Abstract
A linearly linked triscalix[4]arene, in which each calix[4]arene unit possesses both axial chirality and inherent chirality, was synthesized by repeated use of two stereoselective Williamson etherification on calix[4]arene. In the crystal lattice of the biscalix[4]arene intermediate, there are interesting alternate layers of biscalixarene molecules and solvent molecules in the ab plane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multicalixarenes are oligomers of defined molecular weight and architecture, formed by the covalent bonding of multiple calixarene units. According to the linkage pattern, multicalixarenes can be classified as linear multicalixarenes [1] and hyperbranched multicalixarenes such as calixarene dendrimers [2]. As an aggregate of multiple calixarene units linked via covalent bonds, multicalixarenes are not only of synthetic interest, but also capable of realizing specific function such as ion-binding [3,4,5,6], DNA binding [7], and gene transfection [7]. The controlled synthesis of multicalixarenes usually relies on definite and highly efficient reactions such as etherification, esterification, amidation, Friedel–Crafts alkylation, Glaser coupling, Sonogashira coupling, ring-closing metathesis (RCM), click reaction, etc [1]. To date, though a third-generation calixarene dendrimers consisting of calixarene units up to 21 has been synthesized [8], linear multicalixarenes with ≥4 calixarene units are rarely seen [9,10,11,12].
Meanwhile, Most of the linear multicalixarenes are comprised of achiral calixarenes and achiral linkers. Representative exceptions include the multicalixarene series containing peptidic linkers [13,14,15] and the biscalixarenes containing one inherently chiral calixarene unit [16,17,18]. Linear multicalixarenes with each calixarene units being inherently chiral have not been reported previously.
The assembly of multiple chiral calixarene units in a linear manner by covalent bonds to form a well organized architecture has the potential to demonstrate novel properties which are otherwise unattainable for individual calixarene components, due to the synergistic effects of the neighboring calixarene units [19] or by generating higher-ordered structural features [13, 14]. Meanwhile, as a transition specie between chiral calixarene monomer and chiral calixarene-based polymers [20,21,22,23,24,25,26], the study of such chiral calixarene oligomers with definite architecture is helpful to the understanding of chiral polymers made up of numerous chiral calixarene units.
Recently, we have developed a highly efficient method to generate inherent chirality [27] in calix [4] arene starting from a 1,2-substituted calix[4]crown containing an axially chiral BINOL moiety (reaction 1, Diagram 1) [28]. This reaction enjoys several merits, including mild condition (1 equiv of Cs2CO3, 1 equiv of electrophile, DMF, 60–80 °C), desirable yield (ca. 80%), easy separation of the resultant diastereomers on a gram scale by column chromatography on silica gel due to their significant difference in polarity, and high diastereoselectivity (up to 72% based on isolated products). The presence of (S)-BINOL moiety favors the formation of cS-form in terms of inherent chirality and that of (R)-BINOL cR-form. The reaction is feasible for numerous electrophiles R1X, including nPrI, BnBr, BrCH2CO2Et, ClCH2CONEt2 etc and proceeds quite fast. The tri-substituted cone products of reaction 1 can be further alkylated with an electrophile R2X to produce the fully substituted calix[4]arenes via reaction 2 (excess Cs2CO3, excess electrophile, DMF, 60–80 °C), which highly stereoselectively furnishes the partial cone (paco) products (reaction 2, Diagram 1).
The high generality of this two reactions tempted us to try the repeated use of them to produce linear multicalix [4] arenes consisting of optically pure calix[4]arene units with combined axial chirality and inherent chirality. We envisaged that reaction 1 is crucial to the covalent bonding of individual calix[4]arene units when R1X is a (multi)calixarene-based electrophile, while reaction 2 is crucial to the formation of appropriate (multi)calixarene-based electrophiles for the purpose of linkage.
Herein we report the synthesis of a linearly linked triscalix[4]arene by sequential use of two stereoselective Williamson etherifications on calix[4]arene, in which each calix[4]arene units is optically pure and possesses both axial chirality and inherent chirality. The structures of were studied with NMR technology, X-ray crystallography, and circular dichroism spectra.
Results and discussion
As depicted in Scheme 1, (R)-BINOL-containing calix[4]crown 1 underwent reaction 1 with BrCH2CH2CH2CO2Et to produce the tri-substituted cone conformer 2 in 68% yield. The diastereoselectivity was determined to be 57%, based on the integration of the OH signals in 1H NMR spectrum of the crude reaction mixture (major/minor diastereomer = 3.66:1). Calix[4]arene 2 then underwent reaction 2 with nPrI for the purpose of protecting the free phenolic hydroxyl group in 2, thus furnishing the fully substituted partial cone conformer 3. Ester 3 was transformed into corresponding tosylate 5 by two additional steps. Upon treatment of Calix[4]crown 1 with calix[4]arene electrophile 5 under the condition of reaction 1, biscalix[4]arene 6 with two chiral calix[4]arene units was successfully produced as a partial cone–cone conformer in 47% yield, with a diastereoselectivity of 59% (major/minor diastereomer = 3.88:1). Biscalix[4]arene 6 was subsequently transformed into a partial cone-partial cone electrophile 9 by reaction 2 (with BrCH2CH2CH2CO2Et) and two additional reactions. A second round of reaction 1 of calix[4]arene 1 with biscalix[4]arene electrophile 9 smoothly afforded triscalix[4]arene 10 as a partial cone-partial cone–cone conformer in 35% yield. The diastereoselectivity decreased to 47% (major/minor diastereomer = 2.79:1).
In order to enhance the efficiency of covalently linking calix[4]arene units, we tested the use of calix[4]arene-based bifunctional electrophile 11 (see supporting information) to react with calix[4]arene 1 for the purpose of constructing the triscalix[4]arene 12 in one step. However, an inseparable mixture was produced, indicating the reaction was complicated by the formation of several possible configurational isomers (Scheme 2). Therefore, though the protocol of linking individual chiral calix[4]arene units one by one by repeated use of reaction 1 and reaction 2 seems a bit tedious, it is reliable after all.
