Abstract
Spiders lack extensor muscles in the main joints of their walking legs (femur–patella and tibia–metatarsus joint, respectively). These leg joints are extended by a rise of hemolymph pressure in the prosoma, caused by contraction of prosomal dorsoventrally running muscles. This leads to volume decrease in the prosoma (made possible by soft and flexible pleurae) and to hemolymph flow into the legs. Inside the legs, hemolymph flows in channels (lacunae) whose cross sections are strongly influenced by intrinsic muscle activity. In hydraulic leg joints, only flexor muscles occur that act antagonistically to hemolymph pressure. The articular membranes in leg joints are bellows-like folded, in this way preventing unfavourable torques opposing joint extension. During maximum activity, perfusion of the prosoma stops because prosomal pressure exceeds systolic heart pressure. In the opisthosoma, a subepidermal muscle-sheet (“abdominal sac”) and several other muscles generate hemolymph pressure that is considerably lower than prosomal pressure during high activity, but may be very close to it during rest or moderate activity. Opisthosomal pressures are sometimes linked to prosomal ones, but sometimes not. This implies (contrary to previous opinions) a temporary hydrostatic separation between pro- and opisthosoma, probably due to a muscular valve at the anterior end of the pedicel. In some spiders, the prosoma forms a stiff capsule unable to generate hemolymph pressure. It is suggested that these spiders produce hemolymph pressure for leg extension mainly in the opisthosoma by contraction of a strongly modified abdominal sac.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Spiders can be seen from a functional viewpoint as semi-hydraulic machines (Manton 1958). Just to name a few functions of a spider’s hydraulic system, walking, running, climbing and jumping, grasping of prey, moulting, leg-autotomy, extrusion of silk or expanding the male palpal organ are performed under locally increased pressure of body fluid (hemolymph). This is made possible by the open circulatory system of spiders where hemolymph leaves the arteries and flows back to the lungs between the internal organs (Foelix 2011, with references therein). Increased hemolymph pressure is generated by activity of the heart in the opisthosoma, and by certain muscles, situated both in the prosoma and in the opisthosoma. While the heart, being able to pump hemolymph both towards anterior and posterior (Paul et al. 1989), is mainly responsible for the “normal” circulation of hemolymph (see Wirkner and Huckstorf 2013), prosomal and opisthosomal muscles generate locally increased hemolymph pressure enabling the spider to perform various hydraulic movements in different body parts. However, we are far from understanding which muscles in which body parts exactly produce this pressure and how these mechanisms work in detail.
Spider locomotion is of special interest in this context. It has been known for a long time that spiders lack extensor muscles in the main joints of their walking legs, i.e. the femur–patella and tibia–metatarsus joint. Contrary to assumptions of earlier authors who considered elasticity of joint membranes or a local rise of hemolymph pressure inside the legs as responsible for leg joint extension, it is now generally acknowledged that extension of these leg joints is based mainly on an increase of prosomal hemolymph pressure (Shultz 1989, with references therein). Hydraulic leg extension saves space in the cuticular leg tubes (as no extensor muscles are required) and enables slender leg segments that are almost exclusively filled with flexor muscles, which in turn may be adaptive with respect to prey capture (Anderson and Prestwich 1975; Rovner 1980).
A dorsoventral compression of the prosoma is made possible by certain dorsoventrally running muscles and by soft and flexible lateral pleurae (Fig. 4.1) as shown in most spiders. Prosomal compression at the onset of activity (Wilson 1970) leads to a volume decrease in the prosoma, increase of hemolymph pressure, hemolymph flow into the legs, and to extension of leg joints (Anderson and Prestwich 1975; Blickhan and Barth 1985; Paul et al. 1989). In this review, I will discuss functional morphology and physiology of the main structures involved in hydraulic leg movements and present new data on spiders that probably use a different hydraulic system for generating hemolymph pressure.
