Abstract
Grazing by domestic stock is widely used in nature reserve management to maintain or restore characteristics of the flora. While the effects on plants are well understood, grazing effects on arthropods are in need of further investigation. We studied the effects of management on grassland arthropod communities at Needingworth, a mixture of grassland and wetland, created after gravel extraction. We hypothesised arthropod abundance and the species richness of Hemiptera and Coleoptera, would be no greater in fenced, ungrazed areas than in cattle-grazed grassland. We used suction sampling to collect grassland arthropods which were initially identified to order level, and then to species or genus level for the Coleoptera and Hemiptera. Abundance of total invertebrates and of all orders, except for Diptera, was greater in ungrazed than grazed grassland. We estimated that the presence of ungrazed grassland resulted in 14.9% greater invertebrate abundance at Needingworth. Community structure showed strong differences in relation to management, particularly in terms number of detritivores. Even the small amount of grassland management at Needingworth had distinct negative impacts arthropod abundance and community structure, and leaving ungrazed areas has the potential to benefit invertebrate biodiversity. We recommend that some grassland patches should remain unmanaged for long periods, as part of a mixed management strategy. Conservation grazing is not the only approach that should be used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has long been recognised that the management of grassland, whether for amenity, nature conservation or aesthetic reasons, has a major impact not only on habitat structure but also on the plant and animal biodiversity which exists there (Curry 1987; Morris 2000; Vickery et al. 2001; Kruess and Tscharntke 2002). This understanding forms the basis for much of the recent emphasis on managing nature reserves and promoting biodiversity through low-level grazing by domestic stock (WallisDeVries 1998; Rook and Tallowin 2003; Dumont et al. 2007; Bucher et al. 2016). However, grazing, or indeed mowing or other management, affects organisms to differing degrees. This was highlighted in a review by van Klink et al. (2015), which indicated that grassland arthropods are typically more negatively affected than plant diversity by increased grazing intensity, and that plant diversity was sometimes a poor predictor arthropod diversity. Therefore it cannot be assumed that grassland management that promotes plant diversity will be beneficial for invertebrates. One problem is that while there is a well developed understanding of the effects of management on plant communities, much less is known about the effects on arthropod communities (Littlewood et al. 2012). For this reason it is important that efforts are made to reduce this knowledge gap through studies into invertebrate responses to grassland management. This is particularly pertinent given the recent well-publicised finding of large-scale decline in flying insect biomass in Europe (Hallmann et al. 2017). If we are to halt and ideally reverse this decline, we need to know how we can modify our habitat management to better serve the needs of invertebrates.
The methodology and outcomes of grassland management are key considerations of nature conservation managers, particularly those working to restore habitats and communities following negative human impact, such as transport infrastructure creation, intensive agriculture, deforestation, quarrying and gravel extraction (Snazell and Clarke 2000; Walker et al. 2004; Öckinger et al. 2006; Tropek et al. 2010; Lenda et al. 2012; Woodcock et al. 2012). Habitat restoration of gravel workings has often been associated with the flooding of gravel pits, creating networks of freshwater lakes and some associated terrestrial habitats such as grassland, scrub and woodland (Armitage 1990). One such site is at Needingworth, in Cambridgeshire, UK, where the focus has been on wetland creation, and which was the location of our study. The conservation focus at Needingworth has been reedbed restoration, but such sites typically also include areas of wet or dry grassland, with varying degrees of management through grazing, mowing and fencing. These grasslands potentially provide a valuable habitat for biodiversity, especially within the context of the considerable intensification of agricultural and other grasslands that has taken place over recent decades (Vickery et al. 2001; Benton et al. 2003; Pärtel et al. 2005).
The aim of our study was to investigate how grassland management affected arthropod abundance and biodiversity in grasslands surrounding reedbed restoration units at Needingworth/Ouse Fen. In doing so we hoped to both extend scientific understanding and to inform local management practice decisions, which will help to further enhance the biodiversity value of this restoration project. In particular, we were interested in to what extent Hemiptera and Coleoptera communities are affected by cattle grazing at the site. For the Hemiptera we had a specific focus on the Auchenorrhyncha (leafhoppers, planthoppers and froghoppers), which are common in grassland and have been found to be useful indicators of management intensity effects (Andrzejewska 1962; Nickel and Hildebrandt 2003).
The grazing at Needingworth is low intensity for biodiversity conservation purposes and consequently in these managed areas the vegetation retains much of its height and structure (Fig. 1).
Our hypothesis was that given the low intensity grazing, overall arthropod populations, as well as the species richness and abundance of Hemiptera and Coleoptera, would be no greater in fenced, ungrazed plots that had been created for the development of scrub, than in cattle-grazed grassland.
Methods
Location and site details
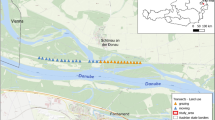
Needingworth/Ouse Fen is located approximately 1 km north of the village of Over, Cambridgeshire, UK. It is the site of a collaboration between Hanson/Heidelberg Cement and the Royal Society for the Protection of Birds (RSPB) in a phased restoration to create a very large reedbed-focused wetland nature reserve, called Ouse Fen (Aggregates Business Europe 2010; RSPB 2011). As gravel extraction is completed in sections of Needingworth, these areas are being converted into wetland habitats, including large areas of reedbed. Open water and reedbeds are the main focus of the RSPB's efforts, given the importance they have for birds, some of which are rare and threatened in the UK (Wotton et al. 2009; White et al. 2014). The site is centred on national grid reference TL377726 (0° 01′ 10″ E, 52° 20′ 04″ N) and in 2014 the area restored after gravel extraction covered a total area of approximately 148 ha. The main habitats within the site are: wetlands, composed of open water, reedbed and marshland vegetation, and a mixture of grassland and developing scrub (Fig. 2). Restoration, by Hanson/Heidelberg Cement and the RSPB, is being carried out in stages. The first area restored, from 2003 onwards (2003 restoration), comprises an area of approximately 69.9 ha and was followed by a further 78.2 ha from 2011 (2011 restoration) (RSPB 2011).