1H NMR spectra
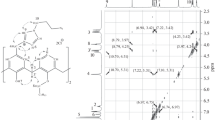
In the 1H NMR spectrum of biscalix[4]arene 6, the eight signals (1.35, 1.33, 1.24, 1.12, 1.08, 1.02, 0.83, and 0.81 ppm) attributable to the eight tert-butyl groups confirmed the existence of two chiral calix[4]arene units in the molecule. Likewise, the ten signals (1.31, 1.252, 1.248, 1.19, 1.13, 1.08, 1.05, 1.00, 0.79, 0.77 ppm, slightly overlapped) attributable to the 12 tert-butyl groups confirmed the existence of three chiral calix[4]arene units in the molecule of triscalix[4]arene 10 (Fig. 1 ). In addition, ESI–MS data confirmed the molecular weights of all the intermediates and products, including that of the biscalix[4]arene 6 (m/z 2093.1 [M+Na]+), and triscalix[4]arene 10 (m/z 3134.8 [M+Na]+).
X-ray crystallographic study
We cultivated single crystals of monocalix[4]arene partial cone conformers 3, 4, and biscalix[4]arene cone-partial cone conformer 6, which are suitable for crystallographic study. Using the (R)-BINOL moiety as an internal reference, the absolute configuration concerning inherent chirality was assigned as cR in all cases (Fig. 2).
Partial cone conformers 3 (Fig. 2) and 4 (Fig. 3) crystallize in P2(1)2(1)2(1) space group. In the solid state, the tilt angles measured between individual phenolic rings (from ring A to ring D) and the mean plane defined by the four bridging methylene carbons are 108.27°, 139.20°, 108.11°, and 82.65° for 3, and 98.37°, 138.27°, 96.55°, and 82.51° for 4. The dihedral angles of the BINOL moiety are 93.39° and 100.45° for 3 and 4, respectively. In the crystal structure of 3, the n-propyl group attached to the inverted ring D tilts inward and bisects the calix[4]arene cavity. Such a self-inclusion phenomenon also exists in its CDCl3 solution, as was evidenced by the pertinent terminal methyl signal appearing at remarkably high field (δ = 0.42 ppm) in its 1H NMR spectrum. In the crystal structure of 4, the n-propyl group attached to the inverted ring D tilts away from the calix cavity. By contrast, in its CDCl3 solution, the n-propyl group points toward the calix cavity, as was confirmed by its terminal methyl signal appearing at 0.38 ppm.
Biscalix[4]arene cone-partial cone conformer six crystallizes in P1 space group (Fig. 4). In the asymmetric unit, there are one biscalix[4]arene molecule and four solvent molecules including three CHCl3 and one CH3OH, which are positioned at one side of the partial cone calix[4]arene unit. In the partial cone calix[4]arene unit, the tilt angles measured between individual phenolic rings (from ring A to ring D) and the mean plane defined by the four bridging methylene carbons are 107.34°, 125.84°, 108.99°, and 72.95°, the dihedral angle of the attached BINOL moiety being 93.54°. The n-propyl group attached to the inverted ring D tilts inward the calix cavity in the solid state, as well as in the CDCl3 solution (δ = 0.36 ppm for the terminal methyl signal). In the cone calix[4]arene unit, the tilt angles measured between individual phenolic rings (from ring A’ to ring D’) and the mean plane defined by the four bridging methylene carbons are 98.29°, 138.51°, 79.52°, 138.39°, suggesting a slightly distorted pinched cone conformation. The dihedral angle of the BINOL moiety is 65.91°, distinctly smaller than that attached to the partial cone calix[4]arene unit. There are one intramolecular hydrogen bond (O⋯O, 2.922 Å) between the phenolic hydroxyl group (ring D’) and the proximal phenolic oxygen (ring A’), and one intermolecular hydrogen bond (O⋯O, 2.852 Å) between the phenolic oxygen on ring D and CH3OH. In biscalix[4]arene 6, the two calix[4]arene units are connected by a butylene spacer at the lower rims in such a manner that the two mean planes defined by the bridging methylene carbons of individual calix[4]arene units adopt a dihedral angle of 17.84°. In the top view the two calix[4]arene skeletons are neither staggered nor overlapped much.
The packing plot revealed the existence of a large number of intermolecular short contacts between CHCl3 molecules, CHCl3 and CH3OH, CHCl3 and BINOL moiety, CHCl3 and calixarene phenolic ring, BINOL moiety and calixarene phenolic ring, BINOL moiety and bridging methylene, BINOL moiety and the glycolic chain, t-butyl groups, t-butyl group and bridging methylene, etc. As a result, in the crystal lattice, there are alternate layers of biscalixarene molecules and solvent molecules in the ab plane, which is rarely seen in calixarene single crystals.
CD spectra
CD spectra of calix[4]arenes 1, 2, 3, biscalix[4]arenes 6, 7, and triscalix[4]arene 10 were measured in CH2Cl2. All the CD spectra resemble that of (R)-BINOL (Fig. 5) [29], with the strong negative cotton band characteristic of the 1B transition of the naphthol chromophore centering at ca. 238 nm, and the two weak positive cotton bands centering at 286 nm (1La transition), and 326 nm (1Lb transition). The CD spectra differ only slightly in intensity at the absorption extrema. Therefore, the axially chiral BINOL moieties in these calix[4]arenes and multicalix[4]arenes determine the shape of the cotton curves and the contribution of inherent chirality is overshadowed. Meanwhile, no clue to high-ordered structures of these chiral calix[4]arenes were observable in CH2Cl2 based on the CD spectra.
Conclusion
This work demonstrated the power of jointly using two stereoselective calix[4]arene-related Williamson etherification to produce multicalix[4]arenes with both axial chirality and inherent chirality. It is highly probable that by varying the R1 and R2 groups according to the need of molecular design, in conjunction with other highly efficient coupling/RCM/click reaction/condensation, the customized partial cone axially chiral/inherently chiral calix[4]arenes could be used in the construction of diverse novel chiral calixarene-based oligomeric/dendritic/polymeric architectures.
