Abstract
In this study, we aimed to investigate the inclusion complexes of the poorly water soluble flavonol, quercetin (QR) and its glycosides quercitrin (QRC) and rutin (RT), formed with β-cyclodextrin (β-CD), 2-hydroxyethyl-β-cyclodextrin (HE-β-CD), 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), and methyl-β-cyclodextrin (M-β-CD) by using UV–Vis spectrophotometric and spectrofluorometric techniques. The formation constants (K f ) of 1:1 stoichiometric inclusion complexes were calculated from Benesi–Hildebrand equation using fluorescence spectroscopic data. Maximum inclusion ability was measured in the case of M-β-CD for rutin and quercitrin. Among CDs, HP-β-CD was most effective for complexing quercetin. The glycosylation of flavonoids considerably affects the binding process. The formation constants of flavonoid-CD complexes decrease after glycosylation. The influence of complexation of quercetin, rutin and quercitrin with native and modified β-CDs on their trolox equivalent antioxidant capacity (TEAC) was studied by the Cupric Ion Reducing Antioxidant Capacity method. It was found that the complexed polyphenols with CDs were much stronger antioxidants than free forms. Antioxidant capacity of HP-β-CD-complexed QR (compared to that of pure QR) was increased by 7.18 % in methanolic solution. Increase in TEAC for M-β-CD-complexed RT and M-β-CD-complexed QRC were measured as 4.30 and 14.8 %, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Native or unmodified cyclodextrins (CDs) are a family of oligosaccharide compounds consisting of 6, 7 or 8 of α-(1-4)-linked d-glycose residues, called α-, β- and γ-CDs, respectively. CDs have hydrophobic inner cavity, and hydrophilic surrounding walls [1]. This hydrophobic cavity allows CDs to form host–guest inclusion complexes with several biological molecular substrates, organic and inorganic guest molecules [2]. In aqueous media, CDs can generate inclusion complexes with lipophilic guest molecules set in the hydrophobic inner cavity [3]. Formation of host–guest inclusion complexes with CDs can relatively enhance the solubility, bioavailability and stability of the guest molecule [1]. The non-polar property of the inner cavity of cyclodextrin allows solubilizing of non-polar solutes, while the polarity of its external part allows water solubility increment of the guest [4]. Various modified β-CD derivatives have been synthesized to enhance their solubility in water, binding ability and molecular selectivity, such as 2-hydroxyethyl-β-cyclodextrin (HE-β-CD), 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), methyl-β-cyclodextrin (M-β-CD), heptakis (2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD), and sulfobutylether-β-cyclodextrin (SBE-β-CD) [5].
Flavonoids are the largest polyphenol family which are found in fruits, vegetables and several food sources. They are useful bioactive substances with anti-tumor, anti-cancer and therapeutic effects [6]. Quercetin is the major flavonoid found in vegetables and fruits, especially presented in glycoside form, as quercitrin, rutin or isoquercitrin. These polyphenols are relatively polar compounds owing to their structures, but they show little solubility in water [7, 8]. Several articles have been recently published on the complexation of flavonoids with CDs, such as kaempferol [5, 9, 10] quercetin [9–17], myricetin [5, 9, 14] galangin [5, 10], 3-hydroxy-flavone [18], morin [18, 19], rutin [11, 17, 20–24], isoquercitrin [25], luteolin [26], chrysin [27], hesperetin, hesperidin [28] and catechin [29]. Fluorescence spectroscopy is widely used to determine the formation constants of corresponding inclusion complexes because of its high sensitivity and selectivity compared to other assays such as proton magnetic resonance (1H-NMR), differential scanning calorimetry, X-ray powder diffractometry, UV–visible spectroscopy (UV–vis), Fourier transform-infrared spectroscopy assays [11, 22, 26, 29–32].
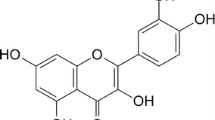
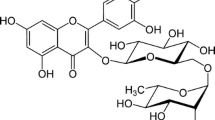
In our previous study, we investigated the antioxidant capacity of inclusion complexes of rosmarinic acid in the presence of α-, β- and modified β-cyclodextrins [32]. In the present study, the inclusion complexation between β-CD, HE-β-CD, HP-β-CD, and M-β-CD with quercetin and its glycosides quercitrin (quercetin-3-O-rhamnoside) and rutin (quercetin-3-O-rutinoside) was analyzed (Fig. 1). The inclusion complexation behaviour after glycosylation was determined using UV–vis absorption and fluorescence techniques. The formation constants (K f ) of inclusion complexes were estimated using fluorimetric data. The related alteration in antioxidant capacity of these compounds was studied by the CUPRAC assay.