In the 2003 restoration, grassland covered 31.9 ha, or 46.7% of the total area. Of this 23.7 ha (74.1% of the grassland) has been managed by grazing. In 2014, the year of sampling, there was a herd of between 31 and 36 cattle, representing 18.6 to 21.6 livestock units (LU), which is typical of the level of grazing management in this part of the reserve. The whole grazed area was divided by a ditch and fencing into two separate sections. The cattle were free to move within each section, and were rotated between them, such that over the spring and summer the mean daily cattle density across the site was 0.89 LU ha−1, and the level of grazing was equivalent between the two sections. There was no supplementary feeding of the herd on the reserve. Of the remaining area, 38.0 ha (54%) was covered by open water, reedbed or other wet vegetation. There were 8.3 ha (11%) of ungrazed grassland, which was fenced with the aim of eventual succession into scrub. In most of these ungrazed areas there has been some low-density planting of woody vegetation, such as hawthorn Crataegus monogyna Jacq. (Rosaceae). The ungrazed grassland areas were mostly located around the edges of the site (Fig. 2). Those along the northern edge bordered a narrow, rough track, incorporating a gravel carrying conveyor belt, beyond which was the grassland and wetland of the 2011 restoration. The eastern edge of the site was bordered by a wet ditch, farm track, and then intensively farmed arable fields. The western edge was without a ditch, had a rarely used track, then a hedgerow and an intensive arable field. The southern boundary consisted of a hedgerow, beyond which was a narrow farm track and then various agricultural fields. The ungrazed area away from the boundary was mostly surrounded by grazed grassland, with part adjoined to an area of open water.
Habitat areas were calculated using satellite photographs from the Ordnance Survey, Get a Map (Ordnance Survey 2014) and the Google Maps Area Calculator Tool (Daft Logic 2014).
Invertebrate sampling and identification
Invertebrates were sampled from 16 grassland locations (Fig. 2) on three dates, 6 June, 25 June and 21 July 2014, using a Vortis suction sampler (Arnold 1994). A sample was taken on each date from each of eight fenced ungrazed areas. These were paired with a sample from an adjacent area of grazed grassland. Sampling locations were numbered sequentially in a clockwise direction, starting with grazed/ungrazed (G1/U1) 1 in the south-east corner (Fig. 2) and finishing with G8/U8, the other location on the eastern boundary, just to the north of location 1 (Fig. 2).
Each suction sample consisted of ten 16-s sucks (Brook et al. 2008) covering a total area of 0.2 m2 (10 × 0.02 m2). Invertebrates collected were preserved in 70% ethanol solution.
Invertebrates were initially identified as: Araneae, Coleoptera, Diptera, Hemiptera, Hymenoptera or as ‘other orders’, although Collembola and Acari were excluded. The numbers of each order were counted. Subsequently the Hemiptera and Coleoptera were identified further. Adult Hemiptera were identified either to species level, or when this was not possible, to genus or morphospecies. Nymphs were identified to species level whenever possible but some could only be assigned to morphospecies, genus, or in the case of some very early instars, to family. Adult Coleoptera specimens were initially identified to family level, and then subsequently to species level. Based on these identifications, adult Hemiptera and Coleoptera were assigned to trophic guilds. Similarly, the numbers of parasitic and herbivorous Hymenoptera were distinguished.
All invertebrates, with the exception of Diptera, were classified as belonging to one of five trophic groups: detritivores, herbivores (chewers), herbivores (suckers), predators, and parasites. For some groups, such as Araneae all individuals are predators. For other groups with a mixed strategy, Coleoptera, Hemiptera and Hymenoptera and other orders, all specimens were examined and grouped according to trophic guild.
Statistical methods
Statistical modelling of invertebrate data was carried out using R version 2.15.1 (R Core Team 2017). Prior to modelling data were summed from the three sample dates for each paired site to give a single data point for each sampling location.
Grazed-ungrazed comparisons were made using generalised linear mixed models, with the lmer function from the lme4 package (Bates et al. 2015). In each model the response variable was the abundance (i.e. number of individuals) of invertebrates, either in total or by order, with habitat type (grazed or ungrazed grassland) being the single explanatory variable. Sample pair was used as a random effect, and Poisson error structure was defined in all models. As well as abundance, the number of Hemiptera species and the number of Coleoptera species were also modelled. Generalised linear mixed models were used in the same way, following the trophic guild ordination (see below) to compare paired grazed and ungrazed samples in terms of abundance of each of the five trophic guilds.
Community structure for all 16 sampling locations was investigated using non-metric multidimensional scaling (NMDS), with the metaMDS function of the vegan package (Oksanen et al. 2017). The ordination used Bray–Curtis dissimilarity to compare the community structure at different sample locations. Three separate ordinations compared communities in terms of invertebrate trophic group, Hemiptera species (n = 29) and Coleoptera species (n = 12), with only groupings or taxa (species, genus or morphospecies) with a total of 10 or more individuals included. Sample locations were used as categorical variables and applied to the ordination using the envfit function, which gave a goodness of fit statistic based on 1000 random permutations of the data. Similarly, trophic group and Hemiptera or Coleoptera taxa were applied to the ordinations with envfit, giving an indication of how the communities differed.
