Abstract
There is considerable controversy around the patterns and processes that influence spatial variation in taxonomic composition in mountain environments. We analysed elevational variation in the taxonomic composition of epigaeic spider assemblages across five mountains in north-western Patagonia (Argentina) to examine the relative importance of dispersal (distance) limitation and environmental heterogeneity on a regional scale. The distance limitation hypothesis predicts greater taxonomic similarity between sampling sites separated by short geographical distances than between mountain peaks separated by longer distances, a lack of indicator species of macro-habitats, and weak associations between spider species composition and environmental gradients. Alternatively, the environmental heterogeneity hypothesis predicts that taxonomic differentiation will occur over short distances along elevation gradients in association with the turnover in major habitats and change in environmental conditions, and that indicator species will be present. We collected spiders using 486 pitfall traps arranged in fifty-four 100-m2 grid plots of nine traps separated by ~ 100 m of elevation, from the base to the summit of each mountain. Multivariate analyses identified spider assemblages that were associated with macro-habitats rather than with mountains. Local environmental variation (mainly in vegetation cover), precipitation and soil characteristics influenced the spatial variation in species composition. Characteristic indicator species showed high specificity and fidelity to macro-habitats, whereas vulnerable species showed high specificity and low fidelity to mountains or macro-habitats. We conclude that, on a regional scale, species adaptation to environmental gradients plays a more important role than dispersal limitation in structuring the taxonomic composition of spider assemblages. Moreover, the presence of indicator species suggests that spiders have a great potential as ecological indicators for evaluating the response of montane biodiversity to future climatic change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountains are distinctive geofeatures of landscapes where substantial interactions between physical, biotic and evolutionary factors occur over relatively short distances, which promote the origin and maintenance of biological diversity (Bailey et al. 2017; Graham et al. 2014; Körner 2007; Ruggiero and Hawkins 2008). Mountain building is often associated with significant environmental changes (e.g. in drainage patterns and atmospheric circulation) that may either increase landscape fragmentation and spatial isolation (i.e., separating populations and favoring species diversification), or connect previous isolated areas to favor migration and reducing extinction, all of which promotes high species diversity (Hoorn et al. 2013). The strong environmental gradients that occur with elevation lead to the vertical subdivision of mountains into bioclimatic belts, making mountains complex ecological entities (Walter 1978; Körner et al. 2011). In general, environmental conditions become less favorable for life towards the summit, and the increase in climatic constrains, along with a reduction in land area above the tree line, often reduces the number of species and/or changes species composition due to the special adaptations required to live in such harsh environments (Hodkinson 2005; Körner 2007).
Mountains influence the spatial variation in species composition of many different arthropod groups (e.g., ants: Gillette et al. 2015; Werenkraut et al. 2015; butterflies: Leingärtner et al. 2014; ground-dwelling beetles: Werenkraut and Ruggiero 2013; spiders: Bowden and Buddle 2010; Chatzaki et al. 2005). Nonetheless, there is considerable controversy around the patterns and processes that account for the spatial variation in taxonomic composition in montane environments. The neutral theory assumes that all individuals are ecologically equivalent, and that niche differences among species are not important (Hubbell 2001). This theory predicts that taxonomic dissimilarity between sampling sites will be a function of dispersal limitation, correlated with geographical distance separating sampling sites (= dispersal limitation hypothesis), and that beta diversity will not change systematically along environmental gradients (Chase and Myers 2011). An alternative hypothesis, framed within niche theory, proposes that spatial variation in taxonomic composition is driven by environmental heterogeneity and adaptation of species to environmental conditions [e.g. Chase and Leibold (2003) and Chase and Myers (2011) for discussion]. This theory predicts biologically meaningful relationships between the spatial variation in species composition and environmental gradients.
Evidence from insect distributions appears to contradict the distance limitation hypothesis. The spatial variation in species composition is higher among nearby bioclimatic belts along elevation gradients than among mountain systems separated by large distances (e.g., butterflies and orthopterans in Grammos and Rodopi separated by 426 km: Zografou et al. 2017; ground-dwelling beetles on mountains in north-western Patagonia separated by 40–100 km: Werenkraut and Ruggiero 2013; ants in Hengduan mountains, China: Liu et al. 2017). These studies suggest that, on a regional scale, the variation in mountain insect assemblages may be associated more with adaptation of species to local environmental conditions than by dispersal limitation imposed by distance (e.g., ants: Liu et al. 2017). However, there are still too few studies contrasting the role of geographic distance and environmental variables in accounting for the structure of arthropod assemblages to consider this evidence as conclusive.
Spiders are a good model taxon to address these issues. They are among the most abundant and diverse generalist predators in terrestrial ecosystems (Cardoso et al. 2011; Turnbull 1973; Wise 1995). Their abundance and richness are associated with prey availability (e.g. Carvalho et al. 2015), which is often highly correlated with habitat structural complexity and primary productivity (Birkhofer and Volkmar 2012; Uetz 1991; Spears and MacMahon 2012). Spiders are agile animals due to a powerful combined muscle/hydraulic pressure system and attributes of their leg-morphology that allow them to develop a high variety of moving mechanisms (Kropf 2013). Furthermore, many species are able to disperse long distances using silk strands carried by air currents, a process named ballooning (see Foelix 2011). They also have varied hunting strategies, allowing them to have broad stratum and niche use within the environment (Sanders et al. 2014).
