Abstract
Riparian zones (RZs) functionally connect aquatic and terrestrial ecosystems, and have azonal and geographically widespread plant communities that differ from those of the neighboring terrestrial zone (TZ). Although well studied botanically, RZs are not well understood in terms of their terrestrial insect diversity, including grasshoppers. The Cape Floristic Region (CFR) is a global biodiversity hotspot with small rocky rivers running through highly diverse sclerophyllous vegetation. It has high levels of endemism among many taxa, including grasshoppers, making it ideal for testing the effect of azonal vegetation on grasshopper assemblages of the RZ, and determining whether conservation efforts should be focused on the RZ as well as the TZ. We determine grasshopper dispersion patterns along the RZ of an important CFR river, and compare these patterns with those of the TZ to understand the habitat occupancy relative to 27 environmental variables of the zones and geographical distribution of the grasshoppers. Forty percent of individuals we collected were CFR endemics. We found only weak differences in the grasshopper assemblages between the RZ and TZ, apparently driven by deep history, complex geomorphology, stressful environmental conditions, a diverse vegetation and land mosaic, and probable high predator pressure. There were two groups: large-sized, well-flighted, geographically widespread generalists that were overall more abundant in the RZ than TZ, and small, flightless or poorly-flighted, vegetation-specialists which are narrow-range endemics adapted to both RZ and TZ, but still more abundant in the TZ. We conclude that although the vegetation of this riparian zone may require some special conservation attention, this is not so for the grasshoppers which overall are best conserved in the TZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural riparian zones (RZs) are linear landscape elements and ecotones that encompass portions of both aquatic and terrestrial environments (Naiman and Decamps 1990). They are complex and dynamic biophysical systems, which in the larger landscape have a diverse mosaic of habitats, microhabitats and communities (Naiman et al. 1993). RZs are also key landscape features with considerable regulatory control over environmental vitality (Naiman et al. 1992; Reinecke 2013), including light, temperature and nutrient regulation.
It is often unclear how many faunal species are present in RZs as many may use the RZ as a conduit for movement and are present one moment but not the next (Nilsson 1992). Furthermore, RZs are a mediator of community movement between the aquatic and terrestrial zones (TZs), and are through-flow systems between these two zones, as well as along the length of the river. In short, RZs provide connectivity between the aquatic and terrestrial systems as well as along the river for energy flow, matter and organisms (Ward et al. 2002). This landscape connectivity generally enhances population viability for numerous species so enhancing biodiversity maintenance (Samways and Pryke 2016). Alternatively, in threatened habitats, this conduit may serve as an introduction pathway for widespread, generalist, species which can out-compete localized specialists for resources.
Typically, RZ vegetation is categorized globally as azonal. Azonal plant communities are those influenced more strongly by edaphic factors than by local climate, and include wetlands and alluvial vegetation, as well as riparian vegetation. RZ vegetation plays an important role in the functioning of riverine ecosystems (Sieben 2000). This RZ azonal vegetation is highly dynamic and diverse, and provides a wide range of ecological niches (Sieben and Reinecke 2008; Sieben et al. 2009).
There has been little focus on the relationship between RZs and TZs in terms of their terrestrial arthropod assemblages (Paetzold et al. 2005), although there can be an association between terrestrial arthropods and those in the RZ (Nelson 2007; Paetzold et al. 2008; Capello et al. 2012), and certain animal species (Lepidoptera and birds) can be indicative of riparian vegetation quality and health (Nelson and Andersen 1994; Innis et al. 2000; Bryce et al. 2002), with many species particular to RZs (Sabo et al. 2005) and with specialized life history traits (Lambeets et al. 2009).
The Cape Floristic Region (CFR) is a global biodiversity hotspot with a rugged terrain and many small rivers. Characteristically it has high levels of diversity of plants (Linder 2003) and insects (Johnson 1992; Wright and Samways 1998; Picker and Samways 1996; Proçhes and Cowling 2006), including Orthoptera (Adu-Acheampong et al. 2016; Naskrecki and Bazelet 2009; Matenaar et al. 2015).
The CFR is dominated by the Fynbos Biome, with many species of plants being highly localized, and groups of them clustering into ‘centers of endemism’ (Rebelo and Low 1996), as with dragonflies (Grant and Samways 2007, 2011). The main plant families that characterize the fynbos are the Ericaceae, Proteaceae and Restionaceae (Cowling et al. 1989). Endemic CFR grasshoppers have adapted to the fynbos terrain and to the vegetation composition and structure, as well as to the low nutritional value of the fynbos plants. Such grasshopper-plant specialization is prominent in the Lentulidae, including species of Betiscoides, which are flightless and associated with the Restionaceae (Key 1937), where they are camouflaged by their coloration, small size, and slender body shape (Matenaar et al. 2015).
The terrestrial vegetation of the CFR is of a different structure from that of the RZ, even though both zones contain endemic and fynbos specific plants. The RZ is usually dominated by larger plant structures (trees) than the TZ (Sieben 2000). In general, fynbos is composed of a high turnover of patchily-distributed, bushy and herbaceous plant species (Sieben et al. 2009) that are adapted to nutrient poor soils, having low productivity, and providing little nourishment (high fibre to protein and water ratio) for phytophagous insects (Giliomee 2003).
