Abstract
The inclusion complexes of four flavonols with modified cyclodextrins (CDs) have been investigated. The effect of heptakis (2,6-di-O-methyl) β-cyclodextrin (DM-β-CD) and 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) on the aqueous solubility of flavonols, namely, galangin, kaempferol, quercetin, and myricetin was investigated, respectively. The increased solubility of all flavonols in the presence of CD was evidenced. The NMR experiment and molecular modeling studies showed that flavonols interact with each modified CD through different binding modes. Flavonols can complex with CDs largely by two binding modes. The first one is that B-ring of flavonols is oriented toward secondary rim of CD. The second one is that A-ring of flavonols is oriented toward secondary rim of CD. Whereas only the first mode was observed in DM-β-CD complexes, both the first and the second mode were observed in HP-β-CD complexes in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoids are polyphenolic compounds that usually exist in plants as secondary metabolites [1]. They possess strong antioxidative activity as well as other potential effects including anti-inflammatory, anti-cancer, and anti-viral [2]. Flavonols constitute a major group within the flavonoids present in several foodstuffs, such as apples, cherries, and other green vegetables. It was reported that kaempferol, quercetin, and myricetin reduced the risk of pancreatic cancer [3]. However, owing to their phenolic nature, flavonoids are poorly water soluble, and their scarce absorption is well known [1]. This aspect reduces their bioavailability.
In recent years, cyclodextrin complexation has been successfully used to improve solubility, chemical stability, and bioavailability of a number of poorly soluble molecules. Cyclodextrins (CDs) are cyclic α-(1 → 4)-linked glucopyranose oligomers having six (α), seven (β) and eight (γ) glucopyranose units and adopt a truncated cone structure with hydrophobic cavity. The non-polarity of the interior cavity of the cyclodextrin makes it ideal for solubilizing nonpolar solutes, whereas the polarity of its exterior helps it and its guest to become soluble in water [4]. This property accounts for the great interest in cyclodextrins. However, unmodified β-CD has poor water solubility and is unsafe due to its nephrotoxicity [5, 6]. Therefore, several modified and relatively safe than β-CD have been synthesized and used [6], such as heptakis (2,6-di-O-methyl) β-cyclodextrin (DM-β-CD) and 2-hydroxypropyl-β-cyclodextrin (HP-β-CD). DM-β-CD and HP-β-CD were used to improve the aqueous solubility of four flavonols (Fig. 1). These flavonols share common structure except B-ring with from no to three hydroxyl group. Phase solubility, 1H-NMR and molecular modeling studies were performed to elucidate the complexation phenomena between these modified CDs and flavonols.
Experimental
Materials
Galangin, kaempferol, quercetin, myricetin, heptakis (2,6-di-O-methyl) β-cyclodextrin (DM-β-CD), and 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) (M.S. = 1) were purchased form Sigma–Aldrich Inc. St. Louis, MO. They were employed without further purification. All other materials were analytical grade, and all water used double-distilled and deionized.
Phase solubility studies
Phase solubility studies were performed according to the method of Higuchi and Connors [7]. A fixed initial amount of flavonols (1 mM), exceeding their solubility, was added to unbuffered aqueous solutions of cyclodextrins (0.0–6.0 mM) in capped vials, then sonicated for 40 min. Vials were sealed to avoid changes due to evaporation and maintained for 24 h, shielded from light to prevent degradation of the molecules. At the equilibrium (about 24 h), the aliquot from each vial was filtered through a PVDF 0.2 μm filter (Whatman) and diluted in 70% ethanol/water. Each sample was analyzed by UV–Vis spectrophotometry (UV 2450, Shimadzu Corporation) to evaluate the concentration of the flavones dissolved. The experiment was carried out in triplicate, solubility data were averaged and used to calculate the stability constant. The stability constants (K C) of the flavonol–cyclodextrin complexes were calculated from the straight lines portion of the phase solubility diagrams according to Higuchi–Connors equation: K C = slope/S 0(1−slope), where S 0 is the solubility of flavonols in water.
1H-NMR studies
1H-NMR spectra were obtained at 300 K using a Bruker AMX spectrometer operating at 500 MHz. Inclusion complexes for 1H-NMR studies were prepared freeze–drying method, which is the best to prepare the inclusion complexes [8]. Each complex was dissolved in 50% v/v dimethylsulfoxide-d 6 (DMSO) in D2O. Chemical shifts were expressed in ppm relative to those of the HOD signal (4.58 ppm from 3-(trimethylsilyl)-propionic acid-d 4 (TSP)). The chemical shift displacements were calculated according to the formula: Δδ = δ(free)−δ(complex), where δ(free) is the chemical shift of flavonols without CDs, and δ(complex) is the chemical shift of flavonols with CDs.