Some ground spider (Gnaphosidae) assemblages in Crete changed little along elevational gradients across different mountains, suggesting the presence of an homogeneous and balanced fauna that only changed at the highest elevations where few spider species can live (Chatzaki et al. 2005). However, because spider families have different ecological strategies and environmental requirements, patterns of variation for a single family do not necessarily scale up to taxonomically heterogeneous species assemblages. Indeed, evidence from diverse spider assemblages is still inconclusive with respect to the influence of dispersal limitation and environmental gradients. For instance, spiders from the Cederberg Mountains in South Africa showed significant but limited elevational distance decay in species composition, with species restricted to certain elevation ranges, and dispersal limitation playing only a limited role in generating the elevational distance decay in similarity (Foord and Dippenaar-Schoeman 2016). In mountains of the northern Yukon Territory in northwestern Canada, spider species composition varied more with latitudinal distance and by mountain than with elevation among mountains (Bowden and Buddle 2010).
The association between spider composition and environmental gradients is also unclear. On a large spatial scale, in Central Europe, a strong relationship between spider assemblage composition and environmental gradients (mainly moisture and shading) indicated that niche differences play an important role in structuring spider assemblages (Entling et al. 2007). However, on a local scale, the associations between spider species distribution and environment is debatable (e.g. Mallis and Hurd 2005), and both spatial proximity and environment have been found to be important determinants of similarity across micro-habitats (Barton et al. 2017). Our study will contribute further evidence based on a regional analysis that considers local variation in spider species composition over several elevation gradients at temperate latitudes of the southern hemisphere.
In this study, we disentangle the role of geographical distance and environmental gradients to account for the spatial variation in the composition of local epigaeic assemblages on a regional scale. We analyse epigaeic spider assemblages from five mountains in NW Patagonia (Argentina), separated between 40 and 100 km on a west-east environmental gradient, to evaluate whether variation in spider composition among mountains is greater than among macro-habitats separated by short distances along the elevation gradient. We analyse the specificity and fidelity (sensu Dufrêne and Legendre 1997) of spider species to mountains and macro-habitats, and test the association between elevational variation in taxonomic similarity and climate, vegetation and soil variables. The distance limitation hypothesis predicts greater taxonomic similarity between sampling sites separated by short distances on the elevation gradient than between mountain peaks separated by comparatively larger distances. Also, it predicts a lack of indicator species for macro-habitats, and weak associations between spider species composition and environmental gradients. Alternatively, the environmental heterogeneity hypothesis predicts that taxonomic differentiation will occur over short distances along an elevation gradient in association with the elevational replacement of major habitats and changes in environmental conditions, and the preference of some species for macro-habitats, leading to the presence of indicator species.
Methods
The present study is part of a larger project we developed to examine the environmental correlates of spatial variation in the richness, abundance and composition of different arthropod groups (beetles, ants and spiders) across five mountains in northwestern Patagonia. Thus, the area of study, fieldwork and sampling protocols, and estimates of environmental correlates are the same as in our previous papers (Werenkraut and Ruggiero 2013, 2014; Werenkraut et al. 2015).
Area of study
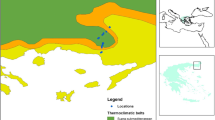
Our study was conducted on five mountains located within the Nahuel Huapi National Park, in north-western Patagonia, Argentina (Fig. 1), during four sampling periods in the southern summer season (January and March 2005 and 2006). The Andean mountains stop the humid winds coming from the Pacific, generating a strong west-east gradient in mean annual precipitation that decreases from more than 3000 mm in the west of Argentina to ca. 300 mm in the eastern Patagonian steppes (Barros et al. 1983; Jobbágy et al. 1995; Paruelo et al. 1998). This strong precipitation gradient is associated with substantial changes in vegetation, leading to the transition from the Subantarctic forests to the Patagonian steppes (Cabrera and Willink 1980; Veblen et al. 1992; Paruelo et al. 1998).
Vegetation on mountain peaks towards the western (wetter) extreme of the gradient (López, Bayo and La Mona: Fig. 1), include forests dominated by Nothofagus dombeyi and N. pumilio growing from 1000 to 1600 m.a.s.l. In general, forests of N. dombeyi grow at lower elevations, decreasing in dominance with elevation. N. pumilio predominates as elevation increases, being the dominant species at the altitudinal treeline where it changes from erect trees to krummholz trees (stunted forests) (Cabrera and Willink 1980; Daniels and Veblen 2004). In the eastern (drier) extreme of the gradient (Pelado and Challhuaco: Fig. 1), vegetation includes semi-arid scrub vegetation and forests of Austrocedrus chilensis and N. antarctica at the lowest elevations, sometimes intermingled with shrublands that originated after severe fires (Veblen et al. 1992; Paritsis et al. 2014). Temperature and precipitation have a significant influence on treelines in northern Patagonia (Daniels and Veblen 2004). Above 1600 m.a.s.l., plant species composition and the physiognomy of plant assemblages change, leading to the high-elevation Andean steppes. The vegetation here lacks trees and is composed of xerophytic shrubs and herbs that are adapted to cold and windy conditions (Ferreyra et al. 1998).