Grasshopper assemblages of the CFR cover a spectrum of species from those that are narrow range endemics to those that are widespread in Africa (Schlettwein and Giliomee 1987). So, here we focus on occupancy patterns of grasshoppers in the RZ, and ask whether the grasshopper assemblages are the same or different from those in the adjacent TZ. However, grasshoppers are not common in natural habitat of the CFR (Schlettwein and Giliomee 1987; Matenaar et al. 2014), with rarity a common feature among all species, especially the endemics, making sampling challenging. As the CFR RZ plant assemblages are azonal, thus containing a diversity of structural types to which grasshoppers have a known association, we hypothesize that the local RZ herbivorous grasshoppers will have a higher proportion of geographically widespread species than specialized narrow-range endemics in comparison with the TZ. If this is the case, the RZ grasshoppers may not require special protection separate from those in the TZ.
Sites and methods
Study area and sites
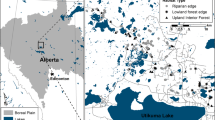
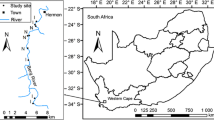
Our study was conducted along the Lourens River (-34.027651°S 18.959923°E), Somerset West, Western Cape Province, South Africa (Fig. 1). The region has a winter rainfall, and in the mountains there is a mean annual rainfall of 1200 mm, whereas at lower elevations the amount is 915 mm. The area is relatively windy with occasional very strong winds, and wind direction is usually from the south-east or north-west, averaging 4–6.5 m/s. The area is dominated by mountain fynbos, with pockets of afromontane forests in the ravines, Boland granite fynbos, shale Renosterveld and Lourensford alluvium fynbos. Boland granite fynbos is an endangered vegetation type with medium-dense to open tree vegetation within tall, dense proteoid shrubland. Both shale Renosterveld and Lourensford alluvium fynbos are critically endangered vegetation types. Shale Renosterveld has tall, open shrublands and grasslands, whereas Lourensford alluvium fynbos is composed of low to medium-dense shrubland with a short graminoid understory (Mucina and Rutherford 2006).
Study map showing the eight study locations with their four zones (one riparian zone and three terrestrial zones). The grey area represents agriculture, the black lines and area represent rivers and dams, black circle riparian zone, dark grey terrestrial zone 1, light grey terrestrial zone 2, open circles terrestrial zone 3
The RZ included the wet bank zone closest to the river, where the vegetation began, which included bedrock and sand, and where there were considerable water fluctuations, typically low levels in summer and high in winter. We chose eight sampling locations along the river over a distance of 2000 m and covering an elevation range of 291–525 m above sea level. Each location was at >200 m from the next location. Sampling was done on the accessible south side of the river (Fig. 1). Each location was made up of four zones: riparian (i.e. RZ) and three non-riparian zones (here termed terrestrial zones (TZ) 1–3), with each zone parallel to the other. Each zone was 35 m (the actual sampled area, excluding the spaces between transects; see below) in width and 25 m in length (width referring to the distance perpendicular to the river, whereas length refers to the distance parallel to the river). Specifically, the RZ extended from the river’s edge up to 35 m away from that edge [to encompass the marginal, lower and upper zones with alluvial soils (Reinecke 2013)], then there was a gap of 45 m (to avoid spillover effects between the two zones, RZ and TZ) before the first of three TZ transects began (i.e. TZ1 started 80 m from the river’s edge). The 35 m-wide transect was maintained for consistency, and so TZs1, 2 and 3 were all 35 m wide and separated by gaps of 65 m making arbitrary 100 m-wide transects parallel to the river i.e. TZ1 was separated from TZ 2 which in turn was separated from TZ 3 by a 65 m gap. This meant that TZ2 started 180 m and TZ3 started 280 m from the river’s edge.
Each zone (RZ, TZ1, TZ 2 and TZ 3) had seven sampling units (SUs) in the form of 5 m × 25 m (width × length) transects, where grasshopper sampling took place. This meant that the sampling area was 7 × 5 (35) × 25 = 875 m2. These SUs were in a line along each transect and were 2.5 m apart from each other. To summarize the sampling design, each location was made up of four zones (1 RZ, 3 TZs) with seven SU’s each, adding up to 28 SUs per location and 224 SUs in total. Each zone covered a sampling area of 875 m2, each location covered a sampling area of 3,500 m2 (875 m2 × 4) and the entire study covered a sampling area of 28,000 m2 (3,500 m2 × 8).
Grasshopper sampling
Grasshoppers were sampled on warm, sunny and wind-free days, with a minimum temperature of 20 °C, between 9h00 and 17h00. All SUs were sampled twice, to sample early season and late season species, between September 2014 and June 2015, and sampling was across zones at each location to ensure comparison. The aim was to sample all grasshopper individuals in the SUs (5 m × 25 m) using a combination of sampling methods to ensure that the maximum number of individuals were sampled. Grasshoppers were sampled along each transect of every location, walking the length (25 m) of the SU and then walking back along the same SU, resulting in a sampled length of 50 m (indirectly having a transect of 5 × 50 m). The reason for this double pass was that some grasshoppers are more elusive than others and could only be detected when returning along the length of the SU (25 m). Only adults were used in analyses to ensure correct identification.