Molecular modeling studies
Molecular modeling was carried out using the Insight II molecular modeling package (Accelrys, San Diego) on Pentium PC. The molecular structure of β-CD was obtained from crystal structure. DM-β-CD, HP-β-CD, and flavonols were built using the Builder module of the Insight II program by adding to β-CD 14 methyl in position 2 and 6 (DM-β-CD) and 7 hydroxypropyl groups on the primary hydroxyl groups of β-CD as shown by Mura et al. (HP-β-CD) [9]. The obtained models were optimized using a protocol of conjugated gradients to avoid steric hindrance. After thorough minimization, docking experiments were carried out using “Dock” modules in Insight II and the CVFF force field for docking and scoring [10]. The flavonols were initially set above the center of the cavity of CD with a distance of ~10 Å. During the course of docking simulations, flavonols could make a maximum translational movement of 1 Å and a maximum rotation of 180° around the x, y, and z axes. Each cycle began with a random change of up to 5 df among them. The resulting new conformation was subjected to 100 iterations of conjugated gradient energy minimization. After the energy minimization, the resulting structure was accepted based on the Metropolis Monte Carlo criteria [11]. Conformations within 0.1 Å RMSD of pre-existing ones were discarded to avoid accepting similar configurations. To mimic solvent screening, a distance-dependent dielectric constant of (ε = 4r, where r = interatomic distance in Å) was used [12]. The docking simulations were performed until energy convergence and up to 50,000 trials. Ten lowest scored conformations of accepted one were selected from each simulation of complexes to use conformational analysis.
Results and discussion
Phase solubility studies
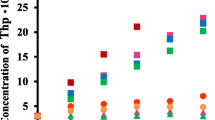
The phase solubility diagram is a widely accepted method for evaluation of the effect of CD complexation on the guest solubility [13]. The diagram is obtained by measuring the concentration of guest in the presence of increasing concentrations of the cyclodextrins. Recently, Bergonzi et al. investigated the solubility of three flavonols (galangin, kaempferol, and quercetin) with native α-, β-, and γ-CD [8]. They reported that α-, β-, and γ-CDs did not have any positive influence on solubility of galangin and α- and γ-CDs also did on solubility of quercetin. On the contrary, water solubility of kaempferol increased as a function of the native CDs concentration and only β-CD showed enhancement of water solubility in the case of quercetin. Figure 2 shows that the phase solubility diagrams of obtained for modified CDs with four flavonols. Both DM-β-CD and HP-β-CD were found to enhance water solubility of all flavonols and showed typical AL type diagram [7]. The solubilizing efficiency of CDs is different in each flavonol. DM-β-CD shows more efficient than HP-β-CD in galangin and two modified CDs show similar solubilizing efficiency in kaempferol (Fig. 2a). However, HP-β-CD is more efficient than DM-β-CD in quercetin and myricetin, which have more hydroxyl group in their B-ring than kaempferol (Fig. 2b). The 1:1 guest/cyclodextrin complex is the most common type of association where a single guest is included in the cavity of one cyclodextrin, with a stability constant K C for the equilibrium between the free and associated species [13]. Furthermore, since the slope of the diagram is lower than unit, the stoichiometry of the complexes was assumed to be 1:1 [7]. The stability constants K C were calculated from the straight-line portion of the phase solubility diagram and summarized in Table 1. Kaempferol complexes show higher stability constants than other flavonoid complex in both modified CDs and stability constants are similar in both modified CDs. DM-β-CD shows higher stability constants than that of HP-β-CD in galangin, but on the other hand, HP-β-CD shows higher stability constants than that of DM-β-CD in quercetin and myricetin which contain more hydroxyl groups than kaempferol. The overall values of stability constant is largely higher than that of α-, β-, and γ-CD complexes, which has been reported below 400 M−1 [8]. These results are reflecting an enhancement of binding and solubility of flavonols with an increase in substitution and hydrophilicity of the CDs. DM-β-CD and HP-β-CD possesses a deeper cavity than native β-CD. Therefore, the solubilization efficiency of these modified CDs improved than that of β-CD. And furthermore, binding mode of complex can be altered. The hydrophobicity of DM-β-CD is greater than that of HP-β-CD because there are fewer hydroxyls in DM-β-CD than in HP-β-CD. Therefore, the stability constant of CDs is different according to the character of guest.