Materials and methods
Chemicals and instruments
Quercetin, rutin, quercitrin, neocuproine (2,9-Dimethyl-1,10-phenanthroline) (Nc), β-CD, M-β-CD, HE-β-CD, HP-β-CD, methanol (MeOH), NaH2PO4 and Na2HPO4 were purchased from Sigma Chemical Co. (Steinheim, Germany). Copper(II) chloride dihydrate, ethyl alcohol absolute (EtOH), ammonium acetate (NH4Ac) were supplied from Merck (Darmstadt, Germany).
Varian Cary Bio 100 UV–vis spectrophotometer (Mulgrave, Victoria, Australia) using quartz quvettes with 10 nm optical path was used for UV–vis spectrophotometric measurements. The fluorescence measurements were recorded by using Agilent Cary Eclipse Fluorescence spectrometer (Mulgrave, Victoria, Australia) with a 1 nm spectral resolution.
Preparation of solutions
CUPRAC assay reagent solution
Ten mM CuCl2 solution and 1 M pH = 7 ammonium acetate solution were prepared in distilled water; 7.5 mM neocuproine solution was prepared in absolute ethanol.
Inclusion complex solution
Aqueous inclusion complex samples were prepared according to the following literature [25, 30, 31]. Half milliliter of 1 mM methanolic antioxidant solution, different volumes (0.5, 1, 2, 4, 8 mL) of 10 mM freshly prepared CDs solution, and 1 mL of 0.5 M phosphate buffer solution (pH 7.0) were added to each test tube, and diluted to 10 mL final volume with distilled water. These solutions were shaken and incubated for 30 min at 25 ± 1 °C, and protected from daylight before analysis. Methanolic stock solutions of antioxidants were kept in the dark at +4 °C until use.
Spectrometric measurements of free and complexed flavonoids
The absorption spectra of quercetin and its glycoside solutions (50 μM) at pH = 7.0 in each of CD solutions within a concentration range of 0-8 mM were monitored between 220 and 400 nm at 25 ± 1 °C. UV–vis absorption of the free form of flavonoids and their complexes (in the presence of 8 mM CD) were monitored in the spectral range of interest. UV–vis absorption of each CD solution was also monitored to detect any absorption (data not shown).
The fluorescence spectra of QR, RT and QRC in the presence or absence of CDs at pH = 7.0 were monitored. Slit widths were set to 10 nm for excitation and emission. For all compounds, emission spectra were recorded in the 480–580 nm interval with excitation at 440 nm. CD solutions were also recorded alone by using the specified conditions to detect any intensity.
The intensity of fluorescence at any wavelength (F) can be related to the initial concentration of CDs (Eq. 1):
where F max is the intensity of fluorescence when all polyphenol has been complexed with CDs (ΔF = F max − F 0) and F 0 is the fluorescence intensity of free polyphenol. The stoichiometry and the formation constants (K f ) of host–guest inclusion complexes were calculated using the modified Benesi–Hildebrand equation from fluorescence experimental data [33].
CUPRAC assay measurements
The cupric ion reducing antioxidant capacity assay developed by Apak et al. is based on the reduction of a chromogenic reagent, cupric neocuproine complex (Cu(II)-Nc), by antioxidant compounds to the cuprous chelate (Cu(I)-Nc) [34]. Trolox equivalent antioxidant capacities (TEAC) of inclusion complexes were determined according to the CUPRAC assay by mixing 1 mL Cu(II) aqueous solution, 1 mL ethanolic neocuproine solution, 2 mL NH4Ac aqueous solution, inclusion complex sample solution or flavonoid standard solution (x = 0.2–1 mL) and (1–x) mL H2O. Final volume was set to 5 mL. Absorbances were recorded at 450 nm after 30 min against a reagent blank. The molar absorptivities of free and complexed flavonoids according to the CUPRAC method were calculated from the slope of the related calibration line. The TEAC of these flavonoids in methanol solvent medium were found by dividing the molar absorptivity (ε) of free and complexed species to that of trolox (ε TR being 1.58 × 104 Lmol−1 cm−1 under corresponding conditions).