Results
Invertebrates recorded
The total number of invertebrates sampled at Needingworth was 14,589, consisting of 1730 Araneae, 1361 Coleoptera, 3737 Diptera, 5049 Hemiptera, 1564 Hymenoptera and 1148 of other orders.
The number of Hemiptera identified further was 3747, representing 54 identified species (Appendix Table 1). All the Hemiptera that could be identified to species level were associated with grassland, with no arboreal species collected. Five species of which were local or more specific conservation interest: Euscelidius variegatus (Kbm.) (Cicadellidae), nationally notable (B); Psammotettix alienus (L.) (Cicadellidae), Red Data Book K (insufficiently known); Eurybregma nigrolineata Scott. (Delphacidae) and Xanthodelphax straminea (Stål) (Delphacidae), both local; and Ribautodelphax imitans (Rib.) (Delphacidae), Red Data Book K (Stewart 2012; Dittrich and Helden 2016). The 617 adult Coleoptera represented 65 species (Appendix Table 2). There was one species, Cantharis decipiens (Baudi), represented by a single individual collected in the ungrazed habitat, that is associated with areas that have trees or shrubs. All the other Coleoptera that could be identified to species level, are associated with grassland, low herbaceous vegetation or similar open habitats.
Invertebrate abundance
The total number of invertebrates recorded in the ungrazed grassland habitat was significantly higher than that in grazed (z = 30.05, d.f. = 14, P < 0.001) (Fig. 3). Statistical model estimates of the number of individuals per 0.6 m2 over the three sampling dates were: 668.1 and 1117.9 for grazed and ungrazed, respectively. The same pattern was true in five out of the six invertebrate orders (Fig. 4): Araneae (z = 12.34, d.f. = 14, P < 0.001) (model estimates, 70.1 grazed, 131.0 ungrazed), Coleoptera (z = 4.22, d.f. = 14, P < 0.001) (model estimates, 72.4 grazed, 91.1 ungrazed), Hemiptera (z = 28.42, d.f. = 14, P < 0.001) (model estimates, 175.9 grazed, 423.4 ungrazed), Hymenoptera (z = 7.15, d.f. = 14, P < 0.001) (model estimates, 76.7 grazed, 111.0 ungrazed), other orders (z = 20.88, d.f. = 14, P < 0.001) (model estimates, 21.7 grazed, 119.3 ungrazed). The Diptera showed the opposite pattern, with greater abundance in the grazed habitat (z = − 2.86, d.f. = 14, P = 0.004) (model estimates, 237.0 grazed, 215.8 ungrazed) (Fig. 4).
Boxplots showing the overall abundance of grassland invertebrates in grazed and ungrazed grassland at Needingworth. Data indicate totals per sampling location over three dates in June and July 2014, representing a cumulative sampled area of 0.6 m2. Dark horizontal lines show the median, with the upper and lower boxes the 25th and 75th percentiles, respectively. The dashed lines indicate either 1.5 times the interquartile range or the maximum and minimum values if there are no outliers (small circles)
As with abundance, the number of species of Hemiptera sampled was greater in ungrazed than grazed grassland (z = 3.78, d.f. = 14, P < 0.001) (model estimates, 16.2 grazed, 24.9 ungrazed) (Fig. 5). The same pattern occurred with the Coleoptera (z = 2.49, d.f. = 14, P = 0.013) (model estimates, 12.0 grazed, 16.8 ungrazed) (Fig. 5).
Using the invertebrate abundance and habitat area data, it was estimated that if all the grassland were grazed there would be 14.9% less invertebrates on the grassland part of the whole site.
Community structure
The NMDS of trophic guild resulted in a two-dimensional ordination with a final stress (which indicates how well the two-dimensional configuration of points represents the full ordination) of 0.089 (Fig. 6). This value indicates that the two-dimensions are a very good representation of the full ordination. The community structure showed significant differences between different habitat classifications (r2 = 0.604, P < 0.001), largely based on the first axis of the NMDS, with no overlap between grazed the ungrazed locations. All trophic groups showed a significant fit to the ordination. The fit was most strong for detritivores (r2 = 0.856, P < 0.001) and showed relatively weak relationships for predators (r2 = 0.501, P = 0.014), parasites (r2 = 0.430, P = 0.019), and for both sucking (r2 = 0.359, P = 0.050) and chewing herbivores (r2 = 0.429, P = 0.026). Detritivores, predators and sucking hebivores were associated with ungrazed, with parasites and chewing herbivores fitted between the two management categories. Generalised linear mixed modelling indicated there were significantly more individuals in ungrazed areas for all guilds (chewers z = 2.44, d.f. = 14, P = 0.015 (model estimates, 8.3 grazed, 12.0 ungrazed), detritivores z = 17.13, d.f. = 14, P < 0.001 (model estimates, 4.1 grazed, 81.8 ungrazed), parasites z = 6.80, d.f. = 14, P < 0.001 (model estimates, 73.0 grazed, 104.7 ungrazed), predators z = 12.82, d.f. = 14, P < 0.001 (model estimates, 107.3 grazed, 184.4 ungrazed), suckers z = 24.56, d.f. = 14, P < 0.001 (model estimates, 126.5 grazed, 306.2 ungrazed)), with the contrast between the two sub-habitats being most marked for detritivores (Fig. 7). The dominant detritivore taxon sampled were woodlice (Isopoda), with 591 from three species (Armadillidium vulgare, Philoscia muscorum and Porcellio scaber) out of the total of 719 detritivores (82.2% of all detritivores).