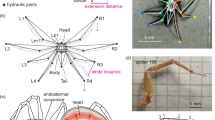
Simplified schematic illustration of the putative main elements of a spider’s hemolymph pressure producing system. It is not yet clear, if the musculi laterales, or the endosternal suspensor muscles, or both generate hemolymph pressure. Muscles in red, endosternite in yellow, pleura in green, hemolymph flow during activity in blue. Note that only a minor hemolymph flow from prosoma to opisthosoma occurs during activity. as abdominal sac, es fourth dorsolateral endosternal suspensor muscle, ml musculi laterales, pl pleura
2 Muscles Generating Hemolymph Pressure in the Prosoma
It is not yet clear what muscles in the spider’s prosoma actually generate hemolymph pressure. Wilson (1970) attributed the function of hemolymph pressure production to the so-called “musculi laterales” (Brown 1939; “carapace compressor” of Whitehead and Rempel 1959). These muscles are running from the dorsolateral region of the carapace to the pleura where they insert at sclerites in the pleural membrane (Fig. 4.1) or possibly also on cuticular folds of the lateral borders of the carapace (Brown 1939; Whitehead and Rempel 1959 and others).
Most subsequent authors followed Wilson’s suggestion (e.g., Anderson and Prestwich 1975; Palmgren 1978; Prestwich 1988a; Paul et al. 1989, 1994; Paul and Bihlmayer 1995). Later, Shultz (1991, 1993) proposed that suspensor muscles of the endosternite (a mesodermal skeletal plate in the prosoma of most arachnids; Fig. 4.1) generate most of the prosomal pressure in “hydraulic” arachnids. Shultz’ hypothesis is based on detailed morphological and electromyographical investigations in a whipscorpion, Mastigoproctus giganteus. The endosternal suspensor muscles connect the endosternite with the carapace (dorsal and dorsolateral suspensors) or (in whipscorpions, see below) with sternum and coxal processes (ventral suspensors). It was found that during unrestrained locomotion of the whipscorpion, the third portion of the musculi laterales contracts in a step-coupled pattern while the activity of one dorsal endosternal suspensor muscle correlates with changes in the prosomal pressure baseline. Therefore, endosternal suspensor activity should provide the general level of pressure in the prosoma during activity. The function of the musculi laterales was ascribed to “controlling of coxal movements by acting as an antagonist to prosomal pressure at the prosoma–coxa joint” (Shultz 1991: 29).
Unfortunately, no such data exist for spiders. It may be problematical to transfer the conclusions from whipscorpions to spiders without restrictions, because the construction of the prosoma is considerably different in the two groups: first, only one dorsal endosternal suspensor (out of four pairs connecting endosternite and carapace) was tested (Shultz 1991, 1993), but these muscles are mostly reduced to a single and often tiny pair in spiders (Fig. 4.2) or are even totally absent (e.g., in Harpactea, Dysderidae, Palmgren 1978). Second, the endosternite of whipscorpions is attached to the sternum by a pair of endosternal suspensors; this is not the case in the vast majority of araneomorph spiders where ventral suspensors are totally lacking (Brown 1939; Whitehead and Rempel 1959; Firstman 1973; Palmgren 1978, 1980). So, their endosternite hangs “freely” in the prosomal cavity and is suspended only ventrolaterally by extrinsic coxal muscles. Third, the sternum is large in most spiders and tiny in whipscorpions; the leg coxae (especially coxae II–IV) cover the ventral surface of the prosoma of whipscorpions almost totally and are limited in movements, while in most spiders the coxae originate in the soft pleura and are extremely mobile. So, Wilson’s (1970) argument should still be taken into account, as long as no better data on prosomal hemolymph pressure production in spiders are available: The activity of endosternal suspensor muscles that act as prosomal compressors for fluid pressure generation would strongly interfere with coxal movements in spiders and thus be dysfunctional.