Results and discussion
UV spectroscopy measurements
The UV–visible absorption spectra of related flavonoids in the presence of different CD species at 8 mM final concentration were recorded (Fig. 2a–c). The electronic spectrum of quercetin shows an intensive band at λmax = 270 nm and less intensive band at λmax = 377 nm, along with a shoulder at 256 nm in an aqueous phosphate buffer solution (pH 7.0) containing 2 % (v:v) methanol. Absorption band shifted towards longer wavelength (from 377 nm to 382 nm) in the presence of CDs (Fig. 2a). An absorbance enhancement and bathochromic shift at the wavelength of 256 nm were recorded. The absorbances measured at 256 nm in the spectra of QR-HE-β-CD, QR-HP-β-CD and QR-M-β-CD complexes increased dramatically. The absorption maxima of rutin appears at 270 and 367 nm and rutin gives a shoulder at 259 nm due to complex formation. The absorption maxima blue shifted from 270 to 263 nm and from 367 to 364 nm. Absorption band at wavelength 259 nm disappeared in the presence of M-β-CD and QR-M-β-CD complex gives a wide absorption band at 263 nm (Fig. 2b). Quercitrin has two absorption bands at 265 nm and 353 nm. In the presence of β-CD, a shoulder at 258 nm was produced while the absorbance was decreased. A blue shift (2–7 nm) was monitored in the presence of modified β-CDs (Fig. 2c). A decrease in absorbance was observed for rutin and quercitrin due to complexation, as similar to the resveratrol-HP-β-CD complexation [35] and rosmarinic acid-CD complexation [32] studies unlike quercetin. A bathochromic or hypsochromic shift in spectrum shows that the main chromophore group of polyphenol is surrounded by a more hydrophobic environment due to the formation of inclusion complexes [18]. Calabro et al. indicated that the UV spectrum of rutin-β-CD was quite different than that of free rutin [21]. In this study, both absorption bands in the UV spectrum of rutin shifted to longer wavelengths (from 256 to 260 nm and from 351 to 355 nm), and the intensity of these maxima decreased from 1.44 to 1.24 due to inclusion complexation. The red shifts observed in the presence of β-CD shows the displacement of chromophores to a more hydrophobic environment upon host binding. Gornas et al. investigated spectral changes in chlorogenic acid and caffeic acid due to complexation with β-CD [30]. Spectroscopic studies indicated that upon the addition of β-CD, the maximum absorption peak shifted to a longer wavelength and the absorbance decreased with increasing CD concentration, suggesting that the chromophore of chlorogenic or caffeic acid is incorporated in the hydrophobic environment of β-CD [30].
(a) UV–vis spectra of 50 μM aqueous solution of quercetin free and with 8 mM different CDs (25 °C, pH = 7.0). (b) UV–vis spectra of 50 μM aqueous solution of rutin free and with 8 mM different CDs (25 °C, pH = 7.0). (c) UV–vis spectra of 50 μM aqueous solution of quercitrin free and with 8 mM different CDs (25 °C, pH = 7.0)
Fluorimetric measurements
The fluorescence spectra of aqueous solutions of QR, RT and QRC recorded in the presence of β-CD and derivatives are shown in Fig. 3a–c). The maximum wavelength of excitation of all three compounds was set to 440 nm. In the case of QR, emission maximum was found at 527 nm. The fluorescence intensity of quercetin was significantly enhanced with increasing of CD concentration. The maximum emission wavelength of QR was shifted from 527 to 540 nm in the presence of M-β-CD solution (Fig. 3a). The emission maximum of RT was 520 nm and shifted only 2-3 nm due to complex formation (Fig. 3b). The emission maximum of QRC, originally seen at 523 nm, shifted only 2–3 nm due to complex formation (Fig. 3c). When fluorescent molecules in aqueous solution are included in the CD cavity, a bathochromic shift in the emission spectra with an enhancement in the fluorescence intensity of polyphenols was recorded due to the inclusion of a part or all of the fluorescent molecule into the hydrophobic cavity and the resulting change of its surrounding environment [30, 36]. Fluorescence emission spectra of 50 μM aqueous solutions of quercetin and its glycosides having variable M-β-CD concentrations between 0 and 8 mM at pH 7.0 were given in Fig. 4a–c. An enhancement in fluorescence intensity shows stronger interaction of QR, RT and QRC with the hydrophobic interior cavity of the cyclodextrin than with the aqueous phase. It seems that the interactions between phenyl hydroxyl groups and the interior part of CD makes the complex more rigid. Smulevich et al. showed that the fluorescence spectra of 1,8-dihydroxyanthraquinone in the presence of native CDs (α-, β- and γ-CD) was changed by the effect of inclusion [37]. A red shift from 571 to 595 nm of the emission band was observed along with an increase in the intensity of emission when 1, 8- dihydroxyanthraquinone complexed with γ-CD by hydrogen bond formation. The fluorescence emission spectra confirmed that the complex formed with either β-CD or γ-CD was more soluble than 1,8-dihydroxyanthraquinone and that the binding of the chromophore was in monomeric form with β-CD and γ-CD. Due to its larger cavity size, the change in spectral data was maximum in γ-CD. The spectral change was less in β-CD, and no inclusion could be observed in case of α-CD [37].