NMDS plot of invertebrate community structure as defined by trophic guild. Grassland category are shown as follows: grazed 2003 restoration (open circles and dotted lines); ungrazed 2003 restoration (filled triangles and solid lines). Trophic guild vector abbreviations: det, detritivores; chew, chewing herbivores; suck, sucking herbivores; pred, predators; and ps, parasites. Sampling locations are labelled as G1-G8 (grazed) and U1-U8 (ungrazed)
At the species level, ordination of Hemiptera communities was described in two dimensions with a final stress of 0.159 (Fig. 7a), indicating a good representation of the full ordination. As with trophic groups the sub-habitats differed significantly (r2 = 0.430, P < 0.001), with no overlap between the grazed and ungrazed locations. When applied to the ordination, seven species showed a significant pattern (Fig. 7a). Five of these were clearly associated with ungrazed (aphid species G (Aphididae); Anoscopus serratulae, Aphrodes makarovi and Arthaldeus pascuellus (Cicadellidae); Himacerus major (Nabidae); and one with grazed, Atheroides serratulus (Chaitophoridae). The ordination vector for one species, aphid species D (Aphididae), was fitted between the grazed and ungrazed categories, indicating no association with either grazed or ungrazed grassland.
The NMDS ordination for Coleoptera was described in a two-dimensional solution, with a final stress of 0.157 (Fig. 7b), and again there was no overlap between grazed and ungrazed locations and a significant difference in communities (r2 = 0.355, P < 0.001). The fit of species to the habitat category was more even than for Hemiptera, with two species associated with grazed (Amischa analis (Staphylinidae) and Neocrepidodera ferruginea (Chrysomelidae)) and three with ungrazed (Megasternum concinnum (Hydrophilidae), Mocyta amplicollis (Staphylinidae) Stenus clavicornis (Staphylinidae)). Two species were fitted between grazed and ungrazed: Cantharis lateralis (Cantharidae) and Trechus quadristriatus (Carabidae).
Inspection of all three ordinations (Figs. 6 and 8) revealed no discernible pattern to indicate that closely located sampling locations were more similar to each other than sites that were more distant, nor any pattern that grouped locations related to their proximity to different types of habitat adjoining the restoration site. Similarly there was no indication of spatial autocorrelation between paired grazed-ungrazed sampling locations.
NMDS plots of a Hemiptera species and b Coleoptera species community structure. Grassland category are shown as follows: grazed (open circles and dotted lines); ungrazed (filled triangles and solid lines). Sampling locations are labelled as G1-G8 (grazed) and U1-U8 (ungrazed). Hemiptera species abbreviations: AD aphid species D (r2 = 0.536 P = 0.009); AG aphid species G (r2 = 0.334 P = 0.047); Am Aphrodes makarovi (r2 = 0.650 P = 0.002); Ans Anoscopus serratulae (r2 = 0.418 P = 0.020); Ap Arthaldeus pascuellus (r2 = 0.387 P = 0.033); Ats Atheroides serratulus (r2 = 0.548 P = 0.006); Hm Himacerus major (r2 = 0.572 P = 0.002). Coleoptera species abbreviations: Aa Amischa analis (r2 = 0.440 P = 0.023); Cl Cantharis lateralis (r2 = 0.450 P = 0.029); Mc Megasternum concinnum (r2 = 0.796 P < 0.001); Ma Mocyta amplicollis (r2 = 0.603 P = 0.005); Nf Neocrepidodera ferruginea (r2 = 0.507 P = 0.009); Sc Stenus clavicornis (r2 = 0.493 P = 0.025); Tq Trechus quadristriatus (r2 = 0.400 P = 0.042).
Discussion
The greater overall abundance of invertebrates and species richness of Hemiptera in ungrazed compared with grazed areas of restored grassland indicates that even grazing intensity classified as lenient or extensive, such as the 0.89 LU ha−1 applied at Needingworth can result in significant reductions in the abundance of individuals and species relative to ungrazed grassland. Our findings add to a growing body of work, which suggests that there is tangible biodiversity benefit to leaving some areas of grassland unmanaged, at least for a number of years (Kruess and Tscharntke 2002; Dumont et al. 2007; Dennis et al. 2015). High intensity grazing has been shown in a number of studies to reduce invertebrate abundance (Morris 1969, 1971, 1973; WallisDeVries 1998; Sheridan et al. 2008) but our work shows that there is a negative impact of even low intensity conservation grazing.
Even so patterns of increased invertebrate numbers with reduced grazing are not always detected. For example, Helden et al. (2015) found no effects from reduced grazing in intensively grazed cattle paddocks. While, Bucher et al. (2016), reported that fallow fields had fewer leafhoppers and spiders than pastures. Similarly, Gossner et al. (2014) found no relationship between grazing intensity and the level of herbivory. However the balance of evidence indicates that arthropod communities are generally negatively affected by large herbivore grazing (van Klink et al. 2015).
Ungrazed grasslands, whether large-scale or in small patches, such as grass islets (Helden et al. 2010) are less disturbed and typically have longer vegetation, more food resources for herbivores, with greater niche diversity, reduced temperature variation and increased shelter compared to grazed areas (Morris 2000; Kormann et al. 2015). Compared to an intensively grazed sward, they may also show greater plant diversity, and many studies have demonstrated that there is a positive link between plant and invertebrate diversity (Siemann et al. 1998; Schaffers et al. 2008; Woodcock et al. 2012). The understanding that less managed vegetation benefits biodiversity, has led to ungrazed grasslands having been set up along field margins and water courses to increase biodiversity in agricultural systems (Woodcock et al. 2007; Sheridan et al. 2008; Cole et al. 2008; Anderson et al. 2013; Fritch et al. 2017). In effect the same has occurred at Needingworth, albeit with the intention of eventually creating scrubland, with the areas fenced off from grazing facilitating an increase in invertebrate populations. Although the ungrazed grasslands sampled contained small numbers of woody saplings, their density was very low. Consequently, the invertebrates, as indicated by the Hemiptera and Coleoptera, were overwhelmingly grassland associated, and there was no evidence that the woody vegetation affected their communities.