Grasshoppers were also sampled by flushing (Gardiner et al. 2002), active searching (especially within the restio stands), direct observation, and with supplementary sweep netting (Gardiner et al. 2005). We swept along the SU over the vegetation 20 times every 3 m in one direction. This was also repeated on the way back. The net was checked for any grasshopper individuals after every sweep. Sampled individuals were retained with details of date, elevation and GPS coordinates, and placed in a freezer for 2–3 days, and then later pinned. Specimens were identified to species level using all available literature, especially Dirsh (1965), Spearman (2013) and Eades et al. (2015).
Environmental variables
In all, 27 environmental variables were recorded (Table 2) which relate to distance to the river, cover (vegetation of various types, bare ground and leaf litter), vegetation height (of various vegetation types), elevation, certain combinations of these, and zone (RZ, T1, T2, T3). Vegetation composition, cover and average height were recorded at each SU. Vegetation composition and associated variables were classified into different growth forms: trees, herbaceous plants, shrubs, restio stands, geophytes, ferns (mostly Pteridium aquilinum), dead biomass litter, rock cover and bare ground cover. Bare ground, dead biomass litter, as well as rock cover, were included into vegetation composition and cover, but not height. This overall approach was taken as it is known that vegetation architecture (composition, structure, cover and height) significantly influences grasshopper species presence/absence (Fartmann et al. 2012; Crous et al. 2014; Joubert et al. 2016). Vegetation height was measured in every SU at 5 m intervals with a measuring stick and the average height of each growth form was pooled separately in each 25 m-long SU (i.e. there was a total of 224 × 6 = 1,344 vegetation sample points). The average cover was estimated while walking along the SU (transect). Elevation was recorded at each SU using a Polaris Navigation GPS application version 7.92.
Statistical analyses
The non-parametric species estimators Chao2 and Jackknife2 were calculated in PRIMER 6 (PRIMER-E 2009) to assess the representativeness of sampling. Additionally, we calculated the grasshopper conservation index (GCI) for each zone in each location (n = 28). GCI is a method for estimating the conservation value of a site on the basis of the conservation value of the individual grasshopper species at that site. Scores are assigned to each grasshopper species on the basis of their traits. The more endemic, sedentary, and rare a species, the more sensitive it is considered to be, and the higher its GCI score. To calculate GCI for a site, the GCI values of each individual species occurring at that site are summed. The higher the value, the higher the conservation priority of the site. Standardized GCI (GCIn) is calculated in a similar way but the sum of all species’ GCIs is then divided by the species richness at the site, and the resulting value is a measure of the mean species value at the site (see Matenaar et al. 2015 for details of calculation).
In order to model the response of the grasshopper assemblages to the environmental variables, Generalized Linear Mixed Models (GLMMs) were calculated using the MASS package in R (R Development Core Team 2015), using the penalized quasi-likelihood estimation method and data fitted to a Poisson distribution (Bolker et al. 2009). These data were tested for spatial autocorrelation using a semivariogram. When a random, dummy variable was exponentially correlated to longitude and latitude it improved the semivariogram (Dormann et al. 2007). Correlated longitudinal and latitudinal data were used as the random variable to overcome spatial autocorrelation in the data for all GLMM.PQL analyses. These analyses were done for five categories of grasshoppers: (1) the overall assemblage, (2) CFR endemic species, (3) geographically widespread species in southern Africa and beyond, and (4) flightless species (a subset of the CFR endemics). As all flightless individuals totaled no more than one per site, we analyzed only species richness for this group. For the four most commonly sampled species (those with abundance >10 individuals), abundances were also analyzed.
For each of the response variables, we created four models: the first had distance to river, elevation, average vegetation cover and height as fixed variables, the second had the same variables as the first model with the first order interactions also included. A third model was created with all distances to river, elevation and the vegetation cover and height categories (per vegetation type) as described above included as fixed variables. These three were analyzed using package GLMM.PQL. The fourth model was a GLMM using a Laplace approximation and data fitted to a Poisson distribution using the lme4 package in R (Bates 2005). This model had the zones (RZ, TZ1-3) described above as the fixed effect and elevation, average vegetation cover and height as random effects. Pairwise Tukey post-hoc tests were performed on all significant results using the multcomp package in R (Hothorn et al. 2008). Finally, a LMM was run on the GCI and GCIn scores with Elevation as a random effect. Pairwise tests were conducted on significant results.
Non-metric multidimensional scaling (nMDS) and distance based redundancy analyses (db-RDA) were performed in Primer 6 to visually assess patterns of similarity in the grasshopper assemblage. To test for differences in assemblage structure among the riparian and three terrestrial zones, permutational multivariate analyses of variance (PERMANOVA) were performed using 9,999 permutations and elevation as a random variable in PERMANOVA (Anderson 2006; PRIMER-E 2009). Distance based linear models (DISTLM) were used to determine the effect that distance to river, elevation, average vegetation cover and height as well as vegetation cover and height (per vegetation type) as described above. All the environmental variables were normalized. nMDSs and PERMANOVAs were based on Bray-Curtis similarity matrices derived from square root transformed abundance data (Anderson 2001).