Emission spectra of 50 μM (a) quercetin (b) rutin (c) quercitrin aqueous solutions having variable M-β-CD concentrations between 0 and 8 mM at pH 7.0 (T: 25 ± 1 °C) and the double reciprocal plot of same data using Eq. (1)
Determination of formation constants of inclusion complexes
The fluorescence enhancement of QR and its glycosides with increasing the concentration of β-CD and derivatives was used to quantify the interaction between polyphenols and CDs. The formation constant is used to determine the binding strength of CD molecules and the alteration in the physicochemical properties of the polyphenolic compounds [38]. The following equilibrium is produced in the fluorescence enhancement with increasing concentrations of CD [14]:

Free flavonoid (FL) and complexed flavonoid (FL-CD) were given as FL* and [FL*-CD] in the excited state. Only fluorescent species are presented in the excited state. Formation constant (K f ) and stoichiometric ratios can be estimated from fluorescence data by the modified Benesi–Hildebrand equation and double-reciprocal plots [32]. Fluorescence intensities of related polyphenols at λem versus incremental M-β-CD concentrations in aqueous solutions at pH 7.0 (T: 25 ± 1 °C) and the double reciprocal plot of same data using Eq. (1) are shown in Fig. 4 (a-c). The reciprocal plots of 1/(F-F0) versus 1/[CD] showed good linearity, indicating that the inclusion complex has a 1:1 stoichiometry. The linear plots of Fig. 4 can be used to determine K f values by dividing the intercepts by the slopes (Table 1). It is noted that the inclusion capacity of cyclodextrins for QR exhibits the order QR-HP-β-CD (4930 ± 525 M−1) > QR-M-β-CD (3560 ± 420 M−1) > QR-HE-β-CD (3085 ± 315 M−1) > QR-β-CD (480 ± 44 M−1). K f value of QR-β-CD complex is in agreement with that of Bergonzi et al. [10] and Jullian et al. [13]. Liu et al. [15] reported the same order for the stability constants of QR-HP-β-CD (3616 ± 28 M−1, 25 °C) and QR-M-β-CD (1935 ± 14 M−1, 25 °C) at pH 7.40 using double-reciprocal plot data. They investigated the effect of medium temperature and pH on inclusion complexation. The results showed a decrease in stability constants with increasing temperature at constant pH. Hydroxyl groups of QR molecules are dissociated into phenolate anions which have a weak binding capacity toward CD molecules by the increment of pH. Therefore, the stability constants are much smaller at physiological pH compared to those at acidic pH. Solubility of QR also increases in these CD solutions with an increase in temperature [16].
In the case of rutin, K f value of RT-M-β-CD (838 ± 94 M−1) was found to be the highest value. K f values of RT-HP-β-CD (562 ± 42 M−1) and RT-HE-β-CD (292 ± 45 M−1) are larger than that of RT-β-CD (122 ± 33 M−1) at 25 ± 1 °C, in accordance with the findings of Calabro et al. [21]. Şamlı et al. [24] found the stability constant of rutin-beta cyclodextrin inclusion complex prepared via co-precipitation method as 262 M−1 at 25 °C calculated by phase solubility analysis. The inclusion capacity of cyclodextrins with quercitrin are given in the following order: QRC-M-β-CD (995 ± 88 M−1) > QRC-HP-β-CD (672 ± 54 M−1) > QRC-HE-β-CD (425 ± 25 M−1) > QRC-β-CD (145 ± 30 M−1) which may indicate that the substituent groups of the chemically synthesized β-CDs allow stronger interactions with these polyphenols than the native β-CD. Methyl or hydroxypropyl substitutions may enlarge the opening of β-CD and demolish the strong intramolecular hydrogen bond network, which allows guest molecules fit into the modified CD cavity more easily and give higher formation constants [39]. The major factor providing complex formation is the release of water molecules from the cavity. In contrast to entropy-driven classical hydrophobic interactions, cyclodextrin complexation is enthalpically-driven process [40]. Water molecules are displaced by more hydrophobic guest molecules present in the solution to attain an apolar–apolar association and decrease of cyclodextrin ring strain resulting in a more stable lower energy state [4]. Strong hydrogen bonds are formed due to complexation, and it increases the hydroxyl bond polarity and decreases the bond dissociation energy [27]. Comparing the K f values of complexes, K f of quercetin is much higher than of its glycosides. For example, K f of QR-HP-β-CD complex is nearly 10-fold higher than those of QRC-HP-β-CD and RT-HP-β-CD complexes. This implies that glycosylation of flavonoids significantly affects the binding process and the formation constants of complexes between flavonoids and CDs decrease after glycosylation, possibly due to weakened H-bonding in the resulting complex. In the study of Yu et al. [20], complexation of quercetin glycosides (quercitrin, rutin and hyperoside) with dimethyl-β-cyclodextrin (DM-β-CD) was investigated by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. The binding constants for all complexes were calculated in the order: DM-β-CD:quercitrin > DM-β-CD:rutin > DM-β-CD:hyperoside. Quercitrin was found to be more favorable for binding with the host molecule than rutin, which is in agreement with our results. They suggested that the binding ability of the guests having the same aglycone and differing only in the glycoside moieties may be affected by the sterical hindrance of the glycoside moiety to the host. Intramolecular interactions of the guest molecules can affect the stability of inclusion complexes. If the interaction between the glycoside moiety and B-ring of the guest is weak, the complex is more stable [20].