Experimental
Calix[4]arene 1
To a suspension of p-tert-butylcalix[4]arene (5.0 g, 7.7 mmol) in DMF (500 mL) was added NaH (60% in mineral oil, 2.16 g, 54.0 mmol) under N2 atmosphere. The reaction mixture was stirred at room temperature for 1.5 h, followed by the addition of (R)- 2,2′-bis(2-tosyloxy-1-ethyloxy)-1,1′-binaphthalene (6.31 g, 9.24 mmol). The reaction mixture was stirred at 60 °C for 1 day and the reaction was quenched by slow addition of methanol. After evaporation of the solvent under reduced pressure, ethyl acetate (EtOAc) was used to dissolve the residue. The insoluble p-tert-butylcalix[4]arene was removed by filtration. After evaporation of EtOAc, the residue was recrystallized with CH2Cl2/petroleum ether. The crude product was subjected to column chromatography on silica gel (petroleum ether/EtOAc 30:1) to give (R)-1 as a white solid. Yield 43% (3.27 g). Mp 202–204 °C. [α]25 D = +133° (c 0.30, CHCl3). 1H NMR (400 MHz, CDCl3, 25 °C): δ 8.99 (s, 1H), 8.53 (s, 1H), 8.06 (d, J = 9.2 Hz, 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.8 Hz, 1H), 7.43 (d, J = 8.8 Hz, 1H), 7.39–7.32 (m, 2H), 7.24–7.19 (m, 2H), 7.13 (d, J = 8.4 Hz, 1H), 7.07 (d, J = 2.4 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H), 6.99 (s, 2H), 6.96 (d, J = 2.4 Hz, 2H), 6.94 (d, J = 2.8 Hz, 1H), 6.90 (d, J = 2.4 Hz, 1H), 4.94–4.88 (m, 1H), 4.75–4.70 (m, 1H), 4.60–4.55 (m, 1H), 4.48 (d, J = 12.4 Hz, 1H), 4.47–4.41 (m, 1H), 4.25 (d, J = 13.6 Hz, 1H), 4.19 (d, J = 12.8 Hz, 1H), 4.17 (d, J = 13.2 Hz, 1H), 4.08–4.03 (m, 3H), 3.96–3.92 (m, 1H), 3.36 (d, J = 13.2 Hz, 1H), 3.34 (d, J = 13.6 Hz, 1H), 3.28 (d, J = 12.8 Hz, 2H), 1.19 (s, 9H), 1.18 (s, 9H), 1.15 (s, 9H), 1.08 (s, 9H). 13C NMR (100 MHz, CDCl3, 25 °C): δ 155.3, 154.1, 151.7, 150.7, 149.6, 148.8, 147.5, 146.6, 143.1, 142.5, 134.7, 134.6, 134.2, 133.6, 133.1, 132.9, 130.23, 130.15, 129.8, 129.6, 129.31, 128.88, 128.7, 128.3, 128.1, 127.9, 126.9, 126.7, 126.57, 126.55, 126.2, 125.9, 125.84, 125.78, 125.7, 125.6, 125.5, 124.2, 124.1, 121.31, 121.25, 118.1, 116.1, 74.7, 73.9, 69.3, 67.9, 34.3, 34.2, 34.1, 34.1, 33.1, 32.9, 32.7, 31.8, 31.7, 31.5, 31.4, 30.9. ESI-HRMS m/z calcd. for C68H74O6Na+ 1009.5378 [M+Na]+, found 1009.5354.
General procedure for the synthesis of 2, 6, and 10
To a solution of (R)-1 and Cs2CO3 in DMF was added an electrophile. The reaction mixture was stirred at 60 °C for a period of time. After evaporation of the solvent under reduced pressure, the residue was partitioned between CH2Cl2 and 3.6% aqueous HCl. The organic layer was dried with anhydrous MgSO4. Column chromatography on silica gel afforded 2, 6, or 10 as a white solid.
Calix[4]arene 2: (R)-1 (4.75 g, 4.81 mmol), Cs2CO3 (1.88 g, 5.77 mmol), DMF (150 mL), and ethyl 4-bromobutyrate (826 µL, 5.77 mol), 60 °C, 4 h. Column chromatography: petroleum ether/ethyl acetate = 25:1. Yield 68% (3.58 g). Mp 165–167 °C. [α]25 D = +102° (c 0.30, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.02 (d, J = 9.2 Hz, 1H), 7.94 (d, J = 8.8 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.68 (d, J = 9.2 Hz, 1H), 7.42 (d, J = 9.2 Hz, 1H), 7.36–7.28 (m, 2H), 7.22–7.17 (m, 3H), 7.12 (s, 2H), 7.10–7.08 (m, 2H), 7.04 (d, J = 2.4 Hz, 1H), 6.51 (d, J = 2.4 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 6.48 (s, 2H), 5.48 (s, 1H), 5.08–5.02 (m, 1H), 4.95–4.89 (m, 1H), 4.52–4.51 (m, 1H), 4.51 (d, J = 12.8 Hz, 1H), 4.36 (d, J = 12.8 Hz, 1H), 4.35–4.32 (m, 1H), 4.25 (d, J = 12.8 Hz, 1H), 4.14 (q, J = 7.2 Hz, 2H), 4.10 (d, J = 12.8 Hz, 1H), 4.06–3.98 (m, 3H), 3.91–3.87 (m, 2H), 3.77–3.73 (m, 1H), 3.30 (d, J = 13.2 Hz, 1H), 3.22 (d, J = 12.8 Hz, 1H), 3.18 (d, J = 13.6 Hz, 1H), 3.16 (d, J = 12.8 Hz, 1H), 2.71 (t, J = 7.6 Hz, 2H), 2.46–2.32 (m, 2H), 1.33 (s, 18H), 1.22 (t, J = 7.2 Hz, 3H), 0.81 (s, 9H), 0.80 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 173.2, 156.5, 154.02, 153.36, 153.0, 151.4, 150.9, 146.3, 145.70, 145.66, 141.8, 136.11, 136.05, 134.6, 134.1, 132.3, 131.91, 131.88, 131.6, 130.1, 130.0, 129.4, 129.31, 129.25, 128.3, 128.1, 126.6, 126.2, 126.0, 125.9, 125.8, 125.5, 125.3, 125.2, 125.0, 124.7, 123.8, 123.6, 121.0, 119.8, 118.8, 114.2, 75.5, 75.0, 71.4, 70.3, 68.4, 60.8, 34.4, 34.1, 33.9, 32.0, 31.9, 31.7, 31.6, 31.5, 31.2, 31.0, 26.0, 14.5. ESI-HRMS m/z calcd. for C74H84O8Na+ 1123.6058 [M+Na]+, found 1123.6044.