Results
Grasshopper species richness, abundance and GCI scores
Despite sampling 28,000 m2 in this CFR global biodiversity hotspot, only a total of ten species (314 individuals) belonging to six subfamilies and two families, were sampled across the entire study. Species accumulation curves showed a near asymptote, with Chao2 estimates of 14.50 (±7.19), Jackknife1 = 12.98 and Jackknife2 = 14.95. Of the ten species sampled, four are endemic to the CFR, eight are South African species, while only two are widespread generalist species. A total of 93 CFR endemic individuals, 201 South African individuals and 20 widespread individuals were sampled. Three of the species captured were flightless CFR endemics (Table 1).
None of the assemblages sampled showed a significant response in either species richness or abundance to distance to river or elevation (Table 2). The overall grasshopper assemblage showed a significantly positive correlation between species richness and vegetation height, elevation, rock cover, bare ground cover, tree cover, shrub cover and restio cover (Table 2). Many of these variables are correlated to distance to river (Supplementary Material 1). The overall abundance was positively correlated to tree cover (Table 2). The widespread species were positively correlated to tree cover only (Table 2). Overall species richness showed an interaction between vegetation height and elevation with high richness at low elevations in low vegetation, and in tall vegetation at higher elevations (Table 2). The CFR and flightless species richness and abundance showed an interaction between distance to river and vegetation height, with high species richness in tall vegetation far from the river (Table 2). When the four zones were analyzed separately, we found that only the CFR endemics had significantly higher species richness in TZ1 vs. the RZ vs. (Table 2; Fig. 2).
The GCI scores showed significant differences between the four zones (χ2 = 11.48, p = 0.0094), with T1 significantly higher than both T2 (z = 5.08, p < 0.001) and T3 (z = 4.80, p < 0.001) (Fig. 3). GCIn showed no significant difference between zones (χ2 = 3.47, p = 0.3242), although there was a general pattern of an increasing score the farther the TZ zone was from the RZ (Fig. 3).
Abundance of the most common species
The four most commonly collected species (>10 individuals were collected per species) were also analyzed individually: Betiscoides sp. (Lentulidae, Lentulinae), was the only CFR endemic species of these four species. Betiscoides sp. abundance showed a significantly positive relationship to elevation and to a combination of all vegetation cover and all height variables (Table 3).
Vitticatantops humeralis (Acrididae, Catantopinae) is a widespread species but confined to South Africa which was significantly negatively influenced by rock cover and leaf litter cover (Table 3). Eyprepocnemis calceata (Acrididae, Eyprepocnemidinae), also a South African species, was significantly more abundant in the riparian zone compared to TZ 1 and TZ 2 although this was not explained by any of the environmental variables or their interaction terms (Table 3; Fig. 2). Acanthacris ruficornis (Acrididae, Cyrtacanthacridinae), an African widespread species, was significantly more abundant in areas with greater restio and fern height (Table 3).
Response of assemblage composition to environmental variables
Overall, distance to river was the strongest explanatory variable for grasshopper species composition (Table 4; Fig. 4). Elevation, fern cover, shrub cover and bare ground cover also significantly influenced the species composition (Table 4; Fig. 4). The zone in which the SU lay also significantly influenced the assemblage (Pseudo-F = 2.31, p = 0.02), with pairwise analyses showing that this was due to the riparian zone having a different assemblage to the three terrestrial zones (RZ-TZ1: t = 1.90, p = 0.015; RZ-TZ2 t = 1.74, p = 0.030; RZ- TZ3 t = 2.74, p < 0.001) and not between the terrestrial zones (TZ1-TZ2: t = 0.51, p = 0.871; TZ1-TZ3: t = 0.123, p = 0.975; TZ2-TZ3: t = 1.31, p = 0.167).
Distance-based redundancy analysis of the best fit model that explains the distribution of the data the best, with all the significant variables in the analyses (circle around the variables describes their plot limit). The site data are riparian zone (black triangles), terrestrial zone 1 (grey triangles), terrestrial zone 2 (grey squares), and terrestrial zone 3 (open circles)
Discussion
In contrast to the strong RZ and azonal patterns in plants (Sieben et al. 2009), we found only weak differences in grasshopper assemblages between the RZ and overall TZ. Also, there was little evidence for higher proportion of widespread generalist species in the RZ as occurs among plants. Our grasshopper findings contrast with high elevation rivers in Germany where the RZ supported two specialized riparian grasshopper species, with one (Bryodema tuberculata) even having been identified as an indicator of high quality riverbanks (Reich 1991).
The CFR endemic and flightless species were most species rich in vegetation far from the river. One South African species (E. calceata) and one widespread species (A. ruficornis) were more abundant in the RZ than in the overall TZ. Despite the relatively weak correlations at species and species group levels, distance from the river was nevertheless the strongest explanatory variable of species composition. This was counter-intuitive since, interestingly, even though some grasshopper species and assemblages in the RZ differed significantly from those in the overall TZ, no differences were apparent among the three TZ zones, indicating that grasshoppers were sensitive to the azonal vegetation and unique microhabitat elements within the RZ, rather than to the distance from the river itself.