According to the data given in Table 1., it was seen that the binding affinity was influenced by the position of glycoside unit and the number of glycosyl groups in the flavonoid structure. The CH3 group of the L-rhamnoside unit of quercitrin extrudes into the space opposite to the C6-O-glu- rhamnoside unit of rutin; the distance between the glycoside moiety and the B-ring is lengthened which results in weaker intramolecular interactions between the terminal L-rhamnoside unit and the B-ring in quercitrin than those in rutin [20]. This is why the stability constants of quercitrin in the presence of CDs are higher than those of rutin. Cao et al. studied the protein binding ability of baicalein and quercetin, and their glycosides, baicalin and quercitrin, by utilizing fluorescence measurements [40]. They stated that the glycosylation of flavonoids decreases the binding affinity with protein and after glycosylation a steric hindrance may take place which weakenes the binding affinity. Moreover, glycosylation may also decrease the hydrophobicity of flavonoids [40, 41]. Xiao and coworkers discussed the influence of glycosylation of flavonoids on the binding affinities for bovine serum albumin (BSA). According to their data, glycosylation of flavonoids can reduce the binding constants for BSA by 1–3 fold of magnitude depending on the conjugation site and the class of sugar [41]. Dangles et al. measured the binding affinities of the BSA complexes of quercetin, rutin and isoquercitrin [42]. The binding constant of rutin-BSA complex was found 11.97 fold lower and the binding constant of isoquercitrin-BSA 7.10 fold lower than that of the quercetin—BSA complex. The presence of a sugar moiety at the C-3 position of quercetin obviously weakens the quercetin—BSA affinity [42, 43].
Antioxidant capacity analysis
Antioxidant capacities of polyphenols in the absence and presence of CDs were measured utilizing the CUPRAC method. This assay has many superiorities over other similar (ET)—based TAC assays. According to this assay, antioxidant capacity can be monitored by utilizing the reduction of a chromogenic copper (II)-neocuproine complex to the bis(neocuproine)copper(I) chelate with maximum absorption at 450 nm. The degree of colour change is correlated to the concentration of antioxidants in the sample [44]. The oxidation reaction of reactive Ar(OH)n groups of polyphenolic antioxidants to the corresponding quinone in the presence of copper(II)-neocuproine complex is shown in Eq. 2.
In this reaction, chromogenic Cu(II)-Nc is reduced to the orange-yellow colored Cu(Nc) +2 chelate showing maximum light absorption at 450 nm. The liberated protons are buffered in ammonium-acetate medium. Although the concentration of Cu2+ ions is in stoichiometric excess of that of neocuproine in the CUPRAC reagent for driving the redox equilibrium reaction given in Eq. 2 to the right, the main oxidant is the Cu(Nc) 2+2 species and not just Cu2+. This is because the standard redox potential of the Cu(II/I)-neocuproine complex is 0.6 V, much higher that of the Cu2+/Cu+ couple (0.17 V) [45]. In consequence, polyphenols are oxidized much more rapidly and effectively with Cu(II)-Nc complex than with Cu2+, and the amount of redox reaction product [i.e. Cu(I)-Nc chelate] is equivalent to that of reacted Cu(II)-Nc [46].