Biscalix[4]arene 6: (R)-1 (1.58 g, 1.60 mmol), Cs2CO3 (2.09 g, 6.40 mmol), DMF (100 mL), and calix[4]arene tosylate 5 (2.03 g, 1.60 mmol), 60 °C, 8 h. Column chromatography: petroleum ether/acetone = 50:1. Yield 47% (1.56 g). Mp 240–242 °C. [α]25 D = +76° (c 0.31, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.02 (d, 1H, J = 9.2 Hz), 7.94 (d, 1H, J = 8.8 Hz), 7.91 (d, 1H, J = 9.2 Hz), 7.90 (d, 1H, J = 7.6 Hz), 7.84–7.79 (m, 4H), 7.72 (d, 1H, J = 9.2 Hz), 7.43 (d, 1H, J = 8.8 Hz), 7.37 (d, 1H, J = 9.2 Hz), 7.34–7.31 (m, 3H), 7.28–7.05 (m, 13H), 7.03 (d, 1H, J = 2.0 Hz), 6.97–6.95 (m, 3H), 6.92 (d, 1H, J = 8.4 Hz), 6.89 (d, 1H, J = 2.4 Hz), 6.83 (d, 1H, J = 2.4 Hz), 6.74 (d, 1H, J = 2.0 Hz), 6.66 (d, 1H, J = 2.4 Hz), 6.57 (d, 1H, J = 2.4 Hz), 6.54 (d, 1H, J = 2.4 Hz), 6.50 (s, 2H), 5.69 (s, 1H), 5.16–5.10 (m, 1H), 5.00-4.94 (m, 1H), 4.56–4.49 (m, 3H), 4.44 (d, 1H, J = 12.8 Hz), 4.39–4.35 (m, 1H), 4.31 (d, 1H, J = 12.4 Hz), 4.20–3.96 (m, 12H), 3.90–3.56 (m, 9H), 3.53–3.47 (m, 1H), 3.42 (d, 1H, J = 13.6 Hz), 3.23 (d, 1H, J = 12.8 Hz), 3.20 (d, 1H, J = 12.4 Hz), 3.19 (d, 1H, J = 13.6 Hz), 3.03 (d, 1H, J = 12.4 Hz), 2.96 (d, 1H, J = 12.8 Hz), 2.67–2.57 (m, 2H), 2.35–2.28 (m, 2H), 2.23–2.13 (m, 2H), 1.35 (s, 9H), 1.33 (s, 9H), 1.26–1.21 (m, 2H), 1.24 (s, 9H), 1.12 (s, 9H), 1.08 (s, 9H), 1.02 (s, 9H), 0.83 (s, 9H), 0.81 (s, 9H), 0.36 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, CDCl3): δ 156.5, 155.7, 155.0, 154.0, 153.9, 153.8, 153.7, 153.4, 153.2, 152.7, 151.4, 150.9, 146.3, 145.8, 145.54, 145.52, 144.5, 144.1, 143.4, 141.7, 136.1, 135.4, 134.6, 134.5, 134.1, 133.4, 133.13, 133.08, 132.8, 132.7, 132.5, 132.0, 131.9, 131.7, 130.5, 130.1, 130.0, 129.7, 129.52, 129.49, 129.4, 129.3, 129.03, 128.97, 128.4, 128.10, 128.05, 128.0, 127.8, 127.6, 126.8, 126.7, 126.4, 126.3, 126.1, 126.0, 125.9, 125.8, 125.62, 125.55, 125.40, 125.35, 125.3, 125.1, 124.7, 124.0, 123.8, 123.54, 123.50, 122.8, 121.1, 120.5, 120.0, 119.4, 118.6, 114.1, 113.1, 76.4, 74.8, 73.74, 73.70, 72.7, 71.4, 71.2, 70.3, 69.9, 68.4, 67.2, 38.9, 38.6, 34.4, 34.2, 34.1, 34.00, 33.99, 33.93, 33.92, 33.85, 32.0, 31.9, 31.8, 31.5, 31.23, 31.21, 31.1, 27.2, 23.6, 10.2. ESI–MS m/z 2093.1 [M+Na]+. Anal. Calcd. for C143H160O12:C 82.94, H 7.79; found C 82.56, H 7.82.
Triscalix[4]arene 10: (R)-1 (108 mg, 0.11 mmol), Cs2CO3 (36 mg, 0.11 mmol), DMF (10 mL), and biscalix[4]arene tosylate 9 (210 mg, 0.09 mmol), 60 °C, 8 h. Column chromatography: petroleum ether/ethyl acetate = 25:1. Yield 35% (100 mg). Mp 249–251 °C. [α]25 D = +55° (c 0.26, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.94–7.77 (m, 12H), 7.62 (d, J = 8.8 Hz, 1H), 7.41–7.28 (m, 8H), 7.22–6.87 (m, 30H), 6.83 (d, J = 2.0 Hz, 1H), 6.75 (d, J = 1.6 Hz, 1H), 6.71 (d, J = 2.0 Hz, 1H), 6.61–6.60 (m, 2H), 6.500 (s, 1H), 6.495 (s, 1H), 6.46 (s, 1H), 6.45 (s, 1H), 5.63 (s, 1H), 5.10–5.04 (m, 1H), 4.93–4.88 (m, 1H), 4.56–4.45 (m, 3H), 4.35–3.49 (m, 46H), 3.31 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 3.14 (d, J = 13.2 Hz, 2H), 3.09 (d, J = 13.2 Hz, 1H), 3.06 (d, J = 12.8 Hz, 1H), 2.99 (d, J = 12.4 Hz, 1H), 2.89 (d, J = 12.4 Hz, 1H), 2.69–2.58 (m, 2H), 2.21–2.13 (m, 4H), 1.87–1.82 (m, 2H), 1.42–1.36 (m, 2H), 1.31 (s, 18H), 1.252 (s, 9H), 1.248 (s, 9H), 1.19 (s, 9H), 1.13 (s, 9H), 1.08 (s, 9H), 1.05 (s, 9H), 1.00 (s, 18H), 0.79 (s, 9H), 0.77 (s, 9H), 0.35 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3): δ 156.4, 155.70, 155.67, 154.9, 154.0, 153.9, 153.6, 153.44, 153.40, 153.35, 153.2, 152.79, 152.75, 151.1, 150.9, 146.3, 145.8, 145.7, 145.4, 144.5, 144.24, 144.18, 144.0, 143.4, 141.6, 136.1, 136.0, 135.6, 135.5, 135.4, 135.3, 134.54, 134.51, 134.47, 134.4, 134.0, 133.5, 133.2, 133.0, 132.94, 132.88, 132.8, 132.7, 132.49, 132.45, 132.4, 132.2, 131.9, 131.7, 131.63, 130.58, 130.5, 130.0, 129.9, 129.7, 129.6, 129.5, 129.42, 129.37, 129.2, 129.0, 128.7, 128.3, 128.1, 128.04, 128.00, 127.95, 127.6, 126.9, 126.72, 126.67, 126.5, 126.3, 126.2, 126.1, 126.0, 125.90, 125.86, 125.74, 125.69, 125.65, 125.62, 125.57, 125.5, 125.42, 125.37, 125.34, 125.25, 125.2, 125.0, 124.6, 124.5, 124.10, 124.06, 123.8, 123.5, 123.2, 123.0, 120.92, 120.87, 120.7, 119.5, 119.3, 119.2, 118.6, 114.0, 112.9, 112.8, 76.4, 74.7, 74.1, 73.9, 73.8, 72.8, 72.5, 71.8, 71.3, 71.11, 71.09, 70.3, 70.2, 69.9, 68.4, 67.3, 67.1, 38.9, 38.8, 38.6, 38.4, 34.4, 34.3, 34.2, 34.1, 34.02, 33.99, 33.94, 33.88, 33.8, 32.1, 32.04, 31.98, 31.9, 31.8, 31.71, 31.65, 31.59, 31.56, 31.5, 31.20, 31.17, 30.4, 30.3, 29.9, 27.4, 27.3, 27.2, 23.5, 22.9, 14.4, 10.1. ESI–MS m/z 3134.8 [M+Na]+. Anal. Calcd. for C215H240O18: C 82.97, H 7.77; found C 82.58, H 7.88.