Regardless of the reason, GCI indicated that the TZ closest to the river (TZ1) was of significantly higher conservation value than the two TZs located farther from the river (TZ2 and TZ3). This can best be explained by the presence in TZ1 of two species with high GCI values [Frontifissia laevata (GCI = 1) and Keya capicola (GCI = 0.78)] and two species with intermediate GCI values [Heteropternis pudica (GCI = 0.67), and Sphingonotus nigripennis (GCI = 0.67)] which were both absent from TZ2 and TZ3 (Table 1). The presence of these additional four species skewed the summed GCI, which is sensitive to species richness, higher for TZ1. However, when species richness was controlled for by calculating GCIn, there was no significant difference among zones. In fact, GCIn displayed a slight (but insignificant) trend towards higher values with increasing distance from the river. Since GCIn is equivalent to the mean score for all species in the zone, this indicates the presence of more species of high conservation value farther away from the river.
The common species were generally evenly distributed across the RZ and three TZs. Overall zone (RZ vs. TZ) played a much less significant role in determining the grasshopper assemblage than did proportional vegetation cover. Percent cover of leaf litter, bare ground, trees, shrubs and restios influenced the distribution of CFR and South African species. Vegetation cover, however, played a much lesser role for the widespread species, which were most strongly influenced by the interaction of vegetation height and elevation, with greatest species richness in short vegetation at low elevations and in tall vegetation at high elevations.
Grasshopper species richness and abundance are known to be influenced by the physical structure and composition (e.g. density and cover) of vegetation, making vegetation a key factor determining the presence and local distribution of grasshopper species in many parts of the world (Gardiner et al. 2002; Gebeyehu and Samways 2002; Squitier and Capinera 2002; Hochkirch and Adorf 2007; Gardiner and Hassall 2009; Bazelet and Samways 2011; Fartmann et al. 2012; Joern and Laws 2013; Joubert et al. 2016). While a small minority of grasshopper species are monophagous herbivores which are present only in association with their preferred host plant (Bidau 2014), the majority are generalist herbivores which are more sensitive to the structure of vegetation than to the specific plant species present (García-García et al. 2008; Gebeyehu and Samways 2002; Bazelet and Samways 2011). Other species occur in association with specific habitat features such as exposed ground or rocky habitats (Crous et al. 2014), or with trees for perching habitats (Bazelet and Samways 2014).
Some grasshopper species (Acrididae) worldwide are known to have an association with aquatic habitats, without having evolved a completely aquatic lifestyle (Amédégnato and Devriese 2008; Bidau 2014). In our study, the widespread E. calceata, a member of the Eyprepocnemidinae, is an example of this, being the only common species which was significantly more abundant in the RZ than in the TZ. In the CFR, the only grasshoppers that have a strong association with freshwater habitats are the acridine, Paracinema tricolor, an inhabitant of wetlands and extensive margins of rivers, and pygmy grasshoppers (Tetrigidae) (Picker et al. 2004), which are associated with damp areas and require specific searches which we did not undertake here. It is therefore not surprising that we did not find these species here along this river with its little marginal wetland vegetation and largely scoured, rocky margins. This is also a reason perhaps why we did not find a specialized riparian grasshopper fauna compared to in Germany where the river banks are more extensive (with floodplains) in their vegetation component, river plain habitats, and by having different, more favorable, river dynamics (Reich 1991).
The second most abundant species in the RZ (although this association was not statistically significant) was the garden locust, A. ruficornis (Cyrtacanthacridinae), a strong flier which is a well-known visitor of gardens and other cultivated areas throughout the CFR. This species is a close relative of the bird locust, Ornithacris cyanea, which is known to use trees as perching sites on South Africa’s East Coast (Bazelet and Samways 2014). The observed association of the garden locust with the RZ can possibly be explained by the presence of trees, which are far more abundant in the RZ than they are in the overall TZ.
The CFR endemic grasshoppers that were sampled belong to the Acrididae and Lentulidae. Most of the CFR endemic grasshoppers were widely dispersed and relatively abundant across both the RZ and TZs, agreeing with García-García et al. (2008) that some grasshoppers can use different habitats. Here however, spatial scale is probably important, with micro-habitats likely to be the key for many of our species. It appears that a mosaic of plant architectures and plant resources are required, with restio stands being perhaps important for functional connectivity, especially as feeding choice might be very narrow for certain species. Other aspects to consider are environmental, with wet windy winters and dry hot summers creating the need for shelter in both seasons (Matenaar et al. 2014).
The flightless lentulid Betiscoides sp. was relatively common and dispersed across both the RZ and TZs, especially where both shrub and herb vegetation types were present as well as its restio food plant. It was absent from fern stands (mostly bracken P. aquilinum), as was largely the case with other CFR endemics, probably because of total dominance and the shading out of under story food plants and absence of sun-basking sites. Ferns often colonize habitats that have been disturbed by wind, water, fire or anthropogenic activities, with them usually colonizing recently disturbed and exposed areas such as riverbanks i.e. RZ (Mehltreter et al. 2010). The presence of fern stands here indicates that there was a disturbance (almost certainly by fire) which by extrapolation has a delayed but reducing effect on CFR endemic grasshopper richness and abundance (Schlettwein and Giliomee 1987), as we found here.
The conservation perspective is interesting in that in the CFR the RZ vegetation is of conservation significance in its own right over the TZ vegetation, with the endemic grasshoppers preferring the TZ. This difference between the RZ and TZ does not hold the same importance for grasshoppers. So while invasion of the RZ by alien trees can lead to local extinction of the endemic dragonflies and other stream insects (with recovery on removal of alien trees) (Samways and Sharratt 2010; Samways et al. 2011) there is no special significance attached to the RZ endemic grasshopper fauna which is better represented in the TZ.
