclopenta[2,1-b:3,4-b′]dithiophene and alkyl thiophene 3-carboxylate. Solar cell fabricated using one of the synthesized polymers (P1-COOR′) have shown a V oc of 0.63 V.
References
Kim DH, Lee BL, Moon H, Kang HM, Jeong EJ, Park JI, Han KM, Lee S, Yoo BW, Koo BW, Kim JY, Lee WH, Cho K, Becerril HA, Bao Z (2009) Liquid crystalline semiconducting copolymers with intramolecular donor-acceptor building blocks for high stability polymer transistors. J Am Chem Soc 131:6124–6132
Allard S, Forster M, Souharce B, Thiem H, Scherf U (2008) Organic semiconductors for solution-processable field effect transistors. Angew Chem Int Ed 47:4070–4098
Zhang M, Tsao HN, Pisula W, Yang C, Mishra AK, Mullen K (2007) Field effect transistors based on benzothiadiazole-cyclopentadithiophene. J Am Chem Soc 129:3472–3473
Chen HY, Hou J, Hayden AE, Yang H, Houk KN, Yang Y (2010) Silicon atom substitution enhances interchain packing in thiophene-based polymer system. Adv Mater 22:371–375
Jung IH, Kim H, Park MJ, Kim B, Park JH, Jeong E, Woo HY, Yoo SH, Shim HK (2010) Synthesis and characterization of cyclopentadithiophene based low band gap band polymers containing electron deficient benzoselenadiazole derivatives for photovoltaic devices. J Polym Sci A Polym Chem 48:1423–1432
Helgesen M, Søndergaard R, Krebs FC (2010) Advanced materials and processes for polymer solar cell devices. J Mater Chem 20:36–60
Soci C, Hwang IW, Moses D, Zhu Z, Waller D, Gaudiana R, Brabec CJ, Heeger AJ (2007) Photoconductivity of a low band gap conjugated polymer. Adv Funct Mater 17:632–636
He Z, Zhong C, Su S, Xu M, Wu H, Cao Y (2012) Enhanced power conversion efficiency in polymer solar cells using an inverted device structure. Nat Photonics 6:591–595
Amanda RM, Jinsong L, Christine L, David K, Jean MJF, Joseph RK, Michael DM (2005) Synthesis, characterization and field effect transistor performance of carboxylate-functionalized polythiophenes with increased air stability. J Mater Chem 17:4892–4899
Martin P, Yang C, Ramesh KK, Ronald LE (1999) Poly(alkyl thiophene 3-carboxylates). Synthesis, properties and electroluminescence studies of polythiophenes containing a carbonyl group directly attached to the ring. Chem Mater 9:2155–2163
Hayashi Y, Sakuragi H, Soga T, Alexandrou I, Amaratunga GAJ (2008) Bulk heterojunction solar cells based on two kinds of organic polymers and fullerene derivative. Colloids Surf A 313–314:422–425
Beaujuge PM, Ellinger S, Reynolds JR (2008) The donor–acceptor approach allows a black- to-transmissive switching polymeric electrochrome. Nat Mater 7:795–799
Chen C-P, Chan S-H, Chao T-C, Ting C, Ko B-T (2008) Low band gap poly(thiophene-phenylene-thiophene) derivatives with broaden absorption spectra for use in high performance bulk heterojunction solar cells. J Am Chem Soc 130:12828–12833
Li J, Ong K-H, Lim S-L, Ng G-M, Tan H-S, Chen Z-K (2011) A random copolymer based on dithienothiophene and diketopyrrolopyrrole units for high performance organic solar cells. Chem Commun 47:9480–9482
Chen C-H, Cheng Y-J, Chang C-Y, Hsu C-S (2011) Donor–acceptor random copolymers based on ladder type nonacyclic units: synthesis, characterization, and photovoltaic applications. Macromolecules 44:8415–8524
Nielsen CB, Ashraf RS, Schroeder BC, Angelo PD’, Watkins SE, Song K, Anthopoulos TD, McCulloch I (2012) Random benzotrithiophene –based donor-acceptor copolymers for efficient organic photovoltaics. Chem Commun 48:5832–5834
Khlyabich PP, Burkhart B, Ng CF, Thompson BC (2011) Efficient solar cells from semirandom P3HT analogues incorporating diketopyrrole. Macromolecules 44:5079–5084
Burkhart B, Khlyabich PP, Thompson BC (2012) Influence of the acceptor composition on physical properties and solar cell performance in semi-random two acceptor copolymers. ACS Macro Lett 1:660–666