The endosternite model of hydraulic pressure generation is based on measurements of a single endosternal muscle in a single species of whipscorpions. Concluding from this that prosomal hemolymph pressure is produced by all dorsal and dorsolateral endosternal suspensors (Shultz 1993) not only in whipscorpions, but also in all arachnids lacking extensor muscles in certain leg joints seems premature. As an example, in Ricinulei this cannot be true as they totally lack contractile endosternal suspensors (Firstman 1973). Finally, the possibility should be taken into consideration, that prosomal hemolymph pressure in spiders may be generated by both muscle sets and even extrinsic and intrinsic leg muscles could play a substantial role (Anderson and Prestwich 1975; Whitehead and Rempel 1959; Palmgren 1981; Shultz 1991). Because of these concerns, musculi laterales and endosternal suspensors are both pictured in Fig. 4.1.
3 Muscles Generating Hemolymph Pressure in the Opisthosoma
In almost all spiders a thin subepidermal muscle sheet, the so-called “abdominal sac,” lies as a continuous layer beneath the epidermis of the opisthosoma (Millot 1949; Fig. 4.1). Noticeably, it is composed of smooth musculature (Vosseler 1891). In its physiological properties, this muscle sheet should resemble to the tonically active smooth muscles of vertebrates (Anderson and Prestwich 1975). Contraction of the abdominal sac leads to a decrease of opisthosomal volume and to an increase of hydrostatic pressure there (Wilson 1970; Anderson and Prestwich 1975; Paul et al. 1994). In addition, up to four pairs of dorsoventrally running muscles can occur in the opisthosoma that fully or at least partly consist of smooth muscle fibres (Millot 1936; Geiler and Beier 1971) and are probably also involved in hydraulic pressure production. Furthermore, it seems obvious that several other muscles are able to compress the opisthosoma and so may contribute to increase its fluid pressure (Robinson and Paim 1969; Wilson and Bullock 1973; Stewart and Martin 1974; Anderson and Prestwich 1975).
4 Hemolymph Pressure in Legs and Prosoma
In an inactive theraphosid (Aphonopelma hentzi sub Eurypelma californicum, Nentwig 2012), the hemolymph pressure in legs is slightly higher than in the prosoma and pressure in the prosoma slightly higher than in the opisthosoma, but the differences are small (Stewart and Martin 1974; Paul and Bihlmayer 1995). These differences are obviously necessary and sufficient for circulation of hemolymph and its reflow to the heart. During activity, the prosoma gets flattened, its volume decreases and the internal hemolymph pressure increases. During “normal” walking, prosomal pressures reach roughly 2–3 times the resting values (Anderson and Prestwich 1975; Paul et al. 1989; Paul and Bihlmayer 1995) and may reach peaks during maximum activity of almost 50 times the resting pressure (i.e., 64 kPa; Table 4.1; Stewart and Martin 1974). In legs, the maximum values may be even higher (Table 4.1; Blickhan and Barth 1985). It seems that many of the published values for “resting pressures” in fact represent pressures of motionless, but more or less alert spiders, probably due to experimental conditions (Table 4.1; Wilson 1970; Stewart and Martin 1974; Anderson and Prestwich 1975). Different levels of alertness could explain the variation in published “resting pressures”; the variation in activity pressures could be due to different levels of activity (Table 4.1). Other factors influencing pressures may be feeding status, water uptake, or blood loss (Paul and Bihlmayer 1995).
Parry and Brown (1959b) roughly calculated the activity pressures in the femur–patella joint and the tibia–metatarsus joint of the fourth leg of the salticid Sitticus pubescens before a jump (i.e., before the leg got fully stretched). The unusually high values varied between 65.3 and 144.0 kPa for the femur–patella joint and between 17.3 and 46.7 kPa for the tibia–metatarsus joint, respectively. The differences between the two joints were attributed to flexor muscle tension (Parry and Brown 1959b). Blickhan and Barth (1985) measured a pressure of 130 kPa in a leg of the ctenid Cupiennius salei during autotomy.