1H-NMR studies
The formation of inclusion complexes can be proved from the changes of chemical shift of guest or CDs in 1H-NMR spectra. Such chemical shift changes may provide valuable insight into the molecular conformation of the inclusion complexes. In the present study, owing to the extremely poor aqueous solubility of flavonols, DMSO was used as a cosolvent, in order to obtain the optimum solubility of both species. The spectra of cyclodextrins are not presented here because of the relatively impure chemical nature and poor spectral resolution of the modified cyclodextrins. Therefore, the present study has only considered the aromatic proton chemical shifts of flavonols. Table 2 lists the variation of the aromatic chemical shifts of flavonols. There are some different aspects between stability constant in purely aqueous solvent system (Table 1) and the degree of chemical shift changes in DMSO-d6/D2O environments. This might be due to the effects of DMSO on the complexes. DMSO can form the inclusion complex with β-cyclodextrin [14] and also act as a competitive guest on the formation of an inclusion complex between cyclodextrin and guest [15]. Furthermore, Zheng et al. show that 20% v/v DMSO drastically lowered the K C values of complexes about 20-fold [16]. The effect of DMSO is more severe in HP-β-CD complex than in DM-β-CD. The degree of chemical shift is reduced largely in HP-β-CD complex (Table 2). However, the reducing the binding strength of the complex in water will not significantly alter the basic model of interaction [16–18]. Upfield shifts are observed for most of the aromatic protons of the A- and B-ring of flavonol. The major induced shielding is observed for A-ring proton on both DM-β-CD and HP-β-CD. However, it is interesting to note that the B-ring proton shows similar degree of chemical shift changes in comparison with A-ring proton in the case of HP-β-CD complexes.
Molecular modeling studies
Based on the results of NMR, it can be inferred that the A-ring of flavonols exhibit significant interaction with the DM-β-CD cavity. In the case of HP-β-CD complex, B-ring as well as A-ring of flavonols shows significant interaction with the HP-β-CD cavity. To further investigate the mode of interaction of complexes, a molecular modeling study was performed [10]. Ten lowest scored conformations were selected from each Monte Carlo docking simulation of complexes to use conformational analysis. There are significant different in binding mode between DM-β-CD and HP-β-CD (Fig. 3). In the case of flavonol–DM-β-CD complexes, the complexes have the B-ring of flavonols oriented toward the secondary rim, while both A- and C-rings remain oriented to the primary rim and inserted on the cavity of CD in all conformations of complexes. This binding mode is well supported the NMR result of DM-β-CD complex (Table 2). However, there are two main binding modes in flavonol–HP-β-CD complexes. One is similar binding mode with DM-β-CD complexes, in which the B-ring of flavonols oriented toward the secondary rim of HP-β-CD. The other is different complex where the flavonols are located in opposite direction with respect to the previous complex. The B-ring of flavonols is oriented toward primary rim of CD. These results are also in good agreement with the experimental data of HP-β-CD complexes (Table 2). Not only A-ring, but also B-ring of flavonols shows significant interaction with HP-β-CDs. This difference of binding mode may affect the efficiency of inclusion complex formation between two different modified CDs.
Inclusion complexes of kaempferol with DM-β-CD (left) and HP-β-CD (right) obtained from molecular docking studies. Two representative structures of each inclusion complex are superimposed on the basis of CDs. The letters indicate the ring of kaempferol of each complex. Left and right side of complexes are secondary rim and primary rim of CDs, respectively
Recently, it is reported a study on the interaction of flavonol–CD complexes [8, 16]. Bergonzi et al. suggested that the interaction of B-ring of quercetin and kaempferol with the β-CD was the most favored [8]. Zheng et al. also suggested similar model of the quercetin–β-CD complex using NMR and molecular dynamic simulations, which the B-ring is more closely associated with the β-CD cavity compared to the A-ring [16]. Similar binding mode has been also reported for (+)-catechin with β-CD [19]. (+)-Catechin is flavan-3-ol type of flavonoid and has basically the same structure of quercetin except the carbonyl oxygen at C4 and unsaturated bond at C2–C3. However, in the case of DM-β-CD and HP-β-CD, A-ring is inserted on the cavity of CD and B-ring is oriented toward the secondary rim [16], which is in agreement with our results. These results are reflecting the clearly different binding mode between native CD and modified CD with flavonoids.
Through the inclusion complex with two modified β-CDs the aqueous solubility of four flavonols is improved and these modified β-CDs are more efficient than native CDs [8]. And furthermore, solubility of flavonols is affected by the OH groups in B-ring and the kind of modified CDs. Thus, modified β-CD may be useful in improving the dissolution and the bioavailability of these flavonols in pharmaceutical formulations. The NMR analysis and molecular modeling showed that flavonols interact with each modified CD through different binding modes. The B-ring of flavonols oriented toward secondary rim is favored in DM-β-CD complex. However, in the case of HP-β-CD complex, the opposite directed interaction which the B-ring of flavonols was oriented toward primary rim is also found.