General procedure for the synthesis of 3, and 7
To a suspension of 2, or 6, Cs2CO3 in DMF was added 20 equiv. of an electrophile. The reaction mixture was stirred at 60 °C for 1 day. After evaporation of the solvent under reduced pressure, the residue was partitioned between CH2Cl2 and 3.6% aqueous HCl. The organic layer was dried with anhydrous MgSO4. Column chromatography on silica gel afforded 3, or 7 as a white solid.
Calix[4]arene 3: calix[4]arene 2 (3.00 g, 2.73 mmol), Cs2CO3 (17.79 g, 54.60 mmol), DMF (10 mL), and nPrI (5.3 mL, 54.60 mmol). Column chromatography: petroleum ether/ethyl acetate = 25:1. Yield 90% (2.80 g). Mp 277–279 °C. [α]25 D = +27° (c 0.28, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 9.2 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 8.4 Hz, 1H), 7.47 (d, J = 8.8 Hz, 1H), 7.38 (d, J = 9.2 Hz, 1H), 7.35 (t, J = 7.2 Hz, 1H), 7.24–7.10 (m, 4H), 7.04 (d, J = 2.4 Hz, 1H), 7.00-6.92 (m, 4H), 6.89 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 2.4 Hz, 1H), 6.75 (d, J = 2.4 Hz, 1H), 6.67 (d, J = 2.4 Hz, 1H), 4.53–4.49 (m, 1H), 4.24 (q, J = 7.2 Hz, 2H), 4.22–4.16 (m, 2H), 4.13–4.03 (m, 2H), 4.05 (d, J = 12.0 Hz, 1H), 4.02 (d, J = 12.0 Hz, 1H), 3.92–3.84 (m, 3H), 3.74–3.48 (m, 6H), 3.06 (d, J = 12.4 Hz, 1H), 2.98 (d, J = 12.4 Hz, 1H), 2.80–2.68 (m, 2H), 2.64–2.50 (m, 2H), 2.33–2.25 (m, 2H), 1.38–1.29 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H), 1.26 (s, 9H), 1.13 (s, 9H), 1.08 (s, 9H), 1.04 (s, 9H), 0.42 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 173.3, 155.8, 155.0, 153.8, 153.7, 152.7, 145.6, 144.5, 144.2, 143.5, 135.4, 135.3, 134.6, 134.5, 133.3, 133.1, 133.0, 132.8, 132.7, 130.6, 129.7, 129.5, 129.1, 128.04, 127.99, 127.9, 127.5, 126.8, 126.6, 126.4, 126.1, 125.9, 125.7, 125.64, 125.56, 125.5, 125.4, 124.0, 123.5, 122.9, 120.69, 120.68, 119.4, 113.1, 73.8, 73.5, 72.7, 71.3, 69.8, 67.2, 60.8, 38.9, 38.5, 34.2, 34.01, 33.99, 33.96, 32.0, 31.8, 31.54, 31.49, 31.12, 31.05, 26.2, 23.6, 14.6, 10.2. ESI-HRMS m/z calcd. for C77H90O8Na+ 1165.6528 [M+Na]+, found 1165.6543.
Biscalix[4]arene 7: biscalix[4]arene 6 (495 mg, 0.24 mmol), Cs2CO3 (1.57 g, 4.80 mmol), DMF (5 mL), and ethyl 4-bromobutyrate (687 µL, 4.80 mmol). Column chromatography: petroleum ether/ethyl acetate = 25:1. Yield 64% (335 mg). Mp 213–215 °C. [α]25 D = +62° (c 0.29, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.8 Hz, 2H), 7.90–7.85 (m, 4H), 7.79 (d, J = 8.4 Hz, 2H), 7.40–7.28 (m, 8H), 7.22–6.90 (m, 20H), 6.75 (d, J = 1.6 Hz, 1H), 6.73 (d, J = 1.6 Hz, 1H), 6.71 (d, J = 2.0 Hz, 1H), 6.70 (d, J = 1.6 Hz, 1H), 4.57–4.47 (m, 2H), 4.28–3.47 (m, 32H), 3.09 (d, J = 12.0 Hz, 2H), 3.02–2.89 (m, 4H), 2.72–2.61 (m, 2H), 2.18 (s, 4H), 2.03–1.95 (m, 2H), 1.92 (t, J = 7.6 Hz, 2H), 1.77–1.70 (m, 2H), 1.29 (t, J = 7.2 Hz, 3H), 1.261 (s, 9H), 1.256 (s, 9H), 1.19 (s, 9H), 1.18 (s, 9H), 1.08 (s, 18H), 1.05 (s, 9H), 1.04 (s, 9H), 0.37 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 173.1, 172.9, 155.72, 155.65, 155.3, 155.0, 154.8, 153.93, 153.86, 153.81, 153.67, 153.66, 152.8, 152.7, 145.68, 145.65, 144.6, 144.5, 144.4, 144.2, 143.6, 143.4, 135.41, 135.36, 134.6, 134.5, 133.4, 133.3, 133.2, 133.1, 133.0, 132.93, 132.88, 132.8, 132.7, 130.6, 130.5, 129.7, 129.5, 129.04, 129.00, 128.1, 127.98, 127.95, 127.6, 127.5, 126.9, 126.7, 126.5, 126.4, 126.2, 125.9, 125.7, 125.6, 125.43, 125.39, 125.3, 124.10, 124.08, 123.5, 123.2, 123.0, 120.9, 120.6, 119.33, 119.29, 113.1, 112.8, 74.0, 73.9, 73.8, 72.8, 72.7, 71.2, 71.1, 70.2, 70.0, 67.3, 67.2, 66.9, 64.1, 60.7, 60.4, 38.9, 38.8, 38.7, 38.6, 34.2, 34.04, 34.00, 33.96, 32.10, 32.05, 31.79, 31.77, 31.5, 31.2, 30.8, 30.7, 27.3, 25.8, 24.3, 23.4, 14.5, 14.4, 10.1. ESI–MS m/z 2207.2 [M+Na]+. Anal. Calcd. for C149H170O14: C 81.91, H 7.84; found C 81.60, H 8.20.