5 Leg Joint Extension
Hemolymph enters the legs by two different pathways. First, it flows into the leg arteries and flows back into the prosoma via venous return channels (see Wirkner and Huckstorf 2013). Two of these channels were found in the femur of Pholcus phalangioides (Pholcidae), a smaller one (ventro-anteriorly situated) and a larger one dorso-posteriorly (Paul et al. 1994). Second, when prosomal pressure increases, hemolymph enters the legs via channels (“lacunae”) between the leg muscles. The best-studied leg joint is the hydraulic tibia–metatarsus joint, a hinge joint with a dorsal axis of rotation and a dorsoventral plane of movement (Blickhan and Barth 1985; see also Zentner 2013, Figs. 34.1 and 34.2). Due to the dorsal axis of rotation, no extensor muscles can occur and therefore hemolymph pressure and flexor muscle forces act antagonistically in the tibia–metatarsus joint. The diameters of the hemolymph channels (and thus their hemodynamic resistances) are strongly influenced by intrinsic muscle activity, showing the interplay of internal forces developed both by muscles and by hemolymph pressure. The tibia is almost completely filled with flexor muscles. A dorsal hemolymph channel supplies the adjacent leg segments, while several dorsoventral channels branch off laterally from the dorsal channel and run towards distal, where they empty at the tibia–metatarsus joint (Fig. 4.3). A muscular ring at the proximal end of the metatarsus can be closed and so prevents hemolymph flow from the dorsoventral channels into the dorsal channel or into the metatarsus, in this way enabling extension of the tibia–metatarsus joint independently from the hemolymph filling of the more distal segments. Muscle rings and joint membrane are able to store both hemolymph and energy (Blickhan and Barth 1985).
Simplified illustration of hemolymph channels in the tibia of a spider. Cross sections with hemolymph spaces (black) at different locations are indicated (modified from Blickhan and Barth 1985)
The joint membrane deserves special interest. By raising hemolymph pressure, the membrane gets inflated. During pressure load, axial tensions develop that would lead to high torques opposing joint extension if the membrane were isotropic (black arrows in Fig. 4.4b). The articular membrane in the tibia–metatarsus joint, however, is bellows-like folded. By the special design of this membrane, axial tensions are resolved radially in tangential stresses applying at the dorsal edge of the tibia, in this way preventing unfavourable opposite torques during joint extension (black arrows in Fig. 4.4a; Blickhan and Barth 1985).
Mechanical design of the articular membrane at the tibia–metatarsus joint. (a) The bellows-like folding of the membrane resolves axial tensions, developed under pressure load, in tangential stresses (see text). (b) A hypothetical isotropic membrane would lead to high torques preventing joint extension (modified from Blickhan and Barth 1985)
Indicators of high hemolymph pressures are erectile leg spines as occurring in many freely hunting spiders (Parry and Brown 1959b; Rovner 1980; Weihmann et al. 2010). This erection may not occur during slower movements and needs ca. 4.6 ms to complete in jumping C. salei spiders. Spine erection always occurs simultaneously in all segments of all legs (evidence for a simultaneous increase in hemolymph pressure in all legs) and is coupled with the first visible movements. However, spine erection may take place between 10 ms before and 14 ms after the first visible movement (Weihmann et al. 2010).
Hemolymph pressure within the tibia also serves other functions. For example, it compensates (by absorbing pressure loads) for potential weaknesses of the thin-walled tibial cuticle that could otherwise easily fail by buckling. Torques sufficient for buckling of the tibia in the region of the tibia–metatarsus joint can be compensated by a hemolymph pressure of ca. 20 kPa that lies well within the physiological range of hemolymph pressure inside the leg (Table 4.1). Hemolymph pressure probably also participates in developing torques in other joints than the femur–patella and tibia–metatarsus joint (Blickhan and Barth 1985).
Recently, Weihmann et al. (2010, 2012) proposed that in large spiders (with a body weight of >3 g) at least propulsion of the hind leg is generated by both hydraulics and activity of proximal flexors in the three basal leg joints (body–coxa, coxa–trochanter and trochanter–femur joint, respectively). By pushing the leg against the substrate, flexor muscle contribution to torques in any distal joint (including the two hydraulic joints) was shown to be dominant over hydraulic pressure contribution in hind leg propulsion (Weihmann et al. 2012).