Although precipitation is the principal factor explaining differences in aboveground primary productivity between the forests (c. 2500 kg ha−1) and steppes (c. 500 kg ha−1) (Paruelo 1998), natural disturbances associated with earthquakes, volcanic activity and fire regimes also influence vegetation types (Kitzberger 2012). For instance, fire regimes vary across the precipitation gradient. In wetter areas, fires can create even-aged cohorts of shade intolerant trees (N. dombeyi, N. pumilio) (Veblen et al. 1992). As precipitation decreases, fires help to maintain shrublands dominated by resprouting species (Veblen et al. 1992; Mermoz et al. 2005; Paritsis et al. 2014) and the boundary between forest and steppe vegetation (Kitzberger 2012 for review). Anthropogenic disturbances include pine plantations and urbanization, mainly towards the drier extreme of the gradient (Kitzberger 2012). However, our study focused on five mountains within relative pristine protected areas in the Nahuel Huapi National Park, thus minimizing the potential effects of anthropogenic disturbances on our findings.
We selected five mountains to encompass the full regional variation in precipitation from east to west: Challhuaco (41°13′S, 71°19′W, Río Negro province, mean annual precipitation at the base = pp: 1100 mm, sampled altitudinal range = sar: 900–2000 m), Pelado (40°56′S, 71°20′W, Neuquén province, pp: ~1220 mm, sar: 800–1800 m), López (41°05′S, 71°33′W, Río Negro province, pp: 1730 mm, sar: 800–1800 m), Bayo (40°45′S, 71°36′W, Neuquén province, pp: 1650 mm, sar: 900–1800 m) and La Mona (40°34′S, 71°42′W, Neuquén province, pp: 1930 mm, sar: 800–1800 m) (Fig. 1).
Spider sampling
A total of 486 plastic pitfall traps (diameter 9 cm; depth 12 cm) were installed in the field, arranged in fifty-four 100 m2 grid plots of nine traps each. Traps were filled with diluted propylene glycol (40%) and a drop of soap. To represent the main habitats found at each elevation, 9–12 plots were established at about 100 m of elevation apart from each other, from the base to the summit on each mountain. The geographical position of each plot was recorded using Global Positioning System (GPS) technology. Pitfall traps were operative from the moment they were established in the field and over 7 days, during four sampling periods, in the southern summer (January and March 2005–2006).
At the end of each sampling period, the contents from the nine pitfalls were pooled into one sample at each plot and preserved in 80% ethyl alcohol. Adult specimens were identified to species/morphospecies level. The abundance of individuals for each species at each plot was computed as the total number of individuals caught at each plot after the four sampling periods. Voucher specimens are held at the National Collection of Archnology (MACN-Ar) in the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Ciudad Autónoma de Buenos Aires, Argentina.
Environmental variables
Climatic variables
To record the temperature at ground level, a HOBOH8 logger (Onset Computer Corporation, Bourne, MA, USA) was mounted on a pole fixed at the centre of each 10 × 10 m sampling plot. Measurements were taken every 2 h during summers of 2005, 2006, and 2007. In 2005, data loggers remained in the field for ca. 3 months during the sampling summer season (January–March). Due to the harsh climatic conditions, some loggers were damaged and their records were lost. To avoid the lack of data from any sampling plot, additional data were recorded during the summer for the two subsequent years. The total number of temperature records per plot varied from 792 to 2148, but the altitudinal patterns of temperature variation obtained at each mountain over the 3 years were similar. Hence, all records obtained at each plot were used to estimate the average minimum daily temperature (Tmin), the average maximum daily temperature (Tmax), the average medium daily temperature (Tmed) and the average daily temperature range (Tvar) at each plot (see Werenkraut 2010 for details).
An ordinal insolation variable was created (Insol = N > NW > NE > W > E > SW > SE > S) to represent the qualitative differences in exposure among plots. To estimate the total precipitation (PRECtot) and the medium precipitation (PRECmed) in each plot, summer (December–March) precipitation data was extracted from high resolution (30 arc-seconds) digital bases (based on Hijmans et al. 2005).
Vegetation variables
The vegetation in each of the 54 plots was sampled and all plants recorded were classified according to their growth form: (1) herbs, (2) shrubs, and (3) trees, as proposed by Ezcurra and Brion (2005).
Vegetation cover
Each of the 10 × 10 m plot was divided into four quadrants. To estimate the proportion of tree canopy cover (TREEcov) in the forest and scrubland plots, a concave spherical densitometer was used. The average of four cover readings, facing a different cardinal direction in each quadrant, was used for each plot. Shrub cover (SHRUBcov) was estimated visually in each of the four quadrants. To estimate the herbaceous cover (HERBcov), a 0.50 × 0.50 m wood frame subdivided into a 25-celled nylon string grid was used. The frame was randomly thrown four times in each plot and the proportion of cells covered by herbs was estimated. The average of these four values was used for each plot.
Plant litter biomass
To estimate dry litter biomass (LITTER) nine 9-cm diameter samples (separated ca. 5 m from each other) per plot were harvested. They were stored in paper bags, oven-dried to constant mass at 60 °C, and weighed.