The antioxidant capacities of QR and RT and QRC in CD-complexed forms according to the CUPRAC assay are given as trolox equivalents in Fig. 5 as follows: TEACQR-HP-β-CD > TEACQR-M-β-CD > TEACQR-HE-β-CD > TEACQR-β-CD > TEACQR and TEACRT-M-β-CD = TEACRT-HE-β-CD > TEACRT-HP-β-CD > TEACRT-β-CD > TEACRT and TEACQRC-M-β-CD > TEACQRC-HP-β-CD > TEACQRC-HE-β-CD > TEACQRC-β-CD > TEACQRC. It was found that flavonoids in encapsulated form are stronger antioxidants than in their free forms. The relative increase in TAC of HP-β-CD-complexed QR was found 7.18 % in methanolic solution compared to that of pure QR, for M-β-CD-complexed RT increase in TAC was 4.30 %, and for M-β-CD-complexed QRC increase in TAC was 14.8 %. Stražišar and coworkers showed that o- and m-coumaric acid exhibited higher antioxidant activity upon inclusion into β-CD cavity [47]. Alvarez-Parilla et al. [11] also demonstrated 7–14 % increment in antioxidant capacity of complexed rutin and chlorogenic acid with β-CD according to the FRAP assay. The authors stated that the redox behaviour of the polyphenol is changed due to the access of o-dihydroxyphenol moiety of the polyphenols into the hydrophobic β-CD cavity and possibly to the stabilization of the semiquinone-radical oxidation product, causing antioxidant capacity enhancement. Experimental data of this study are in aggrement with that of Jullian et al. [13] who stated that radical scavenging capability of quercetin-cyclodextrin inclusion complexes compared to free form was found to be higher. TAC of QR and its glycosides upon CD complexation was slightly increased, as the o-dihydroxyphenyl substitutions enter a more hydrophobic internal cavity of CDs, and this relatively nonpolar environment would be expected to increase resonance stabilization of the aryloxy radicals formed from 1-e oxidation of the o-catechol moiety of QR by enhanced intramolecular H-bonding [48]. The reduction potential for the redox couple formed from 1-e oxidized and reduced forms of polyphenols would decrease, making them stronger antioxidants. These findings are in agreement with that of Calabro et al. [18] who demonstrated β-CD complexes of 3-hydroxyflavone, morin, and quercetin showed the most efficient inhibition in lipid peroxidation assays. Liu et al. [15] investigated the comparison of antioxidant activity of QR, QR-HP-β-CD, QR-SBE-β-CD and QR-M-β-CD complexes as measured by DPPH· radical scavenging assay. According to the EC50 values, the complexed forms of quercetin are more effective than free forms. They emphasized that this increase in antioxidant activity may be attributed to the enhancement of hydrogen atom donating capacity caused by complexation.
Conclusion
In this study, the inclusion complexation between quercetin and its glycosides with β-CD and its derivatized species in aqueous solution was investigated. The limited water solubility of these antioxidants could be overcome by this process. The inclusion process altered the spectral features of quercetin, rutin and quercitrin, resulting in a significant change in UV absorption and fluorescence spectra. Bathochromic or hypsochromic shifts in the presence of CDs indicate that QR and its glycosides are capable of forming inclusion complexes. Inclusion formation constants of complexes in the stoichiometry of 1:1 were calculated by plotting the fluorescence intensities as a function of total CD concentration. Complex formation constants increased gradually from β-CD to the modified β-CD at 25 °C. These data suggest that inclusion in modified β-CDs were more effective than in native cyclodextrins. Formation constants of QR-CD complexes were found nearly four to ten fold higher than that of its glycosides, confirming that inclusion complexation process was influenced by the glycosylation of flavonoids. The presence of glycosyl groups together with the number and position of glycosyls probably affects this process owing to the decrease of binding affinity of flavonoids to the CD molecules. The effect of complexation on antioxidant capacity was examined by comparing the TEAC values of free and complexed forms of flavonoids using one way ANOVA test (SPSS 16.0 for Windows). Enhancement in antioxidant capacity of QR, RT and QRC upon inclusion complex formation with CDs was considered to be statistically significant (p < 0.05). QR-HP-β-CD showed the highest antioxidant capacity due to the more hydrophobic cavity of HP-β-CD compared to that of β-CD [49], possibly due to increased stabilization of semiquinone-radical products in the cavity by H-bonding. This enhancement in antioxidant capacity is believed to be important in food preservation possibly by increasing the shelf-life of plant foods.