6 Hemolymph Pressure in the Opisthosoma
Hemolymph pressure in the opisthosoma is mostly significantly lower than in the prosoma during high activity, while during moderate activity or rest the pro- and opisthosomal values may come very close (Fig. 4.5; Wilson and Bullock 1973; Stewart and Martin 1974; Anderson and Prestwich 1975; Paul et al. 1989, 1994; Paul and Bihlmayer 1995). The circulation pathways in the opisthosoma are complex and depend on the activity level: During rest and recovery, two separate hemolymph circulations exist, at least in theraphosids, see Wirkner and Huckstorf 2013): (1) an anterior circulation loop in the hemolymph spaces of the pedicel and the most anterior part of the opisthosoma transporting hemolymph from the prosoma to the anterior book lungs and further to the heart and (2) a posterior one more behind, transporting hemolymph from the opisthosoma to the heart via the posterior lungs (Paul et al. 1989). During activity, hemolymph from the opisthosoma returns to the heart via both the anterior and posterior lung veins (Paul et al. 1989, 1994; Paul and Bihlmayer 1995). There are no studies about spiders with only one pair of lungs or with tracheae only, in this respect. The high activity values (Table 4.2) shown in the study of Stewart and Martin (1974) must be seen as exceptional at the moment.
Simultaneous measurement of prosomal and opisthosomal hemolymph pressures of Aphonopelma hentzi (Theraphosidae) during phases of locomotor activity. Note that both pressures could sometimes be linked to each other (a) and could sometimes not be linked (b). Pressure oscillations caused by the heartbeat are superimposed, which is seen most clearly on the prosomal traces during rest and moderate activity (modified from Paul et al. 1995)
7 Hydraulic Interaction Between Prosoma and Opisthosoma
The narrow pedicel (representing the first opisthosomal segment) connects the two main body parts of a spider. It contains various muscles, the gut, nerve cord and aorta passing through but does not show any visible valves that could separate hydrostatically the prosoma from the opisthosoma. Therefore, Wilson (1965) concluded that no such separation exists. In addition, Wilson and Bullock (1973) calculated hemolymph volume decrease in the prosoma during high activity and, associated with this, hemolymph volume increase in the opisthosoma, from recording vertical linear movements of the spider’s (Amaurobius ferox, Amaurobiidae) cuticle. Opisthosomal volume increase during activity could not be shown when the pedicel was ligatured. Based on these and other findings (Anderson and Prestwich 1975), it was concluded that the locomotory exhaustion shown by many spiders after maximum activity should be due to loss of hemolymph from the prosoma into the opisthosoma and to lack of oxygen supply for the prosoma through interruption of the normal hemolymph flow. Hemolymph backflow into the prosoma during recovery should be rather slow. The opisthosomal pressure-generating muscles should attenuate the “hemolymph-loss” into the opisthosoma during activity and enhance recovery from the resulting imbalance of body fluid (Wilson and Bullock 1973; Anderson and Prestwich 1975).
However, the “fluid insufficiency hypothesis” did not withstand later results of various authors. In active Aphonopelma, only a marginal hemolymph flow (<0.1 ml, about 3–3.5 % of the total hemolymph volume) into the opisthosoma at the beginning of activity (that was possibly recorded by Wilson and Bullock 1973) can be observed (Fig. 4.1) that quickly returns into the prosoma after activity. A high prosomal-to-opisthosomal pressure difference can be maintained for the whole activity period with no further shifting of hemolymph into the opisthosoma and hemocytes in the anterior ventral part of the opisthosoma stop flowing during activity (Paul et al. 1989, 1994; Paul and Bihlmayer 1995). Prestwich (1988a, b) presented evidence that the locomotory collapse is not related to unbalanced body fluid but to accumulation of anaerobic metabolic by-products and depletion of energy-rich phosphagen sources. Taken together, these data strongly contradict the fluid insufficiency hypothesis. Hemolymph flow between pro- and opisthosoma also depends on the frequency of carapace depression: In relation to hemolymph flow, the pedicel and the structures behind it (e.g., the book lungs) behave like a low-pass filter: Sinusoidal carapace compression at high frequencies (>8 Hz) resulted in <10 % of hemolymph flow (back and forth) as compared to low frequency (i.e. 0.3 Hz) compression (Paul et al. 1989). Finally, 15 araneomorph species of 11 different families were able to maintain forced running for at least 90 s and voluntary running for more than 15 min, without showing the expected locomotory exhaustion (Bromhall 1987).