References
Havsteen, B.H.: The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 96, 67–202 (2002). doi:10.1016/S0163-7258(02)00298-X
Rice-Evans, C.A., Miller, N.J., Paganga, G.: Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20, 933–956 (1996). doi:10.1016/0891-5849(95)02227-9
Nöthlings, U., Murphy, S.P., Wilkens, L.R., Henderson, B.E., Kolonel, L.N.: Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am. J. Epidemiol. 166, 924–931 (2007). doi:10.1093/aje/kwm172
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998). doi:10.1021/cr970022c
Frank, D.W., Gray, J.E., Weaver, R.N.: Cyclodextrin nephrosis in the rat. Am. J. Pathol. 83, 367–382 (1976)
Irie, T., Uekama, K.: Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 86, 147–162 (1997). doi:10.1021/js960213f
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instr 4, 117–212 (1965)
Bergonzi, M.C., Bilia, A.R., Bari, L.D., Mazzi, G., Vincieri, F.F.: Studies on the interactions between some flavonols and cyclodextrins. Bioorg. Med. Chem. Lett. 17, 5744–5748 (2007). doi:10.1016/j.bmcl.2007.08.067
Mura, P., Bettinetti, G., Melani, F., Manderioli, A.: Interaction between naproxen and chemically modified β-cyclodextrins in the liquid and solid state. Eur. J. Pharm. Sci 3, 347–355 (1995). doi:10.1016/0928-0987(95)00025-X
Hyunmyung, K., Karpjoo, J., Hyungwo, P., Seunho, J.: Preference prediction for the stable inclusion complexes between cyclodextrins and monocyclic insoluble chemicals based on Monte Carlo docking simulations. J. Incl. Phenom. Macrocycl. Chem. 54, 165–170 (2006). doi:10.1007/s10847-005-6288-x
Metropolis, N., Rosenbluth, A.W., Rosenbluth, M.N., Teller, A.H., Teller, E.: Equation of state calculations by fast computing machines. J. Chem. Phys. 21, 1087–1092 (1953). doi:10.1063/1.1699114
Chong, L.T., Duan, Y., Wang, L., Massova, I., Kollman, P.A.: Molecular dynamics and free-energy calculations applied to affinity maturation in antibody 48G7. Proc. Natl Acad. Sci. USA 96, 14330–14335 (1999). doi:10.1073/pnas.96.25.14330
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004). doi:10.1038/nrd1576
Aree, T., Chaichit, N.: Crystal structure of β-cyclodextrin-dimethylsulfoxide inclusion complex. Carbohydr. Res. 337, 2487–2494 (2002). doi:10.1016/S0008-6215(02)00335-X
García-Río, L., Hervés, P., Leis, J.R., Mejuto, J.C., Pérez-Juste, J., Rodríquez-Dafonte, P.: Evidence for complexes of different stoichiometries between organic solvents and cyclodextrins. Org. Biomol. Chem. 4, 1038–1048 (2006). doi:10.1039/b513214b
Zheng, Y., Haworth, I.S., Zuo, Z., Chow, M.S.S., Chow, A.H.L.: Physicochemical and structural characterization of quercetin-β-cyclodextrin complexes. J. Pharm. Sci. 94, 1079–1089 (2005). doi:10.1002/jps.20325
Junquera, E., Ruiz, D., Aicart, E.: Role of hydrophobic effect on the noncovalent interactions between salicylic acid and a series of β-cyclodextrins. J. Colloid Interface Sci. 216, 154–160 (1999). doi:10.1006/jcis.1999.6290
Matsui, T., Iwasaki, H., Matsumoto, K., Osajima, Y.: NMR studies of cyclodextrin inclusion complex with ethyl hexanoate in ethanol solution. Biosci. Biotechnol. Biochem. 58, 1102–1106 (1994)
Jullian, C., Miranda, S., Xapata-Torres, G., Mendizábal, F., Olea-Azar, C.: Studies of inclusion complexes of natural and modified cyclodextrin with (+)-catechin by NMR and molecular modeling. Bioorg. Med. Chem. 15, 3217–3224 (2007). doi:10.1016/j.bmc.2007.02.035
Acknowledgments
This study was supported by a grant of the Korea Research Foundation (KRF-2006-005-J03402) and partly by a grant of KOSEF (R01-2008-000-12304-0) in 2008. SDG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H., Choi, J. & Jung, S. Inclusion complexes of modified cyclodextrins with some flavonols. J Incl Phenom Macrocycl Chem 64, 43–47 (2009). https://doi.org/10.1007/s10847-009-9534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9534-9