References
Del Valle, E.E.M.: Cyclodextrins and their uses: a review. Process. Biochem. 39, 1033–1046 (2004)
Cai, Y., Gaffney, S.H., Lilley, T.H., Magnolato, D., Martin, R., Spencer, C.M., Haslam, E.: Polyphenol interactions, Part 4: model studies with caffeine and cyclodextrins. J. Chem. Soc. Perkin Trans. 2, 2197–2209 (1990)
Loftsson, T., Duchêne, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329, 1–11 (2007)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Kahn, A.R., Forgo, P., Stine, K.J., D´ Souza, V.T.: Methods for selective modifications of cyclodextrins. Chem. Rev. 98, 1977–1996 (1998)
Bennick, A.: Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 13, 184–196 (2002)
Di Carlo, G., Mascolo, N., Izzo, A., Papasso, F.: Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 65, 337–353 (1999)
Harbourne, J.B.: The Flavonoids: Advances in Research Since 1986. Chapman & Hall, London (1994)
Mercader-Ros, M.T., Lucas-Abellán, C., Fortea, M.I., Gabaldón, J.A., Núñez-Delicado, E.: Effect of HP-β-cyclodextrins complexation on the antioxidant activity of flavonols. Food Chem. 118, 769–773 (2010)
Bergonzi, M.C., Bilia, A.R., Di Bari, L., Mazzi, G., Vincieri, F.F.: Studies on the interactions between some flavonols and cyclodextrins. Bioorg. Med. Chem. Lett. 17, 5744–5748 (2007)
Alvarez-Parrilla, E., De La Rosa, L.A., Torres-Rivas, F., Rodrigo-Garcia, J., Gonzalez-Aguilar, G.: Complexation of apple antioxidants: chlorogenic acid, quercetin and rutin by β-Cyclodextrin (β-CD). J Incl. Phenom. Macro. 53, 121–129 (2005)
Kim, H., Choi, J., Jung, S.: Inclusion complexes of modified cyclodextrins with some flavonols. J. Incl. Phenom. Macro. 64, 43–47 (2009)
Jullian, C., Moyano, L., Yañez, C., Olea-Azar, C.: Complexation of quercetin with three kinds of cyclodextrins: an antioxidant study. Spectrochim. Acta A 67, 230–234 (2007)
Lucas-Abellán, C., Fortea, M.I., Gabaldón, J.A., Núñez-Delicado, E.: Encapsulation of quercetin and myricetin in cyclodextrins at acidic pH. J. Agric. Food Chem. 56, 255–259 (2008)
Liu, M., Dong, L., Chen, A., Zheng, Y., Sun, D., Wang, X., Wang, B.: Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: spectroscopic study and antioxidant activity. Spectrochim. Acta A. 115, 854–860 (2013)
Dong, L., Liu, M., Chen, A., Wang, Y., Sun, D.: Solubilities of quercetin in three β-cyclodextrin derivative solutions at different temperatures. J Mol. Lipids. 177, 204–208 (2013)
Vijaya Sri, K., Kondaiah, A., Vijaya Ratna, J., Annapurna, A.: Preparation and characterization of quercetin and rutin inclusion complexes. Drug Dev. Indust. Pharm. 33, 245–253 (2007)
Calabro, M.L., Tommasini, S., Donato, P., Raneri, D., Stancanelli, R., Ficarra, P., Ficarra, R., Costa, C., Catania, S., Rustichelli, C., Gamberini, G.: Effects of α- and β-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3- hydroxyflavones. J. Pharm. Biomed. Anal. 35, 365–377 (2004)
Jullian, C., Orosteguis, T., Perez-Cruz, F., Sanchez, P., Mendizabal, F., Olea-Azar, C.: Complexation of morin with three kinds of cyclodextrin. A thermodynamic and reactivity study. Spectrochim. Acta A 71, 269–275 (2008)
Yu, Z., Cui, M., Yan, C., Song, F., Liu, Z., Liu, S.: Investigation of heptakis(2,6-di-O-methyl)-β-cyclodextrin inclusion complexes with flavonoid glycosides by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 21, 683–690 (2007)
Calabro, M.L., Tommasini, S., Donato, P., Stancanelli, R., Raneri, D., Catania, S., Costa, C., Villari, V., Ficarra, P., Ficarra, R.: The rutin/beta-cyclodextrin interactions in fully aqueous solution: spectroscopic studies and biological assays. J. Pharm. Biomed. Anal. 36, 1019–1027 (2005)
Haiyun, D., Jianbin, C., Guomei, Z., Shaomin, S., Jinhao, P.: Preparation and spectral investigation on inclusion complex of beta-cyclodextrin with rutin. Spectrochim. Acta A 59, 3421–3429 (2003)
Nguyen, T.A., Liu, B., Zhao, J., Thomas, D.S., Hook, J.M.: An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 136, 186–192 (2013)
Şamlı, M., Bayraktar, O., Korel, F.: Characterization of silk fibroin based films loaded with rutin-β-cyclodextrin inclusion complexes. J. Inc. Phenom. Macrocycl. Chem. 80, 37–49 (2014)
Wang, Y., Qiao, X., Li, W., Zhou, Y., Jiao, Y., Yang, C., Dong, Y., Inoue, Y., Shuang, S.: Study on the complexation of isoquercitrin with β-cyclodextrin and its derivatives by spectroscopy. Anal. Chim. Acta 650, 124–130 (2009)
Jullian, C., Cifuentes, C., Alfaro, M., Miranda, S., Barriga, G., Olea-Azar, C.: Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized β-cyclodextrin. Bioorganic Med. Chem. 18, 5025–5031 (2010)