With the exception of one location, all the ungrazed grassland at Needingworth was located at the periphery of the site. This raises the question of the potential influence of edge effects. Albrecht et al. (2010) looked at edge effects from ecological compensation areas into adjacent intensively managed agricultural land. They found measurable effects between 100 and 200 m, depending on taxonomic group, into the neighbouring agricultural land. It is probable that Needingworth would have similar effects on the landscape surrounding it, but what might be the reciprocal effect on grasslands within the restoration site? The number of studies on such internal edge effects has been quite limited but there is evidence that for some invertebrates, such as generalist predators, there can be a spillover effect from agricultural areas into more natural areas, which can in turn affect predation dynamics (Rand et al. 2006). If such effects were having an influence at Needingworth is unknown. However, the ordination pattern for guilds did not indicate that ungrazed locations closer to agricultural fields differed in predator numbers, which suggests that if there were such site edge effects, their influence on grassland invertebrate communities was very limited. If they did occur, based on the scale reported by Albrecht et al. (2010), it is likely that their effect would extend beyond the most of the ungrazed areas, encompassing both sampling points of the grazed-ungrazed pairs, and so would not be expected to affect the treatment comparisons made. Moreover, the strong effects of setting aside ungrazed areas at Needingworth, on abundance, diversity and community structure was such that any possible site edge effects would be very hard to detect. However, could there be treatment edge effects around the boundary of the two grassland types? Evidence from a range of studies that invertebrate communities show large differences in community structure, for example in response to vegetation characteristics, over very small distances, measured in metres or even centimetres (Thomas and Marshall 1999; Helden and Leather 2004; Helden et al. 2010, 2018; Hof and Bright 2010; Dittrich and Helden 2012; Anderson et al. 2013). Such strong small scale effects would suggest that edge effects would be mostly at a much smaller scale and at the very extreme periphery of treatments, well away from the sampling locations used in our study.
At present, in the 2003 restoration area, the ungrazed areas account for 25.9% of the grassland. If this were doubled to 51.9%, our data suggest a 14.9% increase in overall invertebrate numbers within the reserve. This would be beneficial not only for the invertebrates themselves but also enhance their contribution to ecosystem processes, being food for other organisms, including birds, and enhancing decomposition, and possibly other roles such as pollination. A greater abundance of invertebrates may enable generally larger populations of species to exist, and larger population sizes tend to increase the stability of populations. By allowing, in particular, larger abundances of uncommon and rare species, management that results in broadly greater numbers of invertebrates are important in enhancing the conservation status of the grasslands. Indeed we identified five species of Hemiptera that are of national conservation interest. Of these, four were only found in the ungrazed grassland. All were found in very small numbers but it is possible that at least some of these species would not be present at Needingworth, if all the grassland areas were grazed. Clearly further sampling and experimental work would need to be done to confirm such a suggestion but that is beyond the scope of the present work. The benefits of ungrazed areas have to be balanced against the opportunity costs of loss of grazing area, such as reduced numbers of those invertebrates which show a preference for shorter swards, reduced feeding opportunities for some ground feeding birds (Vickery and Gill 1999; Whittingham and Devereux 2008), and a reduction in income from renting out grazing rights. Therefore whether an expansion of ungrazed areas is desirable will depend of the relative value of alternatives such as these, and is a matter of judgement for the land managers.
Although there were significantly more individuals of all invertebrates functional groups in ungrazed grassland at Needingworth, by far the strongest effect on community structure was seen in the in detritivore numbers, particularly isopods (woodlice). Increases in herbivores are probably due to the greater amount of plant biomass available for food when management by grazing is reduced but there may also be effects of lower levels of disturbance and changes to plant type or diversity (Morris 2000; Kruess and Tscharntke 2002; van Klink et al. 2015). Terrestrial isopods and other detritivores are probably responding to an increased level of dead organic matter availability, which has been found to be higher in ungrazed grasslands (Curry 1987; Paoletti and Hassall 1999; Morris 2000; Souty-Grosset et al. 2005). However other factors may also be important, such as pH, nutrient availability, and changes in the composition of the plant community (Berg and Hemerik 2004). In addition, the relatively low vagility of isopods and many other detritivores, may mean their recovery from management disturbance is slower than that of highly mobile invertebrates, such as many predators and parasitoids. Detritivores such as isopods are very important within ecosystems, as they feed on dead organic matter and a play a key role in decomposition and nutrient cycling (Paoletti and Hassall 1999). Therefore setting aside of ungrazed areas may be particularly beneficial for maintaining such processes, especially in restored landscapes such as Needingworth where communities have been re-established from an effectively zero baseline. The role of large herbivores such as cattle in mediating decomposition processes in grasslands has been much less studied than the relationship between primary producers and decomposers, but there is evidence that cattle grazing can significantly reduce decomposer biomass (Sankaran and Augustine 2004).