Plant species richness
The number of tree (TREEsp), shrub (Shrubsp), and herb (Herbsp) species found within each plot was counted (Online Resource 1 reports species richness and the list of the most frequently found plant species at each elevation belt).
Soil variables
Soil samples of the top 10 cm of soil at three randomly selected points within each plot were extracted and mixed together. For each plot-sample, soil water pH (pH·H2O; potentiometric determination, Thomas 1996), NaF pH as an indicator of volcanic influence in soil formation (pH.Volc; potentiometric determination, Blakemore et al. 1987a, b), texture (Texture; Klute 1986), organic matter content (OM; Pipette Method, Klute 1986), and humidity (HUM = [(wet weight − dry weight)/wet weight] × 100) was determined.
Three additional soil samples were randomly extracted at each plot using a cylinder of known volume (96.21 cm3), dried at 105 °C for 24 h and sieved to 2 mm. From particles < 2 mm, bulk density was determined as BULDEN = dry weight/cylinder volume. Higher values of Bulden were related to more compacted soils. From samples with larger particles, gravel percentage was estimated (GRAVEL = gravel weight/total weight). BULDEN and GRAVEL estimations were averaged over the three samples. To estimate the topographic slope (SLOPE) four readings were taken and averaged, using a clinometer, at each plot.
Analyses of data
We used the “vegan” package (Oksanen et al. 2016) in R (R Core Team 2016) to conduct a cluster analysis, and an analysis of similarity (ANOSIM; Clarke and Warwick 2001) based on a Bray–Curtis dissimilarity matrix (Bray and Curtis 1957) that allowed the identification of groups of plots with similar species composition, taking into account the species’ identities and their relative abundances. ANOSIM produces an R statistic that is an absolute measure of distance between groups, with a large positive R (up to 1) implying dissimilarity between groups.
A non-metric multidimensional scaling (NMDS) plot was made using the “vegan” package in R (Oksanen et al. 2016). NMDS provides a stress value that reflects how well the ordination summarises the observed relative distances among the samples, with a weaker stress meaning better quality of the representation. A common rule of thumb is that stress below 0.2 represents an adequate fit (Clarke and Warwick 2001).
Associations between taxonomic composition and environment
The influence of environmental variables on the taxonomic composition of species assemblages was assessed by a canonical correspondence analysis (CCA) using the “vegan” package in R (Oksanen et al. 2016). Variance inflation factor (VIF) was calculated for each environmental variable within the model. The variable with the highest value was discarded and then, a new model without that variable was estimated. This process was repeated until the highest VIF value was < 10. A stepwise model selection was applied on the remaining variables to obtain a final explanatory model. The significance of the variation explained by each environmental variable of the selected model was tested using the function “anova.cca”, with 999 permutations.
Identification of indicator species
We used the “indicspecies” package in R (De Cáceres and Legendre 2009) to obtain an indicator value (IndVal: Dufrêne and Legendre 1997) for each species. The IndVal for an i species in the j site is
Specificity ij (i.e., uniqueness) is the proportion of sites of type j with species i and Fidelity ij is the proportion of individuals (abundance) of species i that are in a j type of site. The significance of the IndVal measures for each species was obtained by 999 randomisations (Dufrêne and Legendre 1997). We considered that a species was indicator of a particular mountain or habitat when it had a significant (p < 0.05) IndVal > 25% (Dufrêne and Legendre 1997). Species with IndVal > 70% (subjective benchmark; van Rensburg et al. 1999) were regarded as characteristic indicator species of any macro-habitat or mountain. Species with IndVal values between an arbitrary range of 40–70%, with medium specificity and variable fidelity, were considered as detector indicator species of any macrohabitat or mountain. Species with high specificity and low fidelity were considered as vulnerable (McGeoch et al. 2002 for discussion).
Results
A total of 2964 adult spiders from 18 families and 129 species⁄morphospecies were collected (Online Resource 2). The most abundant families in our study were Linyphiidae (1240 individuals, ≈ 42%), Amaurobiidae (Macrobuninae) (680 individuals, ≈ 23%), and Desidae (Metaltellinae) (378 individuals, ≈ 13%) (Fig. 2a). The richest families were Amaurobiidae (31 species/morphospecies, ≈ 24%), followed by Linyphiidae (23 species/morphospecies, ≈ 18%) and Anyphaenidae (19 species/morphospecies, ≈ 15%).
Dendogram for hierarchical clustering of the 54 sampling sites, using group-average linking of Bray–Curtis similarities. Three main groups are evident: shrublands of dry and moist mountains, plus high Andean regions of the dry mountains (Shrublands+Highlands-d); forests of dry and moist mountains, plus two other plots from dry mountains (Forests); and high Andean region of the three moist mountains plus a high Andean plot from a dry mountain (Highlands-m). Sampling plots are named by the first letter of the mountain name followed by elevation. Mountains L López, P Pelado, B Bayo, M La Mona, C Challhuaco
Similarity in species composition
We identified three main clusters based on species composition: (1) the high Andean region of the three moist mountains: La Mona, Bayo, and López, plus the plot at 2000 m from Challhuaco (hereafter called “Highlands-m”, N = 9); (2) shrublands of dry and moist mountains, plus high Andean regions of the dry mountains, Challhuaco and Pelado (hereafter called “Shrublands+Highlands-d”, N = 10); (3) forests of dry and moist mountains, plus two other plots from dry mountains (hereafter called “Forests”, N = 35; Fig. 2).