Piyakulawat P, Keawprajak P, Jiramitmongkon K, Hanusch M, Wlosewski J, Asawapirom U (2011) Effect of thiophene donor units on the optical and photovoltaic behavior of fluorene based copolymer. Sol Energ Mat Sol Cell 95:2167–2172
Lee PI, Hsu SLV, Lee JF (2011) Efficient bulk heterojunction solar cells with copolymers based on fluorene, dithienylbenzothiadiazole, and thiophene derivatives. Sol Energ Mat Sol Cell 95:1756–1761
Deng Z, Chen L, Chen Y (2013) Novel phenathro carbazole based donor-acceptor random and alternating copolymers for photovolataics. J Polym Sci A Polym Chem 51:4885–4893
Scaria R, Dhawan SK, Chand S (2014) Synthesis of two acceptor random copolymers for organic solar cell applications. Synt Met 191:168–176
Oktem G, Balan A, Baran D, Toppare L (2011) Donor –acceptor type random copolymers for full visible light absorption. Chem Commun 47:3933–3935
Song J, Zhang C, Li C, Li W, Qin R, Li B, Liu Z, Bo Z (2010) Conjugated polymers with broad absorption: synthesis and application in polymer solar cells. J Polym Sci A 48:2571–2578
Zhang G, Fu Y, Qiu L, Xie Z (2012) Synthesis and characterization of thieno[3,4-c]pyrrole-4,6-dione and pyrrolo[3,4-c]pyrrole-1,4-dione-based random polymers for photovoltaic applications. Polymer 53:4407–4412
Jiang JM, Chen H-C, Lin H-K, Yu C-M, Lan SC, Liu C-M, Wei K-H (2013) Conjugated random copolymers of benzodithiophene–benzooxadiazole diketopyrrolopyrrole with full visible light absorption for bulk heterojunction solar cells. Polym Chem 4:5321–5328
Jung JW, Liu F, Russell TP, Jo WH (2013) Semi crystalline random conjugated copolymers with panchromatic absorption for highly efficient solar cells. Energy Environ Sci 6:3301–3307
Jørgensen M, Norrman K, Krebs FC (2008) Stability/degradation of polymer solar cells. Sol Energy Mater Sol Cells 92:686–714
Petersen MH, Gevorgyan SA, Krebs FC (2008) Thermocleavable low band gap polymers and solar cells therefrom with remarkable stability toward oxygen. Macromolecules 41:8986–8994
Helgesen M, Gevorgyan SA, Krebs FC, Janssen RAJ (2009) Substituted 2,1,3-benzothiadiazole- and thiophene-based polymers for solar cells - Introducing a new thermocleavable precursor. Chem Mater 21:4669–4675
Helgesen M, Krebs FC (2010) Photovoltaic performance of polymers based on dithienylthienopyrazines bearing thermocleavable benzoate esters. Macromolecules 43:1253–1260
Manceau M, Helgesen M, Krebs FC (2010) Thermo-cleavable polymers: materials with enhanced photochemical stability. Polym Degrad Stab 95:2666–2669
Krebs FC, Norrman K (2007) Analysis of the failure mechanism for a stable organic photovoltaic during 10000 h of testing. Progr Photovol 15:697–712
Chen CH, Hsieh CH, Dubosc M, Cheng YJ, Hsu CS (2010) Synthesis and characterization of bridged bithiophene-based conjugated polymers for photovoltaic applications: Acceptor strength and ternary blends. Macromolecules 43:697–708
Pomerantz M, Yang H, Cheng Y (1995) Poly(alkyl thiophene 3-carboxylates): synthesis and characterization of polythiophenes with a carbonyl group directly attached to the ring. Macromolecules 28:5706–5708
Peet J, Kim Y, Coates NE, Ma WL, Moses D, Heeger AJ, Bazan GC (2007) Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat Mater 6:497–500
Acknowledgements
We acknowledge Dr. Pritam Mukhopadhyay and Dr.Ajaykumar, AIRF, Jawaharlal Nehru University, New Delhi, for extending their laboratory facilities for NMR measurements. R. S. acknowledges a research fellowship from the University Grant Commission (UGC), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaria, R., Ali, F., Dhawan, S.K. et al. Synthesis and characterization of low band gap random copolymers based on cyclopentadithiophene and thiophene carboxylates for photovoltaic applications. J Mater Sci 50, 555–562 (2015). https://doi.org/10.1007/s10853-014-8611-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8611-7