General procedure for the synthesis of 4, and 8
To an ice cold solution of 3, or 7 in THF was added LiAlH4 in small portions. The reaction mixture was stirred under reflux for 4 h. After cooling to 0 °C, H2O (1 mL), 15% aqueous NaOH (1 mL), and H2O (3 mL) was sequentially added with an interval of 10 min between each addition. After filtration, the solvent was removed under reduced pressure. Column chromatography on silica gel afforded 4, or 8 as a white solid.
Calix[4]arene 4: calix[4]arene 3 (2.80 g, 2.45 mmol), LiAlH4 (558 mg, 14.70 mmol), THF (10 mL). Column chromatography: petroleum ether/ethyl acetate = 5:1. Yield 97% (2.64 g). Mp > 300 °C. [α]25 D = +44° (c 0.30, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.95 (d, 1 H, J = 9.2 Hz), 7.92 (d, 1 H, J = 9.6 Hz), 7.91 (d, 1 H, J = 8.0 Hz), 7.80 (d, 1 H, J = 8.0 Hz), 7.43 (d, J = 8.8 Hz, 1H), 7.39 (d, J = 9.2 Hz, 1H), 7.41–7.34 (m, 1H), 7.25–7.21 (m, 1H), 7.19–7.15 (m, 2H), 7.14–7.10 (m, 1H), 7.04 (d, J = 2.4 Hz, 1H), 7.01–6.96 (m, 3H), 6.92 (d, J = 8.8 Hz, 1H), 6.90 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 2.4 Hz, 1H), 6.75 (d, J = 2.4 Hz, 1H), 6.69 (d, J = 2.4 Hz, 1H), 4.56–4.52 (m, 1H), 4.25–4.17 (m, 2H), 4.15–4.03 (m, 2H), 4.08 (d, J = 12.4 Hz, 1H), 4.00 (d, J = 12.4 Hz, 1H), 3.94–3.82 (m, 5H), 3.75–3.56 (m, 5H), 3.45–3.42 (m, 1H), 3.05 (d, J = 12.4 Hz, 1H), 3.00 (d, J = 12.8 Hz, 1H), 2.73–2.61 (m, 2H), 2.11–1.96 (m, 2H), 1.86–1.75 (m, 2H), 1.41–1.27 (m, 2H), 1.26 (s, 9H), 1.13 (s, 9H), 1.08 (s, 9H), 1.05 (s, 9H), 0.38 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 155.7, 155.0, 154.0, 153.9, 153.7, 152.8, 145.6, 144.5, 144.1, 143.5, 135.4, 135.3, 134.60, 134.55, 133.4, 133.1, 133.0, 132.84, 132.78, 132.7, 130.7, 129.7, 129.6, 129.1, 128.1, 128.0, 127.8, 127.5, 126.9, 126.7, 126.4, 126.2, 125.8, 125.7, 125.64, 125.60, 125.43, 125.35, 124.2, 123.6, 123.2, 121.0, 119.4, 113.0, 74.1, 73.7, 72.8, 71.2, 70.2, 67.2, 63.1, 38.9, 38.6, 34.2, 34.01, 34.00, 33.96, 32.0, 31.8, 31.6, 31.5, 31.2, 31.1, 29.8, 27.3, 23.6, 10.2. ESI-HRMS m/z calcd. for C75H88O7Na+ 1123.6422 [M+Na]+, found 1123.6429.
Biscalix[4]arene 8: biscalix[4]arene 7 (335 mg, 0.15 mmol), LiAlH4 (58 mg, 1.53 mmol), THF (5 mL). Column chromatography: petroleum ether/ethyl acetate = 5:1. Yield 88% (287 mg). Mp 225–227 °C. [α]25 D = +72° (c 0.24, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.94–7.78 (m, 8H), 7.71–6.90 (28 H, m), 6.76 (d, J = 1.6 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.72 (d, J = 2.0 Hz, 1H), 6.70 (d, J = 2.0 Hz, 1H), 4.58–4.53 (m, 1H), 4.41–4.37 (m, 1H), 4.29–3.50 (m, 32H), 3.37–3.23 (m, 2H), 3.12 (d, J = 12.4 Hz, 1H), 3.10 (d, J = 12.4 Hz, 1H), 3.01 (d, J = 12.4 Hz, 1H), 2.89 (d, J = 12.4 Hz, 1H), 2.75–2.63 (m, 2H), 2.15 (s, 4H), 1.53–1.36 (m, 6H), 1.27 (s, 9H), 1.24 (s, 9H), 1.20 (s, 9H), 1.18 (s, 9H), 1.09 (s, 18H), 1.07 (s, 9H), 1.05 (s, 9H), 0.39 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 155.7, 155.5, 155.0, 153.93, 153.90, 153.8, 153.7, 153.6, 153.5, 152.81, 152.76, 145.7, 145.4, 144.5, 144.4, 144.2, 143.5, 143.4, 135.4, 135.24, 135.19, 134.6, 134.5, 133.5, 133.4, 133.2, 133.1, 132.99, 132.97, 132.94, 132.88, 132.7, 132.6, 132.4, 130.6, 130.4, 129.7, 129.6, 129.5, 129.2, 129.0, 128.1, 128.0, 127.5, 126.9, 126.72, 126.65, 126.5, 126.4, 126.22, 126.17, 126.0, 125.9, 125.8, 125.7, 125.54, 125.49, 125.34, 125.31, 124.1, 124.0, 123.6, 123.5, 123.3, 122.7, 120.9, 119.9, 119.5, 119.3, 113.9, 112.8, 74.99, 73.95, 73.8, 72.8, 72.6, 72.0, 71.5, 71.1, 70.2, 69.8, 67.7, 67.2, 62.9, 38.9, 38.8, 38.7, 38.5, 34.2, 34.03, 34.00, 32.1, 32.0, 31.8, 31.6, 31.5, 31.2, 29.6, 27.3, 27.2, 23.6, 10.1. ESI–MS m/z 2165.3 [M+Na]+. Anal. Calcd. for C147H168O13: C 82.39, H 7.90; found C 82.25, H 8.01.