Grazing, and sometimes other approaches such as the management of field margins, is used to increase the structural heterogeneity of grasslands with the aim of increased plant and more general biodiversity (Pykälä 2000; Rook and Tallowin 2003; Humbert et al. 2009). However while plant diversity may be little affected by short-term management effects such as grazing events, evidence suggests that arthropod diversity is generally negatively impacted due to the loss of plant resources outweighing any benefit of increased biotic heterogeneity (van Klink et al. 2015). The same principle can be applied to mowing, which negatively impacts invertebrates and shows an increase of effect with frequency of management (Helden and Leather 2004; Humbert et al. 2009; Cizek et al. 2012; Tälle et al. 2016; Helden et al. 2018). Consequently garden lawns and many other urban grasslands, which are mostly kept short by regular mowing can still maintain a relatively high plant diversity, so long as herbicides and other techniques to discourage broadleaved plants are avoided (Helden and Leather 2004). However invertebrate populations show a very negative response to mowing (Helden and Leather 2004; Helden et al. 2018), which may be because unlike plants that can readily re-grow in situ, invertebrates have to re-colonise following management. The invertebrate abundance and community structure responses we found at Needingworth emphasise how similar outcomes can be apparent with relatively minor differences in grazing. The sensitivity of invertebrates to management, when compared to plants, means that reserve and other land managers need to separately take into account their varied ecological requirements in order to maximise their biodiversity. Indeed it is vital that we take into account the specific needs of invertebrates, if we have any hope of stabilising and if possible reversing their widespread decline (Hallmann et al. 2017).
In relation to grassland management, given the results of our study, we recommend that some areas of grassland are allowed to remain unmanaged for long periods of time. We acknowledge that eventually, without any intervention by human management or other preventative ecological process, any such area will go through a succession to form woodland, so some infrequent management is necessary to preserve grassland. However this could be achieved by strategies such as rotational management to prevent succession (Morris 2000). The maintenance of such infrequently managed grassland plots should be part of a wider strategy to maintain a diversity of management approaches, which would be the optimal strategy for maintaining invertebrate biodiversity on the scale of a nature reserve or landscape (Morris 2000; Biedermann et al. 2005; Nickel and Achtziger 2005; Blake et al. 2011; Tälle et al. 2016).
In conclusion we return to the question of whether conservation grazing is always the answer to grassland management for biodiversity. As we have acknowledged from evidence in the literature, grazing management is both important and effective in conserving biodiversity within restored and other semi-natural grasslands. However as we have found there are also clear benefits to invertebrates of avoiding grazing as a management tool. Therefore we believe that both approaches are valid and important and should be considered within the context of a landscape approach to grassland conservation.
Data availability
Authors: Alvin J. Helden, James Chipps, Stephen McCormack, Luiza Pereira. Title: Invertebrates sampled from Needingworth 2014.xlsx. Repository: Figshare. https://doi.org/10.25411/aru.12167577.
References
Aggregates Business Europe (2010) Restoration at sand and gravel quarry. https://www.aggbusiness.com/sections/health-safety-environment/features/restoration-at-sand-and-gravel-quarry/. Accessed 4 Apr 2017
Albrecht M, Schmid B, Obrist MK et al (2010) Effects of ecological compensation meadows on arthropod diversity in adjacent intensively managed grassland. Biol Conserv 143:642–649. https://doi.org/10.1016/j.biocon.2009.11.029
Anderson A, Carnus T, Helden AJ et al (2013) The influence of conservation field margins in intensively managed grazing land on communities of five arthropod trophic groups. Insect Conserv Divers 6:201–211. https://doi.org/10.1111/j.1752-4598.2012.00203.x
Andrzejewska L (1962) Macrosteles laevis Rib. As an unsettlement index of natural meadow associations of Homoptera. Bull Acad Pol Sci II Sér Sci Biol 10:221–226
Armitage P (1990) Gravel pit restoration for wildlife: a practical manual. Royal Society for the Protection of Birds, Sandy
Arnold AJ (1994) Insect suction sampling without nets, bags or filters. Crop Prot 13:73–76
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
Berg MP, Hemerik L (2004) Secondary succession of terrestrial isopod, centipede, and millipede communities in grasslands under restoration. Biol Fertil Soils 40:163–170. https://doi.org/10.1007/s00374-004-0765-z
Biedermann R, Achtziger R, Nickel H, Stewart AJA (2005) Conservation of grassland leafhoppers: a brief review. J Insect Conserv 9:229–243
Blake RJ, Woodcock BA, Ramsay AJ et al (2011) Novel margin management to enhance Auchenorrhyncha biodiversity in intensive grasslands. Agric Ecosyst Environ 140:506–513. https://doi.org/10.1016/j.agee.2011.02.003
Brook AJ, Woodcock BA, Sinka M, Vanbergen AJ (2008) Experimental verification of suction sampler capture efficiency in grasslands of differing vegetation height and structure. J Appl Ecol 45:1357–1363
Bucher R, Andres C, Wedel MF et al (2016) Biodiversity in low-intensity pastures, straw meadows, and fallows of a fen area—a multitrophic comparison. Agric Ecosyst Environ 234:58–64. https://doi.org/10.1016/j.agee.2016.10.015
Cizek O, Zamecnik J, Tropek R et al (2012) Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J Insect Conserv 16:215–226. https://doi.org/10.1007/s10841-011-9407-6