At the onset of activity, a small prosomal volume increase in Aphonopelma can be observed, probably caused by opisthosomal muscle contraction (Paul et al. 1989). It is followed by a strong prosomal volume decrease caused by prosomal muscle contraction. In this phase, anteriorly directed hemolymph flow generated by the heart has to cope with increasing prosomal pressure. Therefore, the perfusion of the prosoma of Aphonopelma gets reduced during activity and stops between 6.7 and 9.3 kPa, when prosomal pressure exceeds systolic heart pressure (Paul et al. 1989); however, heartbeat is still maintained, causing posteriorly directed perfusion (Fig. 4.5; Paul et al. 1989, 1994), as is also the case in the above-mentioned araneomorph spiders (Bromhall 1987; see also Wirkner and Huckstorf 2013).
Opisthosomal pressures often follow prosomal ones during moderate activity, but the two may also be unlinked or opisthosomal pressure increase can even be absent (Fig. 4.5). These and other results obtained from injecting casting resin into hemolymph spaces indicate an occlusion mechanism at the anterior end of the pedicel, probably consisting of muscles having a valve function (Paul et al. 1994; Paul and Bihlmayer 1995). These muscles, however, have not yet been identified
8 Spiders with an Opisthosomal Hemolymph Pressure Pump
The data presented above stem mainly from a handful of species. It seems probable that the demands on the pressure-generating system of spiders vary tremendously between different spider taxa, depending on their lifestyle, ecological demands, favoured prey capture strategy, anatomical constraints, etc. An example for such an anatomically constrained taxon is given here and concerns spiders with a strongly sclerotized body, especially with a sclerotized pleura.
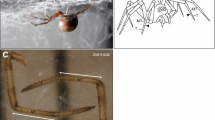
In spiders of the family Tetrablemmidae (and in species of different other families), the pleura is strongly sclerotized and immovably connected to both carapace and sternum (Fig. 4.6a). In this way the prosoma forms an extremely stiff capsule. Such a capsule seems unable for hemolymph pressure generation, as one precondition for the necessary volume decrease, a soft and flexible pleura, is missing. Simple deformation tests in freshly killed specimens of the tetrablemmid Perania nasuta by using a forceps show, that a dorsoventral compression of the prosoma is practically impossible because of the hardiness of the cuticle. Not even minuscule leg movements can be elicited by doing so and the cuticle rather breaks than to deform even slightly. However, already a minor dorsoventral compression of the opisthosoma immediately resulted in well-defined extension of all eight legs in the femur–patella joint and the tibia–metatarsus joint.
Female of Perania nasuta (Tetrablemmidae) in lateral view. (a) Prosoma, legs and hairs omitted. Note the inflexible and strongly sclerotized pleura. (b) Opisthosoma. Note the cuticular folds with lateral longitudinal rows of sclerites. ds dorsal scutum, lf lateral folds with sclerites, p pedicel tube, sp spinnerets, vs ventral scutum
The opisthosoma shows a dorsal and a ventral scutum and several longitudinal furrows laterally (Fig. 4.6b). In these furrows, sclerites are situated that serve as muscle attachments. The smooth subepidermal muscle layer in the opisthosoma (“abdominal sac”) is strongly modified in Perania and in another species of tetrablemmid, Indicoblemma lannaianum. The abdominal sac is unusually strongly developed and partitioned into powerful dorsoventrally running muscles that attach at the lateral sclerites and at the dorsal or ventral scutum, respectively (Fig. 4.7). Obviously, a contraction of these muscles leads to a volume decrease in the opisthosoma and to an increase of hemolymph pressure. I propose that in Tetrablemmidae and in other armoured spider taxa with a similar sclerotisation of the body, it is the modified abdominal sac that mainly generates the hemolymph pressure necessary for leg joint extension. Detailed work about these problems will be published elsewhere.