ANOSIM confirmed significant differences, although with low R values, in the taxonomic composition of spider assemblages between some moist and dry mountains (Table 1a). However, the highest taxonomic dissimilarity was among macro-habitats, most strongly between Forests and Highlands-m (Table 1b). Non-metric multidimensional scaling also showed a clearer taxonomic distinction among habitats rather than among mountains (compare Figs. 3, 4).
Associations between taxonomic composition and environment
The eigenvalues of the first two CCA axes were 0.653 and 0.552, respectively. These two axes together explained about 48% of the constrained variance in the species assemblage–environment relationship (axis 1: 25.9%, axis 2: 21.9%). The first axis showed that an environmental gradient of increasing BULDEN (correlation coefficient, r = 0.342), pH.Volc (r = 0.609), and Tmed (r = 0.343), and of decreasing TREEcov (r = − 0.699) accounts for the separation between spider assemblages in the Forests and those in the Highlands-m and the Shrublands+Highlands-d (Fig. 5). The second axis was mostly associated with increasing values of PRECtot (r = 0.6481), and decreasing values of TREEsp (r = − 0.430) and Tmed (r = − 0.361) (Fig. 5), separating spider assemblages in Higlands-m and (humid) Forests from those in (dry) Forests and Shrublands+Highlands-d. The Highlands-m assemblage was associated with low TREEcov and TREEsp, and the Shrublands+Highlands-d assemblage with high Tmed and SLOPE, and low PRECtot. The Forests assemblage was associated with the decrease in BULDEN, pH.Volc, and the increase in TREEcov (Fig. 5).
Canonical correspondence analysis ordination (biplot) of spider assemblages in relation to environmental variables. Arrows indicate the magnitude and direction of change of environmental variables that significantly explain changes in spider assemblages composition (P < 0.05). Circles = Forests, squares = Highlands-m, triangles = Shrublands+Highlands-d
Indicator species
We did not identify characteristic species of mountains or indicator species of Pelado. There were only one detector species in Bayo and three vulnerable species in Bayo, La Mona and Challhuaco (Table 2). Vulnerable species showed high specificity, i.e. concentration of individuals to mountains, although low fidelity (i.e., frequency of occurrences) within each mountain.
IndVals of macrohabitat indicator species ranged from 48.0 to 80.6%, showing a very high specificity and various degrees of fidelity of species to habitats (Table 3). We identified characteristic species (IndVal > 70%) of Forests, Highlands-m and Shrublands+Highlands-d. Vulnerable species were identified in the Forests and Shrublands+Highlands-d but not in Higlands-m (Table 3).
Discussion
Epigaeic spider distribution in northwestern Patagonia suggests that, on a regional scale, species adaptation to environmental gradients plays a more important role than dispersal limitation in structuring species assemblages (e.g., Carvalho et al. 2011; Entling et al. 2007). In our study, taxonomic composition of spiders varied more among macro-habitats on the same mountain than among mountains separated by 40–100 km. Indeed, these assemblages in northwestern Patagonia were associated with broad-scale replacement of macro-habitats that occur on the elevational gradient, on moist and dry mountains along the west-east gradient. Some spider species showed high fidelity and specificity to macro-habitats, serving as characteristic indicator species. In contrast, we did not find characteristic species for mountains. Our findings suggest that high dispersal capabilities promote widespread distributions, which could facilitate the occurrence of spider species inhabiting elevation belts with similar environmental conditions across distant mountains.
Spider assemblages differed among the high Andean steppes of humid mountains, shrublands and high Andean steppes of dry mountains, and forests of dry and moist mountains. In a previous analysis, ground-dwelling beetles showed a similar differentiation among the same macro-habitats, except that shrublands of moist mountains that were more similar to forests than to shrublands of dry mountains and the high Andean steppes (Werenkraut and Ruggiero 2013). This suggests that beetles and spiders may somewhat differ in the way they respond to changes in vegetation structure. Indeed, spiders are known to be very sensitive to the reduction of habitat complexity (Langellotto and Denno 2004). In our mountains, the presence of shrublands with their shorter canopies and substantial change in micro-climatic conditions (i.e. an increase in air temperature and a decrease in relative humidity: Paritsis et al. 2014) appear to be rather unsuitable habitat to spiders that inhabit the neighboring Nothofagus forests.
The replacement of forests, either by the high Andean or the Patagonian steppes, is concurrent with strong changes in environmental conditions that influenced the local variation in species composition. The spider assemblages in northwestern Patagonia showed a differentiation between the cooler and wetter environmental conditions in the dense Nothofagus forests and highlands towards the west, and the diverse forests, shrublands and highlands situated towards the east. Forest spider assemblages inhabit sites with less compact volcanic soils and high tree canopy cover, suggesting that these spiders are sensitive to changes in vegetation structure, shading, and moisture of habitats, as well as changes in soil conditions, as previously found in other regions (e.g. costal dunes in Belgium: Bonte et al. 2002; upland grasslands in northern England: Coulson and Butterfield 1986; Central Europe: Entling et al. 2007). Also, within the forests, there are a high number of web anchor points and litter crevices available for spiders, compared to the high Andean steppes with little to no vegetation and leaf litter. Thus, changes in vegetation structure that occur with the replacement of the forests by the Andean steppes impact ground-dwelling spiders that depend on leaf litter and/or soil structure to make their webs, caves or retreats (Uetz 1991).