Conclusions
The RZ of the CFR river we studied has various features that blend in with that of the overall TZ, and that while botanists may identify a clear RZ (with various sub-zones) (Reinecke 2013), the azonal effect identified by botanists for the overall RZ is present in grasshoppers but is very weak, with changes manifesting only small changes in grasshopper assemblage composition. Like plants, the grasshopper shifts are towards the widespread generalist elements in the RZ and the specialist endemics in the overall TZ. It is the geomorphology, history, ecological dynamics, as well as the land and vegetation mosaics at the small spatial scale, that are all important here. The CFR has seen no impoverishing glaciations for >200 my, and the surface rocks of our RZ as well as the TZs were formed >300 mya, giving much time for adaptation and evolution of species across a greatly uplifted and weathered landscape. Nevertheless, these species (and perhaps others that have since become extinct) would still have been greatly affected in terms of their local distribution and abundance by climate change (e.g. the warm, high sea level of the Pliocene and the cool, low sea level of the Pleistocene maxima). Many plant species have adapted to this, leaving little horizontal bare ground, disfavoring the oedipodines which prefer gravel and dry soil habitats, and which have been largely washed away or eroded over many millennia at our sites.
Despite the intensive sampling and near asymptotes being reached, we found few species (ten) and few individuals (314). This low diversity and abundance in the CFR has already been commented on by Schlettwein and Giliomee (1987). They recorded only about a third the number of species compared to Gandar (1983) in the savanna to the north of the country. Furthermore, we recorded only about a fifth the number of species compared to similar-sized studies by Crous et al. (2013) and Joubert et al. (2016) along the warmer east coast of South Africa. Schlettwein and Giliomee (1987) also found that densities were remarkably low in the CFR, being >18× lower than Gandar (1983) recorded overall in African savanna, and 164× lower than Pfadt (1981) recorded in North American shortgrass prairie.
Natural fires are common in the CFR, leading to a highly fire-adapted flora and fauna (Schlettwein and Giliomee 1987). Local ferns, especially the globally successful bracken, benefit from these fires and establish as thick stands to the exclusion of much of the grasshopper fauna. In turn, fires interplay with the highly rugose geomorphology, patterns of rainfall seepage, poor nutrients, to create a dynamic and rich but highly patchy flora. These drivers have a major effect on the grasshopper assemblages. This mosaic vegetation effect appears to benefit the local assemblages, and largely overrides any azonal effect, although not leading to either a species-rich or abundant local grasshopper fauna. While the plant diversity in our area is extremely high, the same cannot be said for the grasshoppers, with them not having the same level of species spatial turnover as plants.
The CFR, although seemingly a benign climate, represents harsh conditions for many grasshopper species, especially from the effects of wet, windy winters and very dry summers, and many vertebrate predators (e.g. Alexander 2007), as well as frequent fires (Seydack et al. 2007). It appears that the group of CFR endemic grasshoppers may have opted for camouflage and small size alongside possible retreat into thorny fynbos shrubs and crevices to avoid both fire and predators as farther north in the country (Samways 1990). The other group of grasshoppers is the larger, geographically widespread species that fly well and benefit from trees, and can fly away from fires and predators. These benefit from the RZ as additional forage areas and fire refuges, whereas the CFR endemics get the added benefit from the overall TZ through better functional connectivity provided by the natural plant mosaic, especially presence of pioneering restios. It is the TZ that has particular significance for the local grasshopper fauna with no special need to conserve those of the RZ.
References
Adu-Acheampong S, Bazelet CS, Samways MJ (2016) Extent to which an agricultural mosaic supports endemic species-rich grasshopper assemblages in the Cape Floristic Region biodiversity hotspot. Agric Ecosyst Environ 227: 52–60
Alexander G (2007). A guide to the reptiles of Southern Africa. Struik, Cape Town
Amédégnato C, Devriese H (2008) Global diversity of true and pygmy grasshoppers (Acridomorpha, Orthoptera) in freshwater. Hydrobiologia 595:535–543
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Bates D (2005) Fitting linear mixed models in R. Using the lme4 package. R News 5:27–30
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Indicat 11:1259–1269
Bazelet CS, Samways MJ (2014) Habitat quality of grassland fragments affects dispersal ability of a mobile grasshopper, Ornithacris cyanea (Orthoptera: Acrididae). Afr Ent 22:714–725
Bidau CJ (2014) Patterns in Orthoptera biodiversity. I. Adaptations in ecological and evolutionary contexts. J Insect Biodiver 2:1–39