9 Conclusions
Spiders lack extensor muscles in the main joints of their walking legs (femur–patella and tibia–metatarsus joint, respectively). These leg joints are extended by a rise of hemolymph pressure in the prosoma, caused by contraction of prosomal dorsoventrally running muscles (musculi laterales or endosternal suspensor muscles, or both). This leads to volume decrease in the prosoma (made possible by soft and flexible pleurae) and to hemolymph flow into the legs. Inside the legs, hemolymph flows in channels (“lacunae”) whose cross sections are strongly influenced by intrinsic muscle activity. In hydraulic leg joints, only flexor muscles occur that act antagonistically to hemolymph pressure. The articular membranes in leg joints are bellows-like folded, in this way preventing unfavourable torques opposing joint extension. During maximum activity, perfusion of the prosoma stops because prosomal pressure exceeds systolic heart pressure. In the opisthosoma, a smooth subepidermal muscle-sheet (“abdominal sac”) and several other muscles generate hemolymph pressure that is considerably lower than prosomal pressure during high activity, but may be very close to it during rest or moderate activity. Opisthosomal pressures are sometimes linked to prosomal ones, but sometimes not. This implies (contrary to previous opinions) a temporary hydrostatic separation between pro- and opisthosoma, probably due to a muscular valve at the anterior end of the pedicel that is not yet defined anatomically. Only few spider species have been studied with respect to body hydraulics so far, and various deviations of the here described hydraulic system may occur. For example, in some spiders the prosoma forms an extremely stiff capsule that is almost impossible to deform and therefore unable to generate hemolymph pressure. It is suggested that these spiders produce hemolymph pressure for leg extension mainly in the opisthosoma by contraction of a strongly modified abdominal sac.
References
Anderson JF, Prestwich KN (1975) The fluid pressure pumps of spiders (Chelicerata, Araneae). Z Morph Tiere 81:257–277
Blickhan R, Barth FG (1985) Strains in the exoskeleton of spiders. J Comp Physiol A 157:115–147
Bromhall C (1987) Spider heart-rates and locomotion. J Comp Physiol B 157:451–460
Brown RB (1939) The musculature of Agelena naevia. J Morphol 64:115–166
Firstman B (1973) The relationship of the chelicerate arterial system to the evolution of the endosternite. J Arachnol 1:1–54
Foelix RF (2011) Biology of spiders. Oxford University Press, New York
Geiler H, Beier R (1971) Nachweis glatter Muskulatur im Opisthosoma von Araneus diadematus Clerck. Zool Anz 187:434–438
Manton SM (1958) Hydrostatic pressure and leg extension in arthropods, with special reference to arachnids. Ann Mag Nat Hist 13:161–182
Millot J (1936) Métamérisation et musculature abdominale chez les Araneomorphes. Bull Soc Zool France 61:181–204
Millot J (1949) Ordre des Aranéides (Araneae). In: Grassé P (ed) Traité de Zoologie 6. Masson et Editeurs, Paris
Nentwig W (2012) The species referred to as Eurypelma californicum (Theraphosidae) in more than 100 publications is likely to be Aphonopelma hentzi. J Arachnol 40:128–130
Palmgren P (1978) On the muscular anatomy of spiders. Acta Zool Fenn 155:1–41
Palmgren P (1980) Some comments on the anatomy of spiders. Ann Zool Fenn 17:161–173
Palmgren P (1981) The mechanism of the extrinsic coxal muscles of spiders. Ann Zool Fenn 18:203–207
Parry DA, Brown RHJ (1959a) The hydraulic mechanism of the spider leg. J Exp Biol 36:423–433
Parry DA, Brown RHJ (1959b) The jumping mechanism of salticid spiders. J Exp Biol 36:654–664