General procedure for the synthesis of 5, and 9
To a solution of 4, or 8 in CH2Cl2 was added TsCl, NEt3, and DMAP. The reaction mixture was stirred at room temperature for 12 h, followed by removal of the solvent under reduced pressure. Column chromatography on silica gel afforded 5, or 9 as a white solid.
Calix[4]arene 5: calix[4]arene 4 (3.21 g, 2.83 mmol), TsCl (3.24 g, 17.00 mmol), NEt3 (2.39 mL, 17.00 mmol), DMAP (346 mg, 2.83 mmol), CH2Cl2 (20 mL). Column chromatography: petroleum ether/ethyl acetate = 15:1. Yield 94% (3.39 g). Mp 151–153 °C. [α]25 D = +36° (c 0.29, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.96 (d, J = 8.8 Hz, 1H), 7.93 (d, J = 9.6 Hz, 1H), 7.92 (d, J = 6.4 Hz, 1H), 7.84 (d, J = 8.4 Hz, 2H), 7.81 (d, J = 8.4 Hz, 1H), 7.39 (d, J = 9.2 Hz, 1H), 7.36 (d, J = 6.0 Hz, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.28–7.11 (m, 6H), 7.03 (d, J = 2.4 Hz, 1H), 6.99 (s, 2H), 6.97 (d, J = 9.2 Hz, 1H), 6.93 (d, J = 8.8 Hz, 1H), 6.89 (d, J = 2.4 Hz, 1H), 6.85 (d, J = 2.4 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.65 (d, J = 2.4 Hz, 1H), 4.55–4.51 (m, 1H), 4.21 (t, J = 6.4 Hz, 2H), 4.18–4.07 (m, 3H), 4.05–4.02 (m, 1H), 3.98 (d, J = 12.4 Hz, 1H), 3.95 (d, J = 12.0 Hz, 1H), 3.89–3.65 (m, 6H), 3.58–3.52 (m, 2H), 3.42–3.36 (m, 1H), 3.00 (d, J = 12.4 Hz, 1H), 2.99 (d, J = 12.4 Hz, 1H), 3.79–2.66 (m, 2H), 2.35 (s, 3H), 2.05–1.97 (m, 2H), 1.93–1.86 (m, 2H), 1.42–1.31 (m, 2H), 1.26 (s, 9H), 1.11 (s, 9H), 1.07 (s, 9H), 1.04 (s, 9H), 0.41 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 155.7, 155.0, 153.9, 153.8, 153.7, 152.8, 145.7, 145.0, 144.5, 144.2, 143.5, 135.4, 135.3, 134.6, 134.5, 133.4, 133.2, 133.0, 132.9, 132.8, 132.6, 132.5, 130.7, 130.2, 129.8, 129.6, 129.1, 128.1, 128.0, 127.8, 127.4, 126.9, 126.7, 126.4, 126.2, 125.9, 125.7, 125.59, 125.57, 125.5, 125.4, 124.1, 123.6, 123.1, 120.9, 119.4, 113.0, 73.8, 73.4, 72.7, 71.2, 70.4, 70.1, 67.1, 38.8, 38.6, 34.2, 34.00, 33.96, 33.9, 32.0, 31.8, 31.5, 31.13, 31.05, 26.9, 26.1, 23.6, 21.8, 10.2. ESI-HRMS m/z calcd. for C82H94O9SNa+ 1277.6511 [M+Na]+, found 1277.6517.
Biscalix[4]arene 9: calix[4]arene 8 (347 mg, 0.16 mmol), TsCl (186 mg, 0.98 mmol), NEt3 (137 µL, 0.98 mmol), DMAP (20 mg, 0.16 mmol), CH2Cl2 (10 mL). Column chromatography: petroleum ether/ethyl acetate = 8:1. Yield 77% (286 mg). Mp 204–206 °C. [α]25 D = +71° (c 0.24, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 8.8 Hz, 1H), 7.90 (d, J = 8.8 Hz, 1H), 7.88 (d, J = 8.8 Hz, 2H), 7.85 (d, J = 2.8 Hz, 1H), 7.83 (d, J = 2.4 Hz, 1H), 7.79–7.75 (m, 4H), 7.39 (d, J = 1.6 Hz, 1H), 7.37–7.30 (m, 7H), 7.26 (d, J = 2.4 Hz, 1H), 7.22–7.14 (m, 4H), 7.10–7.05 (m, 4H), 7.01 (d, J = 2.0 Hz, 1H), 6.99 (d, J = 2.0 Hz, 1H), 6.97–6.91 (m, 8H), 6.88 (d, J = 2.0 Hz, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.78 (d, J = 2.4 Hz, 1H), 6.74 (d, J = 2.4 Hz, 1H), 6.71 (d, J = 2.0 Hz, 1H), 6.66 (d, J = 2.0 Hz, 1H), 6.64 (d, J = 2.4 Hz, 1H), 4.57–4.52 (m, 1H), 4.44–4.40 (m, 1H), 4.27–3.85 (m, 22H), 3.83–3.70 (m, 7H), 3.64–3.48 (m, 7H), 3.26–3.16 (m, 2H), 3.09 (d, J = 12.0 Hz, 1H), 3.01 (d, J = 12.0 Hz, 1H), 3.00 (d, J = 12.8 Hz, 1H), 2.88 (d, J = 12.0 Hz, 1H), 2.70–2.59 (m, 2H), 2.41 (s, 3H), 2.19–2.11 (m, 4H), 1.40–1.33 (m, 2H), 1.25 (s, 9H), 1.23 (s, 9H), 1.18 (s, 9H), 1.15 (s, 9H), 1.07 (s, 9H), 1.05 (s, 9H), 1.03 (s, 9H), 0.98 (s, 9H), 0.36 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 155.7, 155.6, 155.0, 154.9, 153.93, 153.87, 153.71, 153.67, 153.6, 153.5, 152.8, 152.7, 145.7, 145.5, 144.9, 144.5, 144.4, 144.3, 144.2, 143.6, 143.4, 135.41, 135.38, 135.34, 135.28, 134.6, 134.51, 134.50, 133.4, 133.32, 133.31, 133.1, 133.03, 132.99, 132.96, 132.9, 132.7, 132.5, 132.4, 132.3, 130.6, 130.5, 130.0, 129.7, 129.6, 129.5, 129.1, 129.0, 128.1, 128.0, 127.5, 126.9, 126.71, 126.68, 126.5, 126.2, 126.0, 125.9, 125.8, 125.7, 125.64, 125.56, 125.51, 125.46, 125.32, 125.28, 124.13, 124.05, 123.6, 123.5, 123.3, 122.8, 120.9, 120.2, 119.4, 119.3, 113.4, 112.8, 74.0, 73.9, 73.8, 72.8, 72.6, 71.4, 71.3, 71.1, 70.3, 70.2, 69.8, 67.5, 67.2, 38.9, 38.8, 38.6, 38.4, 34.24, 34.21, 34.04, 34.01, 33.99, 33.94, 33.90, 32.1, 32.0, 31.8, 31.6, 31.5, 31.2, 27.29, 27.27, 26.8, 25.8, 23.6, 21.8, 10.1. ESI–MS m/z 2319.2 [M+Na]+. Anal. Calcd. for C154H174O15S: C 80.52, H 7.63; found C 80.26, H 7.68.