In our study, which sampled at a local spatial resolution (sampling plots of 10 × 10 m) but over a regional spatial extent, we showed that approximately half of the variation in species composition (48%) was accounted for by environmental variables. The influence of vegetation structure and climate has been postulated to influence spider beta diversity patterns on a regional spatial scale, rather than on a local scale (Carvalho et al. 2011). In northwestern Patagonia, patterns in spider species composition on a regional spatial scale responded more to environmental heterogeneity than to distance limitation. For this reason, conservation approaches that consider complementarity between the spider faunas of major habitats, i.e., the forests and high Andean steppes, will be most effective in promoting the conservation of diversity (e.g., Economo 2011 for discussion). Nonetheless, the considerable proportion of the spider composition variation (52%) that remained unexplained in our study may be due to the influence of unmeasured environmental variables or the effect of other processes that influence species’ distributions at fine spatial scales, such as prey availability, biotic interactions or dispersal and stochastic processes (e.g. Carvalho et al. 2011, 2015). These fine-scaled processes remained undetectable at the spatial scale of our study, and could complement the influence of a species sorting process that occurs along environmental gradients.
We confirmed that spiders have a great potential as bioindicators and as indicators of biodiversity responses to environmental change (e.g. Pearce and Vernier 2006). We identified several characteristic spider species (IndVal > 70%) of macro-habitats that are of specific conservation interest. Given their high specificity and fidelity, they could become vulnerable (sensu McGeoch et al. 2002) if large environmental changes influence their habitats. On the other hand, both mountains and macro-habitats included vulnerable species, with high specificity and low levels of fidelity McGeoch et al. (2002), which suggests the potential fragility (Nilsson and Grelsson (1995) for discussion) of mountain spider assemblages in north-western Patagonia to anthropogenic disturbance, at least on the local scale. The named vulnerable species detected in our study have large distributions in adjacent regions (humid forests in Chile, steppes in Argentina; World Spider Catalog 2017), suggesting that our study area covered the margins of their natural distributions. The distribution of the unnamed morphospecies (see Online Resource 2) is not known outside of our study area, highlighting the potential importance of taxonomic studies of little known taxa, especially the Macrobuninae (Amaurobiidae) for ecological studies. Future studies may fine-tune the efficiency of characteristic species as ecological bioindicators over longer periods of time in northwestern Patagonia, and whether they will become locally vulnerable as the consequences of environmental changes (e.g. ash accumulation) produced by recent volcanic eruptions (e.g. Ruggiero and Werenkraut 2014) and future warmer and drier climates predicted for this region (e.g. Masiokas et al. 2008).
References
Bailey JJ, Boyd DS, Hjort J, Lavers CP, Field R (2017) Modelling native and alien vascular plant species richness: At which scales is geodiversity most relevant? Glob Ecol Biogeogr. https://doi.org/10.1111/geb.12574
Barros VR, Cordón V, Moyano C, Méndez R, Forquera J, Pizzio O (1983) Cartas de precipitación de la zona oeste de las provincias de Río Negro y Neuquén. Facultad de Ciencias Agrarias, Universidad Nacional del Comahue, Cinco Saltos, Argentina
Barton PS, Evans MJ, Foster CN, Cunningham SA, Manning AD (2017) Environmental and spatial drivers of spider diversity at contrasting microhabitats. Austral Ecol. https://doi.org/10.1111/aec.12488
Birkhofer K, Volkmar W (2012) The global relationship between climate, net primary production and the diet of spiders. Glob Ecol Biogeogr 21:100–108
Blakemore LC, Searle PL, Daly BK (1987a) Soil pH (Part 2). Methods for chemical analysis of soils, pp 9–12. NZ Soil Bureau Scientific Report Nr. 80. NZ Soil Bureau. Department of Scientific and Industrial Research, Lower Hunt,
Blakemore LC, Searle PL, Daly BK (1987b) Soluble salts (Part 9). Methods for Chemical Analysis of Soils, pp 77–82. NZ Soil Bureau Scientific Report Nr. 80. NZ Soil Bureau. Department of Scientific and Industrial Research, Lower Hunt
Bonte D, Baert L, Maelfait J-P (2002) Spider assemblage structure and stability in a heterogeneous coastal dune system (Belgium). J Arachnol 30:331–343
Bowden JJ, Buddle CM (2010) Spider assemblages across elevational and latitudinal gradients in the Yukon Territory. Canada Arctic 63:261–272
Bray RJ, Curtis JT (1957) An ordination of the upland forest communities of southern Winsconin. Ecol Monogr 27:325–349
Cabrera ÁL, Willink A (1980) Biogeografía de América Latina. Segunda ed. Monografía 13. Serie de biología. Secretaría General de la Organización de los Estados Americanos. Programa Regional de Desarrollo Científico y Tecnológico, Washington D.C.