Paul RJ, Bihlmayer S (1995) Circulatory physiology of a tarantula (Eurypelma californicum). Zoology 98:69–81
Paul RJ, Tiling K, Focke B, Linzen B (1989) Heart and circulatory functions in a spider (Eurypelma californicum): the effects of hydraulic force generation. J Comp Physiol B 158: 673–687
Paul RJ, Bihlmayer S, Colmorgen M, Zahler S (1994) The open circulatory system of spiders (Eurypelma californicum, Pholcus phalangioides): a survey of functional morphology and physiology. Physiol Zool 67:1360–1382
Prestwich KN (1988a) The constraints of maximal activity in spiders I. Evidence against the fluid insufficiency hypothesis. J Comp Physiol B 158:437–447
Prestwich KN (1988b) The constraints of maximal activity in spiders. II. Limitations imposed by phoshagen depletion and anaerobic metabolism. J Comp Physiol B 158:449–456
Robinson GL, Paim U (1969) Opisthosomal musculature of female Araneus diadematus (Araneae: Argiopidae). Can Entomol 101:337–352
Rovner J (1980) Morphological and ethological adaptations for prey capture in wolf spiders (Araneae, Lycosidae). J Arachnol 8:201–215
Shultz JW (1989) Morphology of locomotor appendages in Arachnida: evolutionary trends and phylogenetic implications. Zool J Linn Soc 97:1–56
Shultz JW (1991) Evolution of locomotion in Arachnida: the hydraulic pressure pump of the Giant Whipscorpion, Mastigoproctus giganteus (Uropygi). J Morphol 210:13–31
Shultz JW (1993) Muscular anatomy of the giant whipscorpion Mastigoproctus giganteus (Lucas) (Arachnida: Uropygi) and its evolutionary significance. Zool J Linn Soc 108:335–365
Stewart DM, Martin AW (1974) Blood pressure in the tarantula, Dugesiella hentzi. J Comp Physiol 88: 141–172
Vosseler J (1891) Untersuchungen über glatte und unvollkommen quergestreifte Muskeln der Arthropoden. Laupp, Tübingen
Weihmann T, Karner M, Full RJ, Blickhan R (2010) Jumping kinematics in the wandering spider Cupiennius salei. J Comp Physiol A 196:421–438
Weihmann T, Günther M, Blickhan R (2012) Hydraulic leg extension is not necessarily the main drive in large spiders. J Exp Biol 215:578–583
Whitehead WF, Rempel JG (1959) A study of the musculature of the Black Widow Spider, Latrodectus mactans (Fabr). Canad J Zool 37:831–870
Wilson RS (1962) The control of dragline spinning in the garden spider. Q J Microsc Soc 104: 557–571
Wilson RS (1965) The pedicel of the spider Heteropoda venatoria. J Zool 147:38–45
Wilson RS (1970) Some comments on the hydrostatic system of spiders (Chelicerata, Araneae). Z Morph Tiere 68:308–322
Wilson RS, Bullock J (1973) The hydrostatic interaction between prosoma and opisthosoma in Amaurobius ferox (Chelicerata, Araneae). Z Morph Tiere 74:221–230
Wirkner C, Huckstorf K (2013) The circulatory system of spiders. In: Nentwig W (ed) Spider ecophysiology. Springer, Heidelberg (this volume)
Zentner L (2013) Modelling and application of the hydraulic spider leg mechanism. In: Nentwig W (ed) Spider ecophysiology. Springer, Heidelberg (this volume)
Acknowledgements
Thanks go to P. Schwendinger (Muséum d’Histoire naturelle, Genève, Switzerland) for kindly providing living specimens of P. nasuta, M. Burger (Honolulu, Hawaii) for the serial sections of I. lannaianum, T. Sonderegger and M. Hohn (Bern, Switzerland) for the graphics design.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kropf, C. (2013). Hydraulic System of Locomotion. In: Nentwig, W. (eds) Spider Ecophysiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33989-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-33989-9_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33988-2
Online ISBN: 978-3-642-33989-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)