References
Sameni, S., Jeunesse, C., Matt, D., Harrowfield, J.: Chem. Soc. Rev. 38, 2117–2146 (2009)
Kim, J.S., Lee, S.Y., Yoon, J., Vicens, J.: Chem. Commun. 32 4791–4802 (2009)
Szemes, F., M.G.B. Drew, Beer, P.D.: Chem. Commun. 11, 1228–1229 (2002)
Lambert, J.B., Kang, S.-H., Ma, K., Liu, C., Condie, A.G.: J. Org. Chem. 74, 2527–2532 (2009)
Galán, H., Murillo, M.T., Quesada, R., Escudero-Adán, E.C., Benet-Bucholz, J., Prados, P., de Mendoza, J.: Chem. Commun. 46, 1044–1046 (2010)
Ben Othman, A., Lee, J.W., Wu, J.-S., Kim, J.S., Abidi, R., Thuéry, P., Strub, J.-M., Dorssaeler, A.V., Vicens, J.: J. Org. Chem. 72, 7634–7640 (2007)
Lalor, R., DiGesso, J.L., Mueller, A., Matthews, S. E.: Chem. Commun. 46, 4907–4909, (2007)
Lalor, R., Gunning, A.P., Morris, V.J., Matthews, S. E.: Chem. Commun. 46, 8665–8667 (2010)
Liu, J.-M., Zheng, Y.-S., Zheng, Q.-Y., Xie, J., Wang, M.-X., Huang, Z.-T.: Tetrahedron 58, 3729–3736 (2002)
Lhoták, P., Kawaguchi, M., Ikeda, A., S. Shinkai.: Tetrahedron 52, 12399–12408 (1996)
Chen, C.-F., Lu, L.-G., Hu, Z.-Q., Peng, X.-X., Huang, Z.-T.: Tetrahedron 61, 3853–3858 (2005)
Organo, V.G., Sgarlata, V., Firouzbakht, F., Rudkevich, D.M.: Chem. Eur. J. 13, 4014–4023 (2007)
Xu, H., Kinsel, G.R., Zhang, J., Li, M., Rudkevich, D.M.: Tetrahedron 59, 5837–5848 (2003)
Xu, H., Rudkevich, D.M.: Chem. -Eur. J. 10, 5432–5442 (2004)
Xu, H., Rudkevich, D. M.: J. Org. Chem. 69, 8609–8617 (2004)
Luo, J., Shen, L.-C., Chung W.-S.: J. Org. Chem. 75, 464–467 (2010)
Li, S.-Z., Shi, J., Yang, K., Luo, J.: Tetrahedron. 68, 8557–8563 (2012)
Li, S.-Z., Yang, K., Liu, H.-B., Xia, Y.-X., Zhu, R.-X., Luo, J., Wan, Q.: Tetrahedron Lett. 54, 5901–5906 (2013)
Wan, Y., Mitkin, O., Barnhurst, L., Kurchan, A., Kutateladze, A.: Org. Lett. 2, 3817–3819 (2000)
Yang Y., Swager T.M.: Macromolecules 39, 2013–2015 (2006)
Yang Y., Swager T.M.: Macromolecules 40, 7437–7440 (2007)
Lu Y., Xiao C., Yu Z., Zeng X., Ren Y., Li C.: J. Mater. Chem. 19, 8796–8802, (2009)
Ogoshi T., Nishida Y., Yamagishi T.-A., Nakamoto Y.: Polym. Chem. 1, 203–206 (2010)
Rambo B.M., Kim S.K., Kim J.S., Bielawski C.W., Sessler J.L.: Chem. Sci. 1, 716–722 (2010)
Wiktorowicz S., Aseyev V., Tenhu H.: Polym. Chem. 3, 1126–1129 (2012)
Wiktorowicz S., Tenhu H., Aseyev V.: Macromolecules 46, 6209–6216 (2013)
Szumna, A.: Chem. Soc. Rev. 39, 4274–4285 (2010)
Zhang, W.-Z., Ma, H., Xiang, G.-Y., Luo, J., Chung W.-S.: ChemistrySelect 1, 2486–2491 (2016)
Gudeangadi, P.G., Sakamoto, T., Shichibu, Y., Konishi, K., Nakano, T.: ACS Macro Lett. 4, 901–906 (2015)
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (NSFC) (Grant No. 20902029) and the Fundamental Research Funds for the Central Universities (HUST: No. 2014QN130) for financial support. The Analytical and Testing Center of Huazhong University of Science and Technology is thanked for measurement service.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, H., Zhang, WZ., Luo, J. et al. Synthesis of a linearly linked triscalixarene consisting of calix[4]arene units with combined axial chirality and inherent chirality. J Incl Phenom Macrocycl Chem 89, 91–104 (2017). https://doi.org/10.1007/s10847-017-0722-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0722-8