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS ONE 6:1–10
Carvalho JC, Cardoso P, Crespo LC, Henriques S, Carvalho R, Gomes P (2011) Determinants of beta diversity of spiders in coastal dunes along a gradient of mediterraneity. Divers Distrib 17:225–234
Carvalho LS, Sebastian N, Araújo HFP, Dias SC, Venticinque E, Brescovit AD, Vasconcellos A (2015) Climatic variables do not directly predict spider richness and abundance in semiarid caatinga vegetation, Brazil. Environ Entomol 44:54–63
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, Chicago
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Phil Trans R Soc B 366:2351–2363
Chatzaki M, Lymberakis P, Markakis G, Mylonas M (2005) The distribution of ground spiders (Araneae, Gnaphosidae) along the altitudinal gradient of Crete, Greece: Species richness, activity and altitudinal range. J Biogeogr 32:813–831
Clarke KR, Warwick RM (2001) Change in marine communities. An approach to statistical analysis and interpretation. PRIMER-R, Plymouth
Coulson JC, Butterfield J (1986) The spider communities on peat and upland grasslands in northern England. Holarct Ecol 9:229–239
Daniels LD, Veblen TT (2004) Spatiotemporal influences of climate on altitudinal treeline in northern Patagonia. Ecology 85:1284–1296
de Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Economo EP (2011) Biodiversity conservation in metacommunity networks: linking pattern and persistence. Am Nat 177:E167–E180
Entling W, Schmidt MH, Bacher S, Brandl R, Nentwig W (2007) Niche properties of Central European spiders: shading, moisture and the evolution of the habitat niche. Glob Ecol Biogeogr 16:440–448
Ezcurra C, Brion C (2005) Plantas del Nahuel Huapi: Catálogo de la Flora Vascular del Parque Nacional Nahuel Huapi, Argentina. Red Latinoamericana de Botánica. San Carlos de Bariloche, Argentina
Ferreyra M, Clayton S, Ezcurra C (1998) La flora altoandina de los sectores este y oeste del Parque Nacional Nahuel Huapi, Argentina. Darwiniana 36:65–79
Foelix RF (2011) Biology of spiders. Oxford University Press, New York
Foord SH, Dippenaar-Schoeman AS (2016) The effect of elevation and time on mountain spider diversity: a view of two aspects in the Cederberg mountains of South Africa. J Biogeogr 43:2354–2365
Gillette PN, Ennis KK, Martínez GD, Philpott SM (2015) Changes in species richness, abundance, and composition of arboreal twig-nesting ants along an elevational gradient in coffee landscapes. Biotropica 47:712–722
Graham CA, Carnaval AC, Cadena CD, Zamudio KR, Roberts TE, Parra JL, McCain CM, Bowie RCK, Moritz C, Baines SB, Schneider CJ, VanDerWal J, Rahbek C, Kozak KH, Sanders NJ (2014) The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography 37:001–009
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513
Hoorn C, Mosbrugger V, Mulch A, Antonelli A (2013) Biodiversity from mountain building. Nat Geosci 6:154
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Jobbágy EG, Paruelo JM, León RJC (1995) Estimación del régimen de precipitación a partir de la distancia a la cordillera en el noroeste de la Patagonia. Ecol Austral 5:47–53
Kitzberger T (2012) Ecotones as complex arenas of disturbance,climate, and human impacts: the trans-Andean forest-steppe ecotone of northern Patagonia. In: Myster RW (ed) Ecotones between forest and grassland. Springer, New York, pp 59–88
Klute A (1986) Methods of soil analysis. Part 1. Physical an mineralogical methods. American Society of Agronomy-Soil Science Society of America, Madison
Körner C (2007) The use of “altitude” in ecological research. Trends Ecol Evol 22:569–574
Körner C, Paulsen J, Spehn EM (2011) A definition of mountains and their bioclimatic belts for global comparisons of biodiversity data. Alp Bot 121:73–78
Kropf C (2013) Hydraulic System of Locomotion. In: Nentwig W (ed) Spider ecophysiology. Springer-Verlag, Berlin, Heidelberg, pp 43–56
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10
Leingärtner A, Krauss J, Steffan-Dewenter I (2014) Species richness and trait composition of butterfly assemblages change along an altitudinal gradient. Oecologia 175:613–623
Liu C, Dudley KL, Xu Z-h, Economo EP (2017) Mountain metacommunities: climate and spatial connectivity shape ant diversity in a complex landscape. Ecography. https://doi.org/10.1111/ecog.03067
Mallis RE, Hurd LE (2005) Diversity among ground-dwelling spider assemblages: habitat generalists and specialists. J Arachnol 33:101–109
Masiokas MA, Villalba R, Luckman BH, Lascano ME, Delgado S, Stepanek P (2008) 20th-century glacier recession and regional hydroclimatic changes in northwestern Patagonia. Glob Planet Chang 60:85–100
McGeoch MA, van Rensburg BJ, Botes A (2002) The verification and application of bioindicators: a case study of dung beetles in a savanna ecosystem. J Appl Ecol 39:661–672
Mermoz M, Kitzberger T, Veblen TT (2005) Landscape influences on occurrence and spread of wildfires in Patagonian forests and shrublands. Ecology 86:2705–2715