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bryce SA, Hughes RM, Kaufmann PR (2002) Development of a bird integrity index: using bird assemblages as indicators of riparian condition. Environ Manag 30:294–310
Capello S, Marchese M, de Wysiecki ML (2012) Feeding habits and trophic niche overlap of aquatic Orthoptera associated with macrophytes. Zool Stud 51:51–58
Cowling RM, Gibbs Russell GE, Hoffman MT, Hilton-Taylor C (1989) Patterns of plant species diversity in southern Africa. In: Huntley BJ (ed) Biotic diversity in Southern Africa. Concepts and conservation. Oxford University Press, Cape Town, pp 19–50
Crous C, Samways MJ, Pryke JS (2013) Exploring the mesofilter as a novel operational scale in conservation planning. J Appl Ecol 50:205–214
Crous CJ, Samways MJ, Pryke JS (2014) Grasshopper assemblage response to surface rockiness in Afro-montane grasslands. Insect Cons Divers 7:185–194
Dirsh VM (1965) The African genera of Acridoidea. Anti-Locust Research Centre at the University Press, Cambridge
Dormann CF, Mcpherson JM, Arau MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling WD, Ohlemu R, Peres-Neto PR, Schurr FM, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Eades DC, Otte D, Cigliano MM, Braun H (2015) Orthoptera Species File. Version 5.0/5.0. http://Orthoptera.SpeciesFile.org. Accessed June 2015
Fartmann T, Krämer B, Stelzner F, Poniatowski D (2012) Orthoptera as ecological indicators for succession in steppe grassland. Ecol Indicat 20:337–344
Gandar MV (1983) Ecological notes and annotated checklist of the grasshoppers (Orthoptera: Acridoidea) of the Savanna Ecosystem Project Study Area, Nylsvley. South African National Scientific Programmes Report No. 74: 42 pp. Pretoria, South Africa
García-García PL, Fontana P, Marini L, Equihua-Martínez A, Sánchez-Escudero J, Valdez-Carrasco J, Cano-Santana Z (2008) Habitat association of orthoptera in El Cimatario National Park, Querétaro, México. J Orth Res 17:83–87
Gardiner T, Hassall M (2009) Does microclimate affect grasshopper populations after cutting of hay in improved grassland? J Insect Conserv 13: 97–102
Gardiner T, Hill J, Chesmore D (2005) Review of the methods frequently used to estimate the abundance of Orthoptera in grassland ecosystems. J Insect Conserv 9:151–173
Gardiner T, Pye M, Field R, Hill J (2002) The influence of sward height and vegetation composition in determining the habitat preferences of three Chorthippus species (Orthoptera: Acrididae) in Chelmsford, Essex, UK. J Orth Res 11:207–213
Gebeyehu S, Samways MJ (2002) Grasshopper assemblage response to a restored national park (Mountain Zebra National Park, South Africa). BiodiversConserv 11:283–304
Giliomee JH (2003) Insect diversity in the Cape Floristic Region. Afr J Ecol 41: 237–244
Grant PBC, Samways MJ (2007) Montane refugia for endemic and Red Listed dragonflies in the Cape Floristic Region biodiversity hotspot. Biodivers Conserv 16:787–805
Grant PBC, Samways MJ (2011) Micro-hotspot determination and buffer zone value for Odonata in a globally significant biosphere reserve. Biol Conserv 144:772–781
Hochkirch A, Adorf F (2007) Effects of prescribed burning and wildfires on Orthoptera in Central European peat bogs. Environ Conserv 34:1617–1625
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometr J 50:346–363
Innis SA, Naiman RJ, Elliot SR (2000) Indicators and assessment methods for measuring the ecological integrity of semi-aquatic terrestrial environments. Hydrobiologia 422:111–131
Joern A, Laws AN (2013) Ecological mechanisms underlying arthropod species diversity in grassland. Ann Rev Entomol 58:19–36
Johnson SD (1992) Plant–animal relationships. (ed. by ) pp. 175–205. In: Cowling RM (ed) The ecology of fynbos: nutrients, fire and diversity. Oxford University Press, Cape Town
Joubert L, Pryke JS, Samways MJ (2016) Positive effects of burning and cattle grazing on grasshopper diversity. Insect Conserv Divers 9:290–301
Key K (1937) New Acrididae from South Africa. Ann South Afr Mus 32:135–167
Lambeets K, Vanderhuchte ML, Maelfait J-P, Bonte D (2009) Integrating environmental conditions and functional life-history traits for riparian arthropod conservation planning. Biol Conserv 142:625–637
Linder HP (2003) The radiation of the Cape flora, southern Africa. Biol Rev Cambridge Philos Soc 78: 597–638
Matenaar D, Bazelet CS, Hochkirch A (2015) Simple tools for the evaluation of protected areas for the conservation of grasshoppers. Biol Conserv 192:192–199
Matenaar D, Broder L, Bazelet CS, Hochkirch A (2014) Persisting in a windy habitat: population ecology and behavioral adaptations of two endemic grasshopper species in the Cape region (South Africa). J Insect Conserv 18:447–456
Mehltreter K, Walker LR, Sharpe JM (2010) Fern ecology. Cambridge University Press, Cambridge
Mucina L, Rutherford MC (2006) The Vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
Naiman RJ, Beechie TJ, Benda LE, Berg DR, Bisson PA, MacDonald LG, O’Connor MD, Olson PL, Steel EA (1992) Fundamental elements of ecologically healthy watersheds in the Pacific Northwest coastal ecoregion. In: Naiman RJ (ed) Watershed management: balancing sustainability and environmental change. Springer, New York, pp 127–1885
Naiman RJ, Decamps H (1990) The ecology and management of aquatic-terrestrial ecotones. Parthenon, UNESCO, Carnforth, Paris