Chakraborty, S., Basu, S., Lahiri, A., Basak, S.: Inclusion of chrysin in β-cyclodextrin nanocavity and its effect on antioxidant potential of chrysin: a spectroscopic and molecular modeling approach. J. Mol. Struct. 977, 180–188 (2010)
Tommasini, S., Calabro, M.L., Stancanelli, R., Donato, P., Costa, C., Catania, S., Villari, V., Ficarra, P., Ficarra, R.: The inclusion complexes of hesperetin and its 7-rhamnoglucoside with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Biomed. Anal. 39, 572–580 (2005)
Folch-Cano, C., Jullian, C., Speisky, H., Olea-Azar, C.: Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res. Int. 43, 2039–2044 (2010)
Górnas, P., Neunert, G., Baczyński, K., Polewski, K.: Beta-cyclodextrin complexes with chlorogenic and caffeic acids from coffee brew: spectroscopic, thermodynamic and molecular modelling study. Food Chem. 114, 190–196 (2009)
Li, J., Zhang, M., Chao, J., Shuang, S.: Preparation and characterization of the inclusion complex of baicalin (BG) with β-CD and HP-β-CD in solution: an antioxidant ability study. Spectrochim. Acta A 73, 752–756 (2009)
Çelik, S.E., Özyürek, M., Tufan, A.N., Güçlü, K., Apak, R.: Spectroscopic study and antioxidant properties of the inclusion complexes of rosmarinic acid with natural and derivative cyclodextrins. Spectrochim. Acta A 78, 1615–1624 (2011)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation on the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. 71, 2703–2707 (1949)
Apak, R., Güçlü, K., Özyürek, M., Karademir, S.E.: Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52, 7970–7981 (2004)
Lu, Z., Cheng, B., Hu, Y., Zhang, Y., Zou, G.: Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food Chem. 113, 17–20 (2009)
Singh, R., Bharti, N., Madan, J., Hiremath, S.N.: Characterization of cyclodextrin inclusion complexes—a review. J. Pharm. Sci. Tech. 2, 171–183 (2010)
Smulevich, G., Feis, A., Mazzi, G., Vincieri, F.F.: Inclusion complex formation of 1,8-dihydroxyanthraquinone with cyclodextrins in aqueous solution and in solid state. J. Pharm. Sci. 77, 523–526 (1988)
Tommasini, S., Raneri, D., Ficarra, R., Calabro, M.L., Stancanelli, R., Ficarra, P.: Improvement in solubility and dissolution rate of flavonoids by complexation with β-cyclodextrin. J. Pharm. Biomed. Anal. 35, 379–387 (2004)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliver Rev. 59, 645–666 (2007)
Cao, H., Wu, D., Wang, H., Xu, M.: Effect of the glycosylation of flavonoids on interaction with protein. Spectrochim. Acta A 73, 972–975 (2009)
Xiao, J., Cao, H., Wang, Y., Zhao, J., Wei, X.: Glycosylation of dietary flavonoids decreases the affinities for plasma protein. J. Agric. Food Chem. 57, 6642–6648 (2009)
Dangles, O., Dufour, C., Bret, S.: Flavonol-serum albumin complexation. Two-electron oxidation of flavonols and their complexes with serum albumin. J. Chem. Soc. Perkin Trans. 2, 737–744 (1999)
Bi, S.Y., Ding, L., Tian, Y., Song, D.Q., Zhou, X., Liu, X.: Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 703, 37–45 (2004)
Apak, R., Güçlü, K., Demirata, B., Özyürek, M., Çelik, S.E., Bektaşoğlu, B., Berker, K.I., Özyurt, D.: Comparative evaluation of total antioxidant capacity assays applied to phenolic compounds, and the CUPRAC assay. Molecules 12, 1496–1547 (2007)
Tütem, E., Apak, R., Baykut, F.: Spectrophotometric determination of trace amounts of copper(I) and reducing agents with neocuproine in the presence of copper (II). Analyst 116, 89–94 (1991)
Ozyurek, M., Güçlü, K., Apak, R.: The main and modified CUPRAC methods of antioxidant measurement. Trac- Trend Anal. Chem 30, 652–664 (2011)
Stražišar, M., Andrenšek, S., Šmidovnik, A.: Effect of β-cyclodextrin on antioxidant activity of coumaric acids. Food Chem. 110, 636–642 (2008)
Çelik, S.E., Özyürek, M., Güçlü, K., Apak, R.: Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP Methods. Talanta 81, 1300–1309 (2010)
Piel, G., Piette, M., Barillaro, V., Castagne, D., Evrard, B., Delattre, L.: Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 338, 35–42 (2007)
Acknowledgments
Authors would like to thank Istanbul University Research Fund for the support given to the projects (UDP-43471, BYP-43699, YADOP-43696) and Istanbul University-Application and Research Center for the Measurement of Food Antioxidants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çelik, S.E., Özyürek, M., Güçlü, K. et al. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: influence of glycosylation on inclusion complexation. J Incl Phenom Macrocycl Chem 83, 309–319 (2015). https://doi.org/10.1007/s10847-015-0566-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0566-z