Nilsson C, Grelsson G (1995) The fragility of ecosystems: a review. J Appl Ecol 32:677–692
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2016) vegan: Community Ecology Package
Paritsis J, Veblen TT, Holz A (2014) Positive fire feedbacks contribute to shifts from Nothofagus pumilio forests to fire-prone shrublands in Patagonia. J Veg Sci 26:89–101
Paruelo JM (1998) The climate of Patagonia general patterns and controls on biotic processes. Ecol Austral 8:85–101
Paruelo JM, Jobbágy EG, Sala OE (1998) Biozones of Patagonia (Argentina). Ecol Austral 8:145–153
Pearce JL, Venier LA (2006) The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: a review. Ecol Ind 6:780–793
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ruggiero A, Hawkins BA (2008) Why do mountains support so many species of birds? Ecography 31:306–315
Ruggiero A, Werenkraut V (2014) Legacy patterns in the abundance of epigaeic mountain beetles after the eruption of the Puyehue-Cordon Caulle volcanic complex (NW Patagonia, Argentina). Ecol Austral 24:31–41
Sanders D, Vogel E, Knop E (2014) Individual and species-specific traits explain niche size and functional role in spiders as generalist predators. J Anim Ecol 84:134–142
Spears LR, MacMahon JA (2012) An experimental study of spiders in a shrub-steppe ecosystem: the effects of prey availability and shrub architecture. J Arachnol 40:218–227
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis Part 3. Chemical methods. SSSA Book Series No 5. American Society of Agronomy—Soil Science Society of America, Madison, pp 475–490
Turnbull AL (1973) Ecology of the True Spiders (Araneomorphae). Annu Rev Entomol 18:305–348
Uetz GW (1991) Habitat structure and spider foraging. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure. Volume 8 of the series population and community biology series. Chapman and Hall, London, pp 325–348
van Rensburg BJ, McGeoch MA, Chown SL, van Jaarsveld AS (1999) Conservation of heterogeneity among dung beetles in the Maputaland Centre of Endemism, South Africa. Biol Conserv 88:145–153
Veblen TT, Kitzberger T, Lara A (1992) Disturbance and forest dynamics along a transect from Andean rain forest to Patagonian shrubland. J Veg Sci 3:507–520
Walter H (1978) Vegetation of the earth and ecological systems of the geo-biosphere, 2nd edn, Heidelberg Science Library. Springer, Heidelberg
Werenkraut V (2010) Patrones altitudinales en la diversidad de coleópteros y hormigas epígeos del noroeste de la Patagonia Argentina. Tesis doctoral. Facultad de Ciencias Exactas y Naturales. Universidad Nacional de Buenos Aires
Werenkraut V, Ruggiero A (2013) Altitudinal variation in the taxonomic composition of ground-dwelling beetle assemblages in NW Patagonia, Argentina: environmental correlates at regional and local scales. Insect Conserv Divers 6:82–92
Werenkraut V, Ruggiero A (2014) The richness and abundance of epigaeic mountain beetles in north-western Patagonia, Argentina: assessment of patterns and environmental correlates. J Biogeogr 41:561–573
Werenkraut V, Fergnani PN, Ruggiero A (2015) Ants at the edge: a sharp forest-steppe boundary influences the taxonomic and functional organization of ant species assemblages along elevational gradients in northwestern Patagonia (Argentina). Biodiv Conserv 24:287–308
Wise DH (1995) Spiders in ecological webs, 1st edn. Cambridge University Press, New York
World Spider Catalog (2017) Natural History Museum Bern, version 18.0. http://wsc.nmbe.ch. Accessed 5 Jul 2017
Zografou K, Wilson RJ, Halley JM, Tzirkalli E, Vassiliki K (2017) How are arthopod communities structured and why are they so diverse? Answers from Mediterranean mountains using hierarchical additive partitioning. Biodivers Conserv. https://doi.org/10.1007/s10531-017-1303-2
Acknowledgements
This project is part of P-UE 2016 22920160100008CO developed at INIBIOMA (CONICET/UNCo). We thank the Agencia Nacional para la Promoción de la Ciencia y Técnica (ANPCyT—FONCYT: PICT2013-0539, PICT2015-0283), CONICET and the British Ecological Society that provided financial support during several years, which allowed developing long-term research on the biodiversity of mountains in north-western Patagonia. C. Grismado and L. Piacentini (MACN-Ar) provided assistance in the identification of spiders. C. Reemts reviewed the manuscript to improve our English. The National Administration of National Parks provided the authorization to work and collect arthropod specimens in the Nahuel Huapi National Park. M. Sahores, F. Galossi, C. Galossi and J. Benclowicz were committed assistants during the long days of summer fieldwork.
Funding
This study was funded by ANPCyT—FONCYT: PICT2013-0539, PICT2015-0283.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aisen, S., Werenkraut, V., Márquez, M.E.G. et al. Environmental heterogeneity, not distance, structures montane epigaeic spider assemblages in north-western Patagonia (Argentina). J Insect Conserv 21, 951–962 (2017). https://doi.org/10.1007/s10841-017-0034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-017-0034-8