Cole LJ, Morton R, Harrison W et al (2008) The influence of riparian buffer strips on carabid beetle (Coleoptera, Carabidae) assemblage structure and diversity in intensively managed grassland fields. Biodivers Conserv 17:2233–2245
Curry JP (1987) The invertebrate fauna of grassland and its influence on productivity. II. Factors affecting the abundance and composition of the fauna. Grass Forage Sci 42:197–212
Dennis P, Skartveit J, Kunaver A, McCracken DI (2015) The response of spider (Araneae) assemblages to structural heterogeneity and prey abundance in sub-montane vegetation modified by conservation grazing. Glob Ecol Conserv 3:715–728. https://doi.org/10.1016/j.gecco.2015.03.007
Dittrich ADK, Helden AJ (2012) Experimental sward islets: the effect of dung and fertilisation on Hemiptera & Araneae. Insect Conserv Divers 5:46–56. https://doi.org/10.1111/j.1752-4598.2011.00133.x
Dittrich ADK, Helden AJ (2016) The community ecology of Ribautodelphax imitans (RIBAUT, 1953) (Hemiptera: Delphacidae), a rare UK planthopper in a distinct grassland habitat. Entomol Aust 23:87–96
Dumont B, Rook AJ, Coran C, Röver KU (2007) Effects of livestock breed and grazing intensity on biodiversity and production in grazing systems. 2. Diet selection. Grass Forage Sci 62:159–171
Fritch RA, Sheridan H, Finn JA et al (2017) Enhancing the diversity of breeding invertebrates within field margins of intensively managed grassland: effects of alternative management practices. Ecol Evol 7:9763–9774. https://doi.org/10.1002/ece3.3302
Gossner MM, Weisser WW, Meyer ST (2014) Invertebrate herbivory decreases along a gradient of increasing land-use intensity in German grasslands. Basic Appl Ecol 15:347–352. https://doi.org/10.1016/j.baae.2014.03.003
Hallmann CA, Sorg M, Jongejans E et al (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Helden AJ, Leather SR (2004) Biodiversity on urban roundabouts—Hemiptera, management and the species-area relationship. Basic Appl Ecol 5:367–377
Helden AJ, Anderson A, Sheridan H, Purvis G (2010) The role of grassland sward islets in the distribution of arthropods in cattle pastures. Insect Conserv Divers 3:291–301. https://doi.org/10.1111/j.1752-4598.2010.00106.x
Helden AJ, Anderson A, Finn J, Purvis G (2015) The response of sward-dwelling arthropod communities to reduced grassland management intensity in pastures. Ir J Agric Food Res 54:107–120. https://doi.org/10.1515/ijafr-2015-0012
Helden AJ, Morley GJ, Davidson GL, Turner EC (2018) What can we do for urban insect biodiversity? Applying lessons from ecological research. Zoosymposia 12:51–63
Hof AR, Bright PW (2010) The impact of grassy field margins on macro-invertebrate abundance in adjacent arable fields. Agric Ecosyst Environ 139:280–283
Humbert JY, Ghazoul J, Walter T (2009) Meadow harvesting techniques and their impacts on field fauna. Agric Ecosyst Environ 130:1–8
Kormann U, Rösch V, Batáry P et al (2015) Local and landscape management drive trait-mediated biodiversity of nine taxa on small grassland fragments. Divers Distrib. https://doi.org/10.1111/ddi.12324
Kruess A, Tscharntke T (2002) Contrasting responses of plant and insect diversity to variation in grazing intensity. Biol Conserv 106:293–302
Lenda M, Skórka P, Moroń D et al (2012) The importance of the gravel excavation industry for the conservation of grassland butterflies. Biol Conserv 148:180–190. https://doi.org/10.1016/j.biocon.2012.01.014
Littlewood NA, Stewart AJA, Woodcock BA (2012) Science into practice—how can fundamental science contribute to better management of grasslands for invertebrates? Insect Conserv Divers 5:1–8. https://doi.org/10.1111/j.1752-4598.2011.00174.x
Morris MG (1969) Differences between the invertebrate faunas of grazed and ungrazed chalk grassland. III. The Heteropterous fauna. J Appl Ecol 6:475–487
Morris MG (1971) Differences between the invertebrate faunas of grazed and ungrazed chalk grassland. IV. Abundance and diversity of Homoptera-Auchenorhyncha. J Appl Ecol 8:37–52
Morris MG (1973) The effects of seasonal grazing on the Heteroptera and Auchenorhyncha (Hemiptera) of chalk grassland. J Appl Ecol 10:761–780
Morris MG (2000) The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biol Conserv 95:129–142
Nickel H, Achtziger R (2005) Do they ever come back? Responses of leafhopper communities to extensification of land use. J Insect Conserv 9:319–333
Nickel H, Hildebrandt J (2003) Auchenorrhyncha communities as indicators of disturbance in grasslands (Insecta, Hemiptera)—a case study from the Elbe flood plains (northern Germany). Agric Ecosyst Environ 98:183–199
Öckinger E, Eriksson AK, Smith HG (2006) Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biol Conserv 133:291–300. https://doi.org/10.1016/j.biocon.2006.06.009
Oksanen J, Blanchet FG, Friendly M et al (2017) vegan: Community Ecology Package. R package version 2.4-3. https://CRAN.R-project.org/package=vegan
Paoletti MG, Hassall M (1999) Woodlice (Isopoda: Oniscidea): their potential for assessing sustainability and use as bioindicators. Agric Ecosyst Environ 74:157–165. https://doi.org/10.1016/S0167-8809(99)00035-3
Pärtel M, Bruun HH, Sammul M (2005) Biodiversity in temperate European grasslands: origin and conservation. Grassland science in Europe. Lund University, Sweden, pp 1–14
Pykälä J (2000) Mitigating human effects on European biodiversity through traditional animal husbandry. Conserv Biol 14:705–712
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rand TA, Tylianakis JM, Tscharntke T (2006) Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett 9:603–614. https://doi.org/10.1111/j.1461-0248.2006.00911.x
Rook AJ, Tallowin JRB (2003) Grazing and pasture management for biodiversity benefit. Anim Res 52:181–189
RSPB (2011) Natural England Fund transforms Quarry into Wildlife Haven. https://www.rspb.org.uk/about-the-rspb/about-us/media-centre/releases/details.aspx?id=279548. Accessed 4 July 2017