Naiman RJ, Decamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecol Appl 3: 209–212
Naskrecki P, Bazelet CS (2009) A species radiation among South African flightless spring katydids (Orthoptera: Tettigoniidae: Phaneropterinae: Brinckiella Chopard). Zootaxa 2056:46–62
Nelson SM (2007) Butterflies (Papilionoidea and Hesperioidea) as potential ecological indicators of riparian quality in the semi-arid western United States. Ecol Indicat 7:469–480
Nelson SM, Andersen DC (1994) An assessment of riparian environmental quality by using butterflies and disturbance susceptibility scores. Southwest Nat 39:137–142
Nilsson C (1992) Conservation management of riparian communities. In: Hansson L (ed) Ecological principles of nature conservation. Elsevier, Amsterdam, pp 352–372
PRIMER-E (2009) Primer 6 version 6.1.13 and Permanova + version 1.0.3. Primer-E, Ltd
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic-terrestrial linkages along a braided river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759
Paetzold A, Yoshimura C, Tockner K (2008) Riparian arthropod responses to flow regime regulation and river channelization. J Appl Ecol 45:894–903
Pfadt RE (1981) Density and diversity of grasshoppers (Orthoptera: Acrididae) in an outbreak area on Arizona rangeland. Environ Entomol 11:199–208
Picker M, Griffiths C, Weaving A (2004) Field guide to insects of Southern Africa. Struik, Cape Town
Picker MD, Samways MJ (1996) Faunal diversity and endemicity of the Cape Peninsula, South Africa: a first assessment. Biodivers Conserv 5:591–606
Proçhes S, Cowling RM (2006) Insect diversity in Cape fynbos and neighbouring South African vegetation. Global Ecol Biogeog 15:445–451
R: The R Project for Statistical Computing (2015) The R Project for Statistical Computing. https://www.r-project.org/. Accessed July 2015
Rebelo AG, Low AB (1996) The Vegetation of South Africa, Lesotho and Swaziland. Department of Environmental Affairs and Tourism, Pretoria
Reich M (1991) Grasshoppers (Orthoptera: Saltatoria) on alpine and dealpine riverbanks and their use as indicators for natural floodplain dynamics. River Res Appl 6:333–339
Reinecke MK (2013) Links between Lateral Riparian Vegetation Zones and Flow. PhD thesis. Stellenbosch University, South Africa
Sabo JL, Sponselker R, Dixon M, Gade K, Harms T, Heffernan J, Jani A, Katz G, Soykan C, Watts J, Welter J (2005) Riparian zones include regional species diversity by harbouring different, not more, species. Ecology 85:56–62
Samways MJ (1990) Land forms and winter habitat refugia in the conservation of montane grasshoppers in southern Africa. Conserv Biol 4:375–382
Samways MJ, Pryke JS (2016) Large-scale ecological networks do work in an ecologically complex biodiversity hotspot. Ambio 45:161–172
Samways MJ, Sharratt NJ (2010) Recovery of endemic dragonflies after removal of invasive alien trees. Conserv Biol 24:267–277
Samways MJ, Sharratt NJ, Simaika JP (2011) Effect of alien riparian vegetation and its removal on a highly endemic macroinvertebrate community. Biol Invas 13:1305–1324
Schlettwein CHG, Giliomee JH (1987) Comparison of insect biomass and community structure between fynbos sites of different ages after fire with particular reference to ants, leafhoppers and grasshoppers. Ann Univers Stellenbosch, Ser A3 (Landbouwet) 2(2):1–76
Seydack AHW, Bekker SJ, Marshall AH (2007) Shrubland fire regime scenarios in the Swartberg Mountain Range, South Africa: implications for fire management. Int J Wildl Fire 16:81–95
Sieben EJJ, Mucina L, Boucher C (2009) Scaling hierarchy of factors controlling riparian vegetation patterns of the Fynbos Biome at the Western Cape, South Africa. J Veg Sci 20:1–10
Sieben EJJ, Reinecke MK (2008) Description of reference conditions for restoration projects of riparian vegetation from the species-rich fynbos biome. South Afr J Bot 74:401–411
Sieben EJJ (2000) The Riparian Vegetation of the Hottentots Holland Mountains, SW Cape. PhD thesis. Stellenbosch University, South Africa
Spearman LA (2013) Taxonomic revision of the South African grasshopper genus Euloryma (Orthoptera: Acrididae). Trans Am Entomol Soc 139:1–111
Squitier JM, Capinera JL (2002) Habitat associations of Florida grasshoppers (Orthoptera: Acrididae). Florida Entomol 85:235–244
Ward JV, Tockner K, Arscott DB, Claret C (2002) Riverine landscape diversity. Freshw Biol 47:517–539
Wright MG, Samways MJ (1998) Insect species richness tracking plant species richness in a diverse flora: gall-insects in the Cape Floristic Region, South Africa. Oecologia 115:427–433
Acknowledgements
We thank J. West and S. Reece of Lourensford Wine Estate and J. van Rensburg of Vergelegen Wine Estate for access to sites. S. Fouche, A. Hattingh, B. Haupt, G. Kietzka, A. Pronk and M. Roussouw kindly assisted in the field. Financial support was from the South African DST/NRF Global Change Project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pronk, B.M., Pryke, J.S., Samways, M.J. et al. Range restricted grasshoppers better conserved in a terrestrial zone than in a riparian zone. J Insect Conserv 21, 97–109 (2017). https://doi.org/10.1007/s10841-017-9957-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-017-9957-3