Sankaran M, Augustine DJ (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Ecology 85:1052–1061. https://doi.org/10.1890/03-0354
Schaffers AP, Raemakers IP, Sýkora KV, ter Braak CJF (2008) Arthropod assemblages are best predicted by plant species composition. Ecology 89:782–794. https://doi.org/10.1890/07-0361.1
Sheridan H, Finn JA, Culleton N, O’Donovan G (2008) Plant and invertebrate diversity in grassland field margins. Agric Ecosyst Environ 123:225–232
Siemann E, Tilman D, Haarstad J, Ritchie M (1998) Experimental tests of the dependence of arthropod diversity on plant diversity. Am Nat 152:738–750
Snazell R, Clarke R (2000) The colonisation of an area of restored chalk downland by spiders (Araneae). In: Gajdoš P, Pekár S (eds) Proceedings of the 18th European Colloquium of Arachnology, Stará Lesná, 1999. pp 263–271
Souty-Grosset C, Badenhausser I, Reynolds JD, Morel A (2005) Investigations on the potential of woodlice as bioindicators of grassland habitat quality. Eur J Soil Biol 41:109–116
Stewart AJA (2012) Checklist of Auchenorrhyncha. http://www.ledra.co.uk/species.html. Accessed 4 July 2017
Tälle M, Deák B, Poschlod P et al (2016) Grazing vs. mowing: a meta-analysis of biodiversity benefits for grassland management. Agric Ecosyst Environ 222:200–212. https://doi.org/10.1016/j.agee.2016.02.008
Thomas CFG, Marshall EJP (1999) Arthropod abundance and diversity in differently vegetated margins of arable fields. Agric Ecosyst Environ 72:131–144
Tropek R, Kadlec T, Karesova P et al (2010) Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J Appl Ecol 47:139–147. https://doi.org/10.1111/j.1365-2664.2009.01746.x
van Klink R, van der Plas F, van Noordwijk CGET et al (2015) Effects of large herbivores on grassland arthropod diversity: large herbivores and arthropods. Biol Rev 90:347–366. https://doi.org/10.1111/brv.12113
Vickery J, Gill JA (1999) Managing grassland for wild geese in Britain: a review. Biol Conserv 89:93–106. https://doi.org/10.1016/S0006-3207(98)00134-7
Vickery JA, Tallowin JR, Feber RE et al (2001) The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. J Appl Ecol 38:647–664
Walker KJ, Stevens PA, Stevens DP et al (2004) The restoration and re-creation of species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biol Conserv 119:1–18
WallisDeVries MF (1998) Large herbivores as key factors for nature conservation. In: WallisDeVries MF, Bakker JP, Van Wieren SE (eds) Grazing and conservation management. Kluwer Academic, Dordrecht, pp 1–20
White G, Self M, Blyth S (2014) Bringing reedbeds to Life: creating and managing reedbeds for wildlife. RSPB, Sandy
Whittingham MJ, Devereux CL (2008) Changing grass height alters foraging site selection by wintering farmland birds. Basic Appl Ecol 9:779–788. https://doi.org/10.1016/j.baae.2007.08.002
Woodcock BA, Bullock JM, Mortimer SR, Pywell RF (2012) Limiting factors in the restoration of UK grassland beetle assemblages. Biol Conserv 146:136–143. https://doi.org/10.1016/j.biocon.2011.11.033
Woodcock BA, Potts SG, Pilgrim E et al (2007) The potential of grass field margin management for enhancing beetle diversity in intensive livestock farms. J Appl Ecol 44:60–69
Wotton S, Brown A, Burn A et al (2009) Boom or bust—a sustainable future for reedbeds and bitterns? Br Wildl 20:305–315
Acknowledgements
We are very grateful to the RSPB and Hanson/Heidelberg Cement who gave us access to the Needingworth site. In particular we would like to thank Hilton Law from Hanson, and Chris Hudson and Barry O’Dowd from the RSPB, for their assistance and encouragement. The project was supported by an Anglia Ruskin University, Faculty of Science and Technology Undergraduate Summer Research Placement for James Chipps. Luiza Pereira worked on the project as part of her Science Without Borders scholarship to Anglia Ruskin University. We thank the Anglia Ruskin University Wildlife Society for providing help in fieldwork and the sorting of suction samples. Specifically we would like to thank, Liam Smith, Steve Allain, Mark Goodman and David Pereira for assisting with the suction sampling, and Steve Allain, Claire Cable, Mark Goodman, Toni Mott and David Pereira for sorting samples. The project was awarded first place in the Heidelberg Cement, UK national Quarry Life Awards for 2014.
Funding
The project was supported by an Anglia Ruskin University, Faculty of Science and Technology Undergraduate Summer Research Placement for James Chipps.
Author information
Authors and Affiliations
Contributions
AJH designed the study, did field work; identified the Hemiptera, did the analysis and wrote the paper; JC did field work, co-ordinated student helpers, and sorted samples to order level; LP did field work, and sorted samples to order level; SM identified the Coleoptera.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed involving animals (as above) were approved by the Anglia Ruskin University Ethics Committee and in accordance with the ethical standards of the Anglia Ruskin University, at which the studies were conducted.
Research involving human and/or animal participants
This article does not contain any studies with human participants performed by any of the authors. It involved the collection of invertebrate specimens but the sampling design was such that it covered a very small area of the site (0.001% by area) such that the impact on invertebrate populations was minimal. All procedures performed involving animals (as above) were approved by the Anglia Ruskin University Ethics Committee and in accordance with the ethical standards of the Anglia Ruskin University, at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Helden, A.J., Chipps, J., McCormack, S. et al. Is grazing always the answer to grassland management for arthropod biodiversity? Lessons from a gravel pit restoration project. J Insect Conserv 24, 655–670 (2020). https://doi.org/10.1007/s10841-020-00